Abstract
Furosemide a diuretic is a Biopharmaceutical Classification System class IV drug which shows pH dependent solubility and permeability. Furosemide inhibits water reabsorption in the nephron by blocking the sodium potassium co-transporter in the thick ascending limb of the loop of Henle. It is very poorly soluble in stomach medium and having high permeability through stomach but its solubility increases with pH but is impermeable through intestine due to its permeability limitation. The objectives of this study was to prepare sustained release floating tablets of Furosemide a potent loop diuretics using polymer HPMC K15M, HPMC K100M, EMPRESS SR and study its release profile. The formulation F1, F2, F3contained HPMC K15M and F4, F5, F6 contained HPMC K100M and F7, F8, F9 contained EMPRESS SR. Floating tablet play an important role to increase the gastro-retention time in the stomach. Floating tablets were prepared by direct compression using polymers in different percentage and evaluated for parameter like weight variation, hardness, friability, floating time, dissolution and assay.
Keywords
HPMC K15M, HPMC K100M, EMPRESS SR, Furosemide, Floating Lag Time, Total Floating Time, Dissolution
Introduction
Oral Drug Delivery is most feasible and widely used route of administration due to convenience, cost effectiveness and high patient compliance (1). It is frequently necessary to take a conventional dosage form numerous times a day in order to achieve and maintain the drug concentration in the body within the therapeutic range required for a therapeutic action (2). Since novel dosage forms stay in the stomach longer than conventional dosage forms, the ability to extend and control the emptying time is a major asset. Gastric emptying of dosage forms is a highly variable process. The ability to limit the dose form in the desired region of the gastrointestinal tract is one of these challenges. In order to address this physiological issue, a number of drug delivery methods that extend the duration of stomach retention have been studied (3). Furosemide is slightly yellowish powder, insoluble in water, completely soluble in acetone and very slightly soluble in chloroform (15). Furosemide (FUR) is a potent loop diuretic, it is the first and the prototype drug of high ceiling (loop) diuretics. Furosemide is classified as Biopharmaceutical Classification System (BSC) class IV drug which shows pH dependent solubility and permeability. FUR inhibits water reabsorption in the nephron by blocking the sodium-potassium-chloride co-transporter in the thick ascending limb of the loop of Henle. The absolute bioavailability of furosemide is about 60-70%. It is chemically described as 4-chloro-2-[(furan-2-ylmethyl) amino]-5-sulfamoylbenzoic acid (20). Drug of choice for the reduction of the acute pulmonary edema of heart failure. In acute pulmonary edema because of rapid onset of action particularly when given intravenously therefore this is use in emergency cases (21). It is very poorly soluble in stomach medium and having high permeability through stomach, but its solubility increase with pH but it is impermeable through intestine due to its permeation limitation (22).

Fig 1 structure of furosemide
Floating Drugs Delivery System (FDDS) is a water boat system that rests on liquid and is positioned so that it rests on stomach acid for a long time, increasing the absorption of medications in limited absorption sites. Davis originally described FDDS in 1968 (4,5).
Bulk density should be less than 1 in order to float on stomach acid. There are two types of floating dosage forms i.e.
- Non effervescent and
- Effervescent floating dosage form (4,5).
NON EFFERVESCENT FDDS
These dosage forms come in single units and contain one or more hydrophilic gel-forming polymers. The most widely used excipient is Hydroxypropyl methylcellulose (HPMC), however other options include carrageen, sodium carboxymethyl agar, ethyl cellulose (EC), and Hydroxypropyl cellulose (HPC). The medication is combined with the polymer and often filled in a hard gelatin capsule. The capsule dissolves quickly in the stomach juice, and floating mass is created is formed as the polymers swell and hydrate. (6,7). Non effervescent system further classified into
- Colloidal gel barrier system / hydrodynamically balanced systems (HBS)
- Microporous compartment system
- Alginate beads
- Microballoons / Hollow microspheres
- Layered tablets
EFFERVESCENT FDDS
These are matrix-type systems made with different effervescent ingredients like calcium carbonate, sodium bicarbonate, citric acid, or tartaric acid, and swellable polymers like hydroxypropyl methylcellulose, polysaccharides, and chitosan. These dosage forms are designed such that carbon dioxide is released and trapped in the swelling hydrocolloids when they come into contact with gastric fluid in the stomach. This gives the dosage form into buoyancy state. If the tablet is single layered, the released carbon dioxide may become thoroughly mixed with the matrix of the tablet. (8).
Effervescent system further classified into:
- Gas generating system
- Volatile/Vacuum containing systems (9).
- Advantages of floating drug delivery system (10,11,12).
- Makes the medication more bioavailable when taken orally.
- Enhanced first pass biotransformation.
- Sustained drug delivery/ reduced frequency of dosing.
- Less variation in the drug's plasma levels.
- Enhanced selectivity of receptor activation.
- Provide higher efficiency due to reduced counter-activity of body.
- Extended time over critical (Effective) concentration.
- Reduced negative colonic activity
- Targeted therapy for local ailments within the upper GIT.
- Site specific Drug Delivery.
Limitations of floating drug delivery system (13,14).
- Drugs having solubility or stability problem in GIT aren't suitable for FDDS
- Medicines that undergo first pass metabolism and are well absorbed throughout the GIT, such as propranolol and nifedipine, are not ideal candidates.
- It is also undesirable to use medications that irritate the stomach mucosa.
- This system is not appropriate for medications unstable in the acidic environment.
- High level of fluid in the stomach is required for maintaining buoyancy; float and work efficiently
MATERIALS AND METHODS
Materials
HPMC K15M, HPMC K100M, was obtained as a gift sample from QMED Formulation Pvt.Ltd, chhaling, Bhaktapur. Sodium bicarbonate, Aerosil, Furosemide MCCpH102 Magnesium stearate, Lactose, PVP K30, Empress SR was used from CTL pharmaceuticals Pvt. Ltd. Byasi, Bhaktapur.
Instruments Used
The instrument or equipments used during the work are as follows:
Table 1: List of Instruments used

METHODS
Preformulation
Melting Point
API was filled in capillary tube then placed in melting point apparatus. The time at which API started to melt was noted (15).
Loss on Drying
1gm of Furosemide was taken and placed in hot air oven for 2hrs at 105oC. The initial weight (1gm) of Furosemide was weighted then placed in hot air oven for 2hours at 105oC. After drying again weight of dry Furosemide was weighted (14).
% Loss on Drying = weight before drying - weight after drying × 100 ……equation 1
Weight before drying
Bulk Density, Tapped Density
API was filled up to certain volume in graduated cylinder and the level of volume was noted (bulk volume). Then the cylinder was placed in apparatus for tapping the powder and final volume was measured (16).
Bulk density = Weight of Sample …………………………………equation 2
Volume
Identification Test
A 0.0005% w/v solution of sample in 0.1M NaOH was examined in range of 220nm-360nm in UV spectrophometer (4).
UV Spectrophotometric Study
Furosemide (0.1gm) was accurately weighted and transferred to a 50ml V.F. It was dissolved and diluted to 50ml with pH 5.8 Phosphate buffer to obtained a final concentration of 100µg/ml. Dilution were made to obtained a concentration of 10µg/ml and scanned for ?max in a range of 200-400nm in the spectrum basic mode for 3 consecutive days. (15).
Preparation of calibration curve
The stock solution of Furosemide (100µg/ml) were pipette out into a series of 10ml V.F and diluted with pH 5.8 Phosphate buffer to get final concentration in the range of 2-10µg/ml. The absorbance of the resultant solutions was measured at 270nm (15).
Compatibility study of Furosemide
Compatibility studies were performed using IR spectrophotometer. IR spectrum of pure drug and physical mixture of drug and polymer were studied. Drug- excipients interactions play a vital role with respect to release of drug from the formulation amongst others. FTIR techniques have been used here to study the physical and chemical interaction between drug and excipients used. (17)
Methods
Preparation of Floating Tablet of Furosemide
Furosemide was weighted and sieve through sieve no 80 then, HPMC K15M, HPMC K100M, MCCpH 102, Sodium bicarbonate, Aerosil, Magnesium stearate, Lactose, PVP K30 were weighted and then passed through the sieve i.e. 30 mesh size then mixed all ingredients in polythene for 15mins. Then mixed powder was compressed in Tablet Compression Machine (18, 19). The formulation was shown in table:
Table 2 Formulation table of tablet

Preparation of 0.1N HCL
8.57ml of HCL was taken in 1000ml V.F then volume was maintained with Distilled Water. 51.5 ml of HCL was taken for 6000ml.
Evaluations of Granules/Powder
Bulk Density, Tapped Density
Granules were filled up to certain volume in graduated cylinder and the level of volume was noted (bulk volume). Then the cylinder was placed in apparatus for tapping the powder and final volume was measured (16).
Carr’s index and Hausner’s, s ratio
From the data obtained from bulk density and tapped density Carr’s index and housners ratio was calculated for the flow property and compressibility index.
Carr’s index % = Tapped density - Bulk density ×100 ………. equation 3
Tapped density
Housner ratio (H) = Tapped density …………………………equation 4
Bulk density
Evaluations of Tablets
Assay
Twenty tablets were taken and powdered. The powder equivalent to 0.1gm of FUR was taken in 250ml V.F and dissolved with 150ml of 0.1M NaOH and shake for 10mins then make up the volume with 0.1M NaOH and filtered the solution. 5ml of filtrate was diluted to 200ml with 0.1M NaOH and measured the absorbance of the resulting solution at maxima 271nm.
Weight Variation
20 tablets were weighted individually. Average weight was calculated from the total weight of all tablets. The individual weights were compared with the average weight. The percent deviation was calculated.
?viation = Individual weight -Average weight× 100……………equation 5
Average weight
Dimension
Thickness and diameter of the tablet was measured by using Vernier caliper. Tablet was placed between lower jaws of Vernier caliper then reading was noted.
Hardness Test
Hardness of the tablet was tested using Monsanto’s hardness tester. The tablet was placed between two anvils, force applied to the anvils and the crushing strength that just cause the tablet to break was recorded.
Floating lag time and floating time
The term "floating lag time" refers to the period of time that passes after the tablet is introduced into the medium until it rises to the upper one-third of the dissolution vessel. The word "floating/flotation time/total floating time" refers to the amount of time that the dosage form floats. These tests are typically conducted using dissolution apparatus that contains dissolution medium or Simulated Gastric Fluid (SGF) or 0.1 N HCl (900ml) kept at 37°C (32). The tablets were placed in a 100 ml beaker containing 0.1N HCl. The time required for the tablet to rise to the surface and float was considered as the floating lag time.
Friability
20 tablets were weighted collectively and placed in the chamber of the friability test apparatus. In the friability test apparatus, the tablets were exposed to rolling, resulting from free fall of tablets within the chamber of the friability test apparatus. After 100 rotations (i.e in 4mins), the tablets were taken out of the friability test apparatus and intact tablets were again weighted collectively. Permitted friability limit is 1%.
%Friability = weight of tablet before test - weight of the tablet after test× 100%…equation 6
` Weight of tablet before test
Dissolution
900ml of 0.1N HCL was taken in a dissolution vessel at given temperature (37±0.5) and dissolution was carried out for 8hours at 50rpm. The dissolution was carried out for given time interval where the sample were withdrawn at particular interval and analyzed by UV spectrophotometer at 271nm. 10ml sample of a solution was withdrawn from the dissolution apparatus hourly for eight hours and then were replaced with fresh dissolution medium. The sample was filtered wattman filter paper.
Results and discussion
EVALUATION OF API (FRUSEAMIDE)
Physical Appearance
The physical appearance of the API is as per table 3
Table 3: Physical appearance

Melting Point
Melting point of standard Furosemide was found to be 209.67±0.192°C n=3 as given in table 4
Table 4: Melting point determination

Determination of solubility
The furosemide is found to be insoluble in water, sparingly soluble in ethanol, slightly soluble in chloroform but freely soluble in NaOH, acetone which is shown in table 5.
Table 5: Solubility Test

Note
+ = Insoluble
++ = Poorly Soluble
+++ = Slightly soluble
++++ = Freely soluble
Determination of ?max
Spectrophotometric study was carried out in order to determine the ?max of Furosemide in 0.1M NaOH. 10 µg/ml solution of furosemide in the test medium when scanned for absorption maxima in the range of 200-400nm, exhibited the results as given in table 6 on three consecutive day.
Table 6: Determination of ?max of Furosemide

The scanned ?max were found to be similar as that of reported ?max and the difference in absorbance value for three determinations was found to be similar.
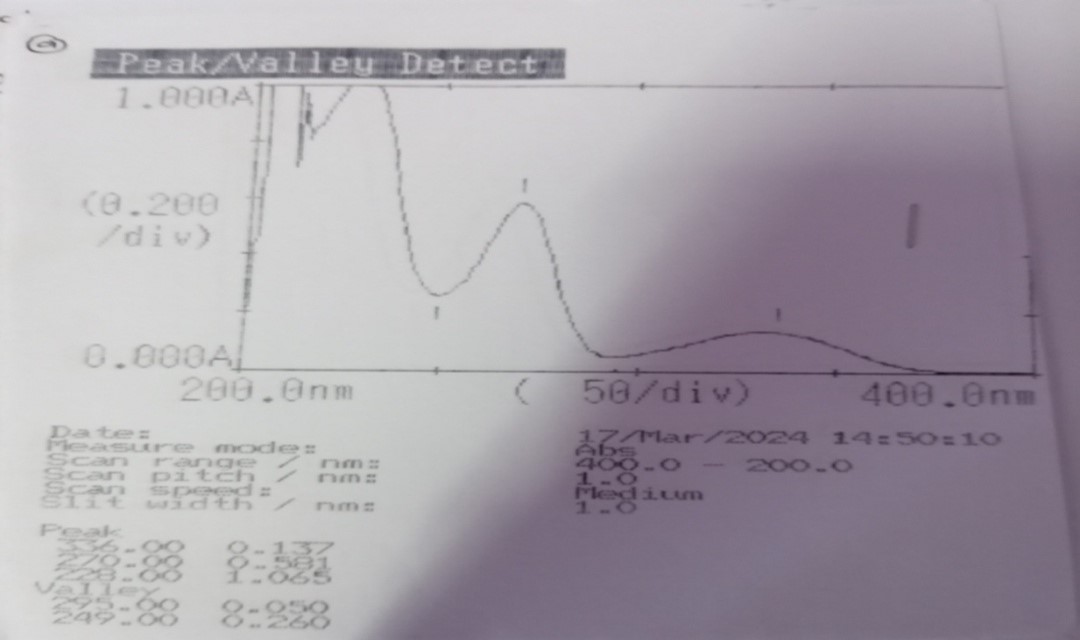
Fig:2 scanned ?max of furosemide
Identification
The ratio of the absorbance of the maximum at about 270nm to that at the maximum at about 228nm is 0.544 which was between the given ranges 0.52 to 0.57 as given in Indian pharmacopeia

Fig 3: Identification chart of furosemide
Preparation of standard calibration curve in phosphate buffer 5.8
The standard calibration curve of maximum absorbance of different concentration is given in table 7.
Table 7: Calibration curves data of furosemide using pH 5.8 phosphate buffers


Fig 3: Standard calibration curve
The linear relationship was obtained in beer-lambert plot of Furosemide in 0.1 M NaOH (y = 0.053x , R2 = 0.997).
Loss on drying (LOD)
Weight of weighing bottle of a sample (a) = 23.3675gm
Weight of the sample & weighing bottle (b) = 24.406gm
Weight of the sample & weighing bottle after drying (c) = 24.406gm
Loss on drying % = 0.0%
Hence, Loss on drying was found to be 0.0% which lies below the 0.5%
Bulk density and Tapped density
The bulk density of the API was found to be 0.3365g/ml whereas tapped density of the granules was found to be 0.6514gm/ml.
Carr’s index and Hausner’s ratio
The Carr’s index of the API furosemide was found to be 48.34%.
Hausner’s ratio of the API powder was found to be 1.936
Compatibility study of drug with polymer and Excipients
The physical mixture of drug Furosemide and polymer shows no interaction with each other as there was no changes found in the base peak of furosemide in FT-IR as shown in graphs below

Fig: 4 Graph showing IR peak of Furosemide

Fig: 5 Graph showing IR peak of API and HPMC

Fig 6 Graph showing IR peak of API and EMPRESS SR

Fig:7 Graph showing IR peak of API and Excipients
valuation of granules/powder
The bulk density and tapped density along with Carr’s index and Hausner ratio of granules are shown in the table
Table 8 bulk density and tapped density of formulated granules

Evaluation of tablet
Uniformity of weight
The weight uniformity of different formulation was determined weight uniformity was determined by the help of analytical balance. The weight ranged between 197mg – 205mg. The mean weight variation tests results are tabulated and given ANNEX I. All the tablet passed weight variation test as the average percentage weight variation was with ±5% i.e. in the pharmacopoeia limits.
Hardness
The hardness of the tablets prepared by direct compression method was maintained within the 5 kg/cm2. The hardness was found to be between 3-6 kg/cm2 was given in ANNEX I.
Thickness and diameter
The thickness of the tablets was found to be 3.5-3.933 and the diameter of the tablets was found to be 8mm and given in ANNEX I.
Friability
The friability was found in all designed formulation in the range 0.39 – 0.77% to all within the approved range (<1>
Floating lag time
Floating lag time was found to be in the range of 25 secs – 50sec and all detail of each formulation are given in ANNEX I.
Total floating time (hour)
Total floating time was found to be in the range of 12 - 14hrs. All the values are given in given table of ANNEX I.
Assay
The Assay of different batches were found to be between the limit mentioned in IP. According to IP, the acceptable criteria for tablet are within 90 - 110%. The assay percentage of all the batches with their respective absorbance values are given in ANNEX I.
Dissolution study
The in – vitro dissolution studies were performed for prepared tablets using 0.1N HCl at 50 rpm andtemperature at 37±0.5°c. The In-vitro tablets dissolution profiles of tables were shown in figure and under ANNEX II. Cumulative % drug release of formulation F1-F9 at 12 hours was found to be in the range 10.5% - 94.9%. The result indicated that among all the polymers F1 formulation which has 7% HPMC K15M has shown retardation of drug release with 94.9% of the drug release rate at the end of 12 hours. F2 with 10.5% HPMC K15M has 92.3% release even at 12 hours which also has shown retardation release profile compared to F1 and F3 with 14% HPMC K15M has 81.6% release till 12 hours which increase time required to release drug in comparison with F1 and F2 formulation. Among the formulation HPMC K100M, F4 has showed the 86 % drug release with 12 hours. F5 has showed 84% drug release at the end of 12 hours and F6 has showed 81% drug release at the end of 12 hours and among the formulation of EMPRESS SR there is release of 95%, 85% and 81% in 12 hours with the polymer concentration of 15%,20%and 25% respectively
From the result it showed that the release rate was higher for formulation containing low level of different polymer compared with other formulations containing lower level, due to higher concentration of polymer, drug may have entrapped within the polymer matrix causing a decrease in rate of drug release.

Fig 9: Dissolution Comparison Graph
CONCLUSION:
Sustained release floating tablets which release drug over a long period of time within therapeutic concentration would be the best possible approach to achieve better and prolonged effect of furosemide. It will increase patient compliance, avoid multiple frequency of dose, avoid night time dosing. The study was concentrated on formulation and evaluation of the tablets and to determine the best formulation among present formulations. The totals of nine formulations were designed. Each of the formulation designed was evaluated for the pre compression parameter and post compression parameter. The result of pre compression parameter that the uniform tablets were obtained with weight variation of ±5% which complies with IP specification limit. The post compression parameter of tablet like friability and assay were evaluated. The result shows that all the formulations pass friability having maximum loss of 0.77% and also assay of all the formulation was within the limit of 90 to 110%. From the result it showed that the release rate was higher for formulation containing low level of HPMC K15M compared with other formulations containing higher level, due to higher concentration of polymer, drug may have entrapped within the polymer matrix causing a decrease in rate of drug release. Floating lag time was increased with increasing concentration of sodium bicarbonate.
The result of the present study indicated that Empress SR showed the good result among all nine of formulation. It showed the best result because the release of drug was increasing constantly which follow the first order reaction where as the increase viscosity of the polymer show the good result. From the result, it can be concluded that increase in concentration of polymer will increase the time required to release drug
Relevant conflict of interest/financial disclosure:
This author declare that the research was conducted in the absence of any commercial or financial relationship that could be constructed as a potential conflict of interest
ANNEX I: Physical Parameter Table

ANNEX II: Release Kinetics (Time Vs Cumulative % drug release)

REFERENCES
- Naz A, Sadia S, Zama MA, Tarannum SF. Design and evaluation of gastroretentive floating tablets of diuretic furosemide.
- Choubey S, Patel A, Patel S, Dwivedi N. Design, development and evaluation of floating microspheres of furosemide by mixed solvency concept.
- Dixit N. Floating drug delivery system. Journal of current pharmaceutical research. 2011;7(1):6-20.
- Dr. Yajaman Sudhakar, Dr. K.N.Jayaveera, Novel Drug Delivery System and regulatory affairs Book, S.CHAND.
- Raymond C Rowe, Paul J Sheskey, Marian E Quinn, Handbook of Pharmaceutical Excipients, Sixth edition, 2009, Pharmaceutical Press
- Menon A, Ritschel W.A., Sakr A. Development and evaluation of a monolithic floating dosage form furosemide. J Pharm Sci. 1994; 83:239-245.
- Oth M, Franz M, Timmermans J, Moes A. The bilayer floating capsule: a stomach directed drug delivery system for misoprostal. Pharm Res. 1992; 9:298-302
- Bhosale AR, Shinde JV, Chavan RS, A Comprehensive Review on Floating Drug Delivery System (FDDS), Journal of Drug Delivery and Therapeutics. 2020; 10(6):174-182
- Vedhahari b.n.et al, 2010. Development of a novel controlled-release system for gastric retention. Pharm Res.1997; 14:815-819
- Dubey J, Verma N, Floating Drug Delivery System: A Review, IJPSR, 2013; 4(8):2893-2899.
- Baviskar P, Patil P, Saudagar RB, Floating Drug Delivery System: A Comprehensive review, Journal of drug delivery and therapeutics, 2019; 9(3-s): 839-846.
- Pakhale NV, Gondkar SB, Saudagar RB, Effervescent Floating Drug Delivery System: A Review, Journal of Drug Delivery and Therapeutics. 2019; 9(3-s):836-838.
- Singh LP et al, Floating effervescent Tablet: A Review, JPBMS, 2011; 5(11):1-6. 11).
- Pawar VK, Shaswat K, Garg G, Awasthi R, Singodia D, Kulkarni GT, Gastroretentive Dosage For ms: A review with special emphasis on floating drug delivery systems, Drug Delivery, 2011; 18(2):97-110.
- Satendra kumar, Arun Kumar Mishra. Preformulation study of furosemide. 2016,8(13):214-222
- Karkhile VG, Karmarkar RR, Sontakke MA, Badgujar SD, Nemade LS. Formulation and evaluation of floating tablets of furosemide. Int. J. Pharm. Res. Dev. 2010;1:1-9
- Deepak Jain, Sofiya Verma, Shashi Bharti Shukla, Alok Pal Jain, Prakash Jain and Priyanka Yadav, Formulation and evaluation of gastroretentive tablets of Furosemide (Evaluation based on drug release kinetics and factorial designs) J. Chem. Pharm. Res., 2010, 2(4):935-978
- Niaz S, Naqvi SB, Asghar MA, Mumtaz N, Khaliq SA. Research paper Formulation and Evaluation of Sustained Release Matrix Tablets of Furosemide Using Different Polymers. RADS Journal of Pharmacy and Pharmaceutical Sciences. 2018 Jun 20;6(2):144-51.
- Kondapuram Parameshwar, Mounika divya Bharath, Muthadi Radhika Reddy, Chindam suresh and T.Anessha, “Formulation and Evaluation of Sustained Released Matrix Tablet of Domperidone, EJPMR, 2017,4(8),509-524.
- Kumar S, Nanda A. Formulation, optimization and in vitro evaluation of gastroretentive mucoadhesive microspheres of furosemide. International Journal of Pharmacy and Pharmaceutical Sciences. 2016;8:392-8.
- Ho KM, Power BM. Benefits and risks of furosemide in acute kidney injury. Anaesthesia. 2010 Mar;65(3):283-93.
- Chittam S, Bhosale A. Development and Evaluation of Floating and Expanding Gastroretentive Film of Furosemide. International Journal of Pharmaceutical Investigation. 2020 Jun 8;10(2):179-83.


 RAM PRASAD KHOTEJA*
RAM PRASAD KHOTEJA*
 Aarti Kori
Aarti Kori



















 10.5281/zenodo.13333791
10.5281/zenodo.13333791