Abstract
Anxiety disorders, including generalized anxiety disorder (GAD), panic disorder, post-traumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD), present a significant public health and economic burden globally. Traditional pharmacotherapies, primarily targeting the ?-aminobutyric acid (GABA) and serotonergic systems, are associated with substantial limitations, including treatment resistance and side effects. Emerging research suggests that dysregulation in glutamatergic neurotransmission may play a key role in anxiety pathophysiology, offering novel therapeutic targets. Neuroimaging studies have identified structural and functional alterations in glutamate-rich regions, such as the hippocampus and amygdala, in individuals with anxiety disorders. Moreover, pharmacological modulation of glutamate receptors, including NMDA, AMPA, and metabotropic glutamate receptors, has demonstrated efficacy in reducing anxiety symptoms. Agents such as D-cycloserine (NMDA partial agonist) and riluzole (glutamate release inhibitor) have shown promise in alleviating symptoms of PTSD and OCD. These findings support the potential of glutamate-based therapies as an innovative approach to treating anxiety disorders. Further research is warranted to elucidate the precise mechanisms and optimize the clinical application of glutamate-targeting treatments.
Keywords
Glutamate, Anxiety Disorders, Neuroimaging, Pharmacotherapy, NMDA Receptor, PTSD, OCD
Introduction
Anxiety disorders, encompassing generalized anxiety disorder (GAD), post-traumatic stress disorder (PTSD), panic disorder, and obsessive-compulsive disorder (OCD), are among the most common and debilitating psychiatric conditions globally [1, 2]. These disorders not only significantly impair individual well-being and daily functioning but also contribute to a substantial economic burden through increased healthcare costs and diminished productivity due to absenteeism and disability. Current pharmacological interventions primarily focus on modulating the ?-aminobutyric acid (GABA) and serotonergic systems, utilizing agents such as benzodiazepines and selective serotonin reuptake inhibitors (SSRIs). However, these treatments are frequently associated with limitations, including partial efficacy, treatment resistance, and adverse side effects such as sedation, cognitive impairment, and the risk of dependency, which can lead to poor adherence and relapse. Emerging evidence has identified the glutamatergic system as a crucial player in the neurobiology of anxiety disorders. Dysregulation in glutamate signaling, particularly within the NMDA (N-methyl-D-aspartate) and AMPA (?-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor pathways, is implicated in the pathogenesis of anxiety-related behaviors. Pharmacological modulation of these receptors presents a novel therapeutic strategy [3-7]. Agents such as D-cycloserine, a partial NMDA receptor agonist, and riluzole, a glutamate release inhibitor, have demonstrated efficacy in attenuating symptoms in anxiety disorders, including PTSD and OCD. These findings highlight the therapeutic potential of targeting glutamatergic transmission, offering a new frontier in the treatment of anxiety disorders that may overcome the limitations of traditional therapies. Continued research is essential to further elucidate the precise mechanisms by which glutamatergic modulation can be optimized to improve clinical outcomes for patients suffering from anxiety-related conditions [8-10].
Mechanistic Role of Glutamate in Anxiety: Receptor Dynamics and Neural Circuitry
Glutamate, the brain's principal excitatory neurotransmitter, plays a vital role in anxiety regulation by influencing key neuronal circuits involved in emotional responses, stress adaptation, and fear processing. The underlying mechanism by which glutamate impacts anxiety is closely linked to its action on both ionotropic and metabotropic glutamate receptors. These receptors include NMDA (N-methyl-D-aspartate), AMPA (?-amino-3-hydroxy-5-methyl-isoxazolepropionic acid), kainate receptors, and various metabotropic glutamate receptors (mGluRs), which are widely distributed across critical brain regions like the amygdala, hippocampus, prefrontal cortex, and anterior cingulate cortex—areas essential for emotional regulation and implicated in anxiety disorders [11-17]. During periods of stress or elevated anxiety, glutamate release tends to increase, resulting in overactivation of these receptors [18, 19]. NMDA receptors, in particular, are crucial for synaptic plasticity, learning, and memory consolidation, including the encoding of fear-related memories [20, 21]. However, chronic overactivation of NMDA receptors can lead to excitotoxicity, causing neuronal damage, which may contribute to the persistence of anxiety in conditions such as post-traumatic stress disorder (PTSD) and generalized anxiety disorder (GAD) [22-24]. Similarly, AMPA and kainate receptors are integral to fast excitatory synaptic transmission and fear responses [25, 26]. Dysregulation of AMPA receptor activity, in particular, has been linked to excessive neural circuit excitation, which manifests as heightened fear responses, hyperarousal, and anxiety-related behaviors [27, 28]. Metabotropic glutamate receptors, notably mGluR2/3 and mGluR5, play an important role in modulating glutamate release and regulating neuronal excitability [29, 30]. Agonists of mGluR2/3 have shown anxiolytic effects by reducing excessive glutamate release, thereby mitigating hyperactivity in anxiety-related brain neurones [31, 32]. This reduction in glutamate signalling helps prevent overstimulation of neural pathways that contribute to anxiety. On the contrary, antagonists of mGluR5 have also demonstrated therapeutic potential in reducing anxiety-like behaviors, likely due to their ability to inhibit glutamate's excitatory effects [33-35]. Chronic dysregulation of glutamate transmission not only alters synaptic activity but also leads to structural changes in the brain. For example, hippocampal atrophy and amygdala hypertrophy, which are frequently observed in individuals with anxiety disorders, exacerbate the cycle of anxiety symptoms. These structural changes reinforce the dysfunction in glutamatergic signaling, contributing to emotional dysregulation. Targeting glutamate signaling pathways offers a promising approach for developing novel treatments aimed at restoring balance in glutamatergic transmission and alleviating the symptoms of anxiety disorders [36-40].
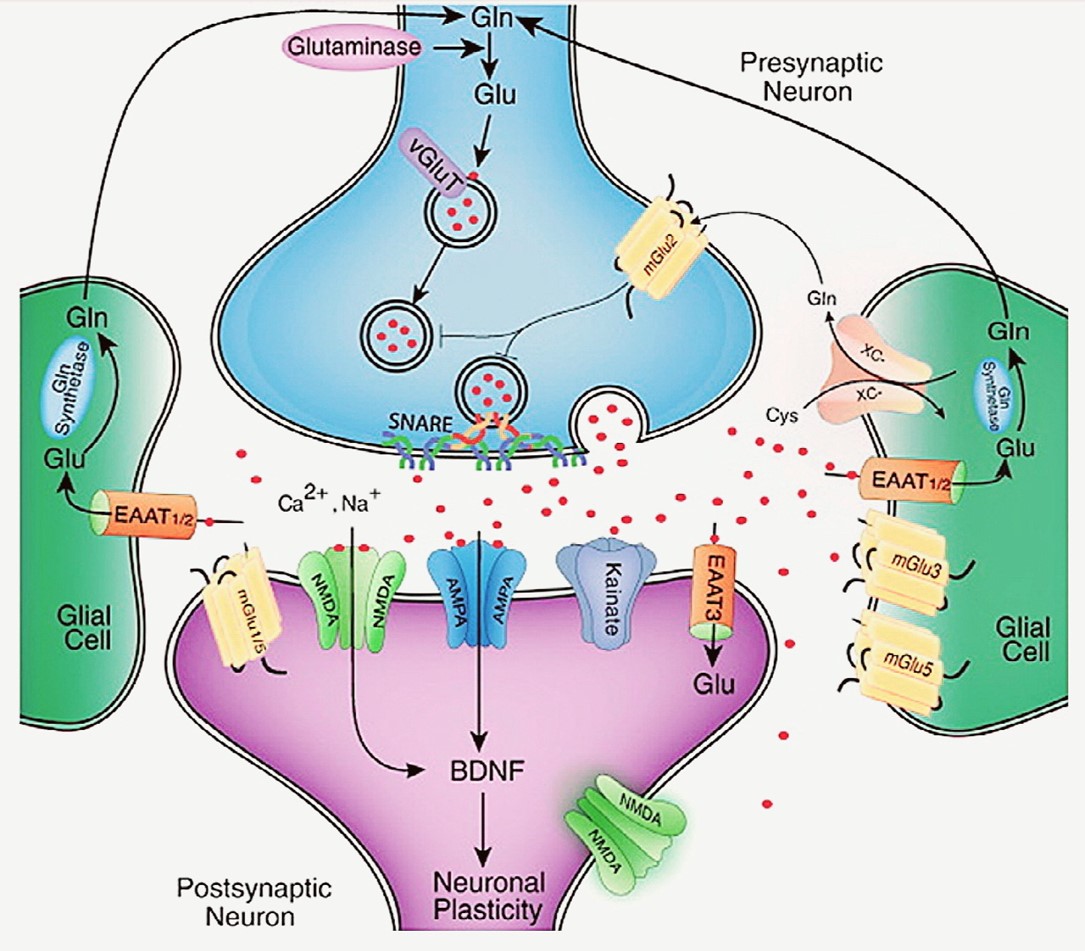
Fig. 1. This is a schematic representation of a glutamatergic synapse which illustrates that the synthesis, release, and regulation of glutamate (Glu), along with its associated receptors and deactivation pathways. Glutamine (Gln), converted to Glu by the enzyme glutaminase, serves as the primary source of synaptic glutamate, though Glu can also be produced from intermediates of the tricarboxylic acid (TCA) cycle. Once synthesized, Glu is transported into presynaptic vesicles by vesicular glutamate transporters (VGluTs) and released into the synaptic cleft via a voltage-dependent mechanism involving SNARE protein complexes. Following its release, glutamate is removed from the extracellular space predominantly by excitatory amino acid transporters (EAATs) located mainly on astroglial cells. In astrocytes, Glu is converted back to Gln by the enzyme glutamine synthetase, and Gln is then exported and taken up by neurons for reuse in synaptic transmission. Another mechanism for Glu recycling involves the system x-C, a cystine/glutamate antiporter on glial cells, which also facilitates Glu reuptake.Glutamate receptors are expressed on both presynaptic and postsynaptic neurons, as well as on glial cells. These receptors are classified into two major categories: ionotropic receptors, including AMPA (?-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid), NMDA (N-methyl-D-aspartate), and kainate receptors, and metabotropic glutamate receptors (mGluRs). The effects of glutamate signaling are determined by the specific receptor subtype and its location within synaptic, perisynaptic, or extrasynaptic sites. Brain-derived neurotrophic factor (BDNF) also plays a role in modulating glutamatergic synaptic function and plasticity.
The Role of Glucocorticoids and Glutamatergic Excitotoxicity in Stress-Induced Neuroanatomical and Behavioural ChangesThe release of adrenal glucocorticoids is a typical physiological response to stress [41, 42]. However, chronic exposure to stress results in prolonged glucocorticoid release, which has been linked to neurotoxic events, including increased glutamate release, particularly in the hippocampus [43, 44]. For example, animal models exposed to repeated immobilization or forced swimming stress show elevated glutamate release and uptake in the hippocampus and prefrontal cortex [45, 46]. Moreover, chronic stress has been associated with altered gene expression, such as increased AMPA receptor mRNA levels in the hippocampus, further emphasizing glutamate's role in stress-induced neurotoxicity [47-50]. Chronic stress-induced glucocorticoid release can lead to hippocampal atrophy, including decreased dendritic branching, neuronal death, and reduced neurogenesis in hippocampal pyramidal cells [51, 52]. Conversely, stress exerts hypertrophic effects in the amygdala, promoting increased dendritic arborization in pyramidal and stellate neurons of the basolateral amygdala and bed nucleus of the stria terminalis [53, 54]. These paradoxical anatomical changes reflect the contrasting roles of the hippocampus and amygdala in stress circuitry, with the hippocampus exerting inhibitory effects on the hypothalamic-pituitary-adrenal (HPA) axis, while the amygdala promotes excitatory regulation [55, 56]. Behavioural studies highlight the divergent effects of stress on hippocampal- and amygdala-dependent learning. Chronic stress impairs hippocampal-dependent spatial learning, as evidenced by deficits in tasks like the radial arm maze and Morris water maze, both of which rely on glutamatergic signalling [57, 58]. In contrast, glutamate release in the amygdala enhances fear-related learning, such as contextual fear conditioning. Pharmacological studies underscore the importance of NMDA receptors in both the hippocampus and amygdala for the acquisition and expression of fear-related memories, suggesting that glutamatergic pathways play a key role in emotional processing under stress. This mechanism is further implicated in disorders like PTSD, where glutamatergic dysregulation may contribute to symptoms such as dissociation and altered perception, similar to the effects of NMDA receptor antagonists like ketamine [59-63]. Various pharmacological agents targeting glutamate receptors and associated pathways have demonstrated significant effects on fear-potentiated startle and other anxiety-related behaviors. NMDA receptor antagonists, such as AP5, have been shown to reduce fear-potentiated startle, while NMDA partial agonists, like D-cycloserine (DCS), exhibit mixed effects, either enhancing or reducing responses depending on the specific behavioral paradigm. For example, DCS reduces fear-potentiated startle but enhances cue-conditioned freezing during extinction. It also affects behaviors in the elevated plus maze (EPM), with both increases and decreases in time spent in open arms, depending on the study [64, 65].
The Role of Glutamate Receptor Agonists and Antagonists in Modulating Stress-Induced Behaviours
Several agents targeting AMPA/kainate receptors exhibit similar variability. Kainic acid, an agonist, decreases fear-potentiated startle, while antagonists like NBQX increase it. AMPA/kainate receptor antagonist LY326325 increases punished drinking and time spent in open arms during the EPM [66, 67]. Topiramate, an AMPA/kainate receptor agonist, reduces stress-induced startle, highlighting its anxiolytic potential [68, 69]. In the metabotropic glutamate receptor (mGluR) category, mGluR5 antagonists, such as MPEP, decrease fear-potentiated startle but increase punished responding, suggesting complex modulation of anxiety behaviors [70, 71]. mGluR2/3 agonists like LY354740 reduce fear-potentiated startle and panic-like responses, such as lactate-induced panic, while also increasing time spent in open arms during the EPM [72, 73]. Additional agents, including sodium channel blockers (e.g., lamotrigine) and glutamate release inhibitors (e.g., riluzole), enhance conditioned emotional responses, indicating their potential in modulating anxiety-related behaviors through glutamatergic mechanisms. These findings collectively demonstrate the diverse and context-dependent effects of glutamate receptor modulation on stress and anxiety responses [74-78].
In addition to this, different pharmacological agents targeting glutamate signalling have shown promise in reducing symptoms associated with various anxiety disorders and phobias [79, 80]. The mGluR2/3 agonist LY354740 has been found to reduce fear-potentiated startle and panic responses during CO2 challenge, while LY544344, another mGluR2/3 agonist, has been shown to alleviate panic responses to CCK-4 challenge [81, 82]. Phenytoin, which inhibits glutamate transmission, has demonstrated a decrease in symptoms of PTSD [83, 84]. Similarly, the NMDA partial agonist D-cycloserine (DCS) has been associated with reductions in PTSD symptoms and specific phobias [85, 86]. Topiramate, an AMPA/kainate receptor agonist, has been shown to decrease symptoms of PTSD and social phobia in multiple studies [87, 88]. Additionally, Riluzole, a glutamate release inhibitor, has shown efficacy in reducing symptoms of obsessive-compulsive disorder (OCD) and generalized anxiety disorder (GAD). These findings support the role of glutamatergic modulation in the treatment of stress and anxiety-related disorders [89, 90].
Table-I. Pharmacological Agents and Their Effects on Anxiety-Related Behaviours
The Role of Glutamate in Anxiety Disorders: Genetic, Clinical, and Pharmacological Evidence
Although limited, human genetic, physiological, and behavioral studies provide preliminary evidence supporting the involvement of the glutamate system in fear and anxiety. Research has identified a significant association between the NMDA receptor subtype 2B (GRIN2B) gene and obsessive-compulsive disorder (OCD), with a specific single nucleotide polymorphism (5072T/G) linked to increased OCD risk and symptom severity. Elevated cerebrospinal fluid (CSF) glutamate levels have also been observed in patients with OCD, adding to the evidence of glutamatergic involvement in anxiety disorders, though the exact relationship between brain and CSF glutamate remains unclear [91-95]. Pharmacological studies further underscore the role of glutamate modulation in anxiety. The mGluR2/3 agonist LY354740 has demonstrated anxiolytic effects, reducing fear-potentiated startle in humans, and its prodrug LY544344 has shown promise in reducing anxiety induced by cholecystokinin tetrapeptide (CCK-4). Moreover, clinical trials targeting glutamate receptors have shown efficacy in treating anxiety disorders such as PTSD, phobias, and OCD. Phenytoin, which reduces glutamate transmission, significantly decreased PTSD symptoms, while D-cycloserine (DCS), an NMDA partial agonist, has been shown to enhance the extinction of fear in patients with acrophobia and PTSD [96-100].
Non-NMDA glutamate receptors also play a role in anxiety. Topiramate, an AMPA/kainate receptor modulator, has shown effectiveness in treating chronic PTSD and social phobia, likely due to its dual modulation of both excitatory (glutamate) and inhibitory (GABA) neurotransmission. Similarly, riluzole, a glutamate release inhibitor, has demonstrated efficacy in treating generalized anxiety disorder (GAD) and treatment-resistant OCD, with promising results in early clinical trials [101-103].
These findings highlight the significant role of glutamate receptor modulation in the treatment of anxiety disorders and suggest that further research into glutamatergic mechanisms could lead to novel therapeutic approaches for these conditions [104, 105].
Glutamate's Role in Anxiety Disorders: Insights from Neuroimaging Studies
Magnetic resonance imaging (MRI) has provided significant insights into the relationship between glutamate and anxiety disorders, albeit indirectly. Structural MRI (volumetric) and functional MRI (fMRI) studies have shown that glutamate-rich brain regions, such as the hippocampus, amygdala, and anterior cingulate cortex (ACC), are often structurally or functionally altered in individuals diagnosed with anxiety disorders [106, 107]. For instance, trauma survivors with post-traumatic stress disorder (PTSD) show reduced gray matter volume in the anterior cingulate and hippocampus. Moreover, studies have shown that combat veterans with PTSD, as well as women with PTSD related to childhood abuse, exhibit reduced hippocampal volume [108, 109]. Although not all studies consistently report hippocampal changes in PTSD, a meta-analysis has confirmed smaller hippocampal volumes in individuals with PTSD compared to healthy and traumatized controls [110]. This reduction in hippocampal volume is consistent with findings from animal studies showing stress-induced atrophy in the hippocampus [111]. Interestingly, in contrast to the hippocampus, the amygdala demonstrates hypertrophy in children and adolescents diagnosed with anxiety disorders, such as generalized anxiety disorder (GAD) and obsessive-compulsive disorder (OCD) [112, 113]. These structural alterations align with functional brain imaging studies, such as positron emission tomography (PET) and fMRI, which have shown altered activity in these regions. For example, patients with panic disorder exhibit abnormal metabolism in the hippocampus and ACC, while veterans with PTSD show altered blood flow in the amygdala [114-116]. Furthermore, fMRI studies have linked hyperactive amygdala responses to negatively biased social cues in individuals with social anxiety disorder, highlighting the role of glutamatergic pathways in emotional processing [117, 118]. Proton magnetic resonance spectroscopy (1H-MRS) has allowed researchers to directly quantify neurochemicals, including glutamate, in vivo. Although much of the focus has been on N-acetylaspartate, recent studies have revealed region-specific changes in glutamate levels in individuals with anxiety disorders [119, 120]. For instance, increased glutamate levels have been detected in the frontal cortex of healthy individuals with higher state-trait anxiety. In patients with social anxiety disorder, elevated glutamate concentrations in the ACC correlate with symptom severity [121, 122]. Similarly, children with OCD exhibit reduced glutamate concentrations in the ACC and elevated glutamatergic activity in the caudate nucleus, with symptom improvement following treatment [123, 124]. These findings suggest a complex tonic-phasic dysregulation of the glutamate system in anxiety, supporting the role of glutamatergic mechanisms in the pathophysiology of anxiety disorders [125].
Economic and Public Health Burden of Anxiety and Related Disorders
Anxiety, stress, and trauma-related disorders present a significant public health challenges in all over the world. This cost is driven by both direct healthcare expenditures, including psychiatric and non-psychiatric services, emergency care, hospitalization, and medication, as well as indirect costs such as reduced workforce productivity and occupational absenteeism. These disorders impose a considerable burden not only on the healthcare system but also on the economy as a whole, underscoring the importance of addressing the needs of affected individuals [126-128].
Prevalence and Associated Comorbidities
Anxiety is a common psychological response that, in normal circumstances, serves an adaptive function by helping individuals manage stress. However, for more than 15 million adults in the U.S. each year, this response becomes maladaptive, resulting in the development of anxiety disorders. These disorders, which include conditions such as generalized anxiety disorder (GAD), social and specific phobias, panic disorder, post-traumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD), are the most prevalent mental health issues in the U.S. Individuals with anxiety disorders frequently experience comorbid conditions such as irritable bowel syndrome (IBS) and hypertension, and they are at increased risk for mood disorders, including depression. These comorbidities compound the impact of anxiety disorders on overall health and well-being [129-132].
Limitations of Current Therapies and the Need for Alternative Approaches
The significant limitations of current treatment options for anxiety disorders, including issues of noncompliance and treatment resistance, highlight the urgent need for novel therapeutic strategies. Emerging research in neuroscience has identified the glutamate system, the brain’s principal excitatory neurotransmitter, as a potential target for new anxiety treatments. Many anxiety and stress-related disorders are thought to arise from hyperactive or overly responsive neural circuits, suggesting that interventions aimed at modulating glutamatergic function could offer a more effective approach to managing these conditions. Investigations into glutamate-modulating drugs hold promise for improving outcomes in individuals with anxiety disorders and advancing our understanding of the underlying mechanisms driving these severe and debilitating mental health conditions [133-135].
CONCLUSION:
In conclusion, anxiety disorders represent a major challenge both in terms of individual health and global public health due to their high prevalence, comorbidities, and significant economic impact. Traditional treatments targeting the GABAergic and serotonergic systems have shown efficacy but are often limited by treatment resistance and adverse effects, underscoring the need for novel therapeutic strategies. Emerging research into the glutamatergic system provides promising insights, with glutamate modulation offering a potential pathway for more effective treatments. Agents like D-cycloserine, riluzole, and others targeting NMDA, AMPA, and metabotropic glutamate receptors have shown encouraging results in alleviating anxiety symptoms, particularly in conditions such as PTSD and OCD. Furthermore, neuroimaging studies reveal structural and functional abnormalities in glutamate-rich brain regions, such as the hippocampus and amygdala, in individuals with anxiety disorders, further supporting the role of glutamatergic dysregulation in the pathophysiology of these conditions. The accumulating genetic, clinical, and pharmacological evidence points to glutamate-based therapies as a potential frontier in the treatment of anxiety disorders. However, more research is required to elucidate the precise mechanisms of glutamatergic modulation and optimize the clinical application of these treatments. By advancing our understanding of glutamate's role in anxiety and refining glutamate-targeting pharmacotherapies, there is hope for more effective and tailored interventions that address the limitations of current therapeutic approaches, ultimately improving the quality of life for those affected by anxiety disorders
REFERENCE
- Watson D. Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. Journal of abnormal psychology. 2005 Nov;114(4):522.
- Uhmann S, Beesdo-Baum K, Becker ES, Hoyer J. Specificity of interpersonal problems in generalized anxiety disorder versus other anxiety disorders and depression. The Journal of nervous and mental disease. 2010 Nov 1;198(11):846-51.
- Godfrey KE, Gardner AC, Kwon S, Chea W, Muthukumaraswamy SD. Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: a systematic review and meta-analysis. Journal of psychiatric research. 2018 Oct 1;105:33-44.
- Troppoli TA, Zanos P, Georgiou P, Gould TD, Rudolph U, Thompson SM. Negative allosteric modulation of GABAARs at ?5 subunit-containing benzodiazepine sites reverses stress-induced anhedonia and weakened synaptic function in mice. Biological psychiatry. 2022 Aug 8;92(3):216.
- Kositsyn YM, de Abreu MS, Kolesnikova TO, Lagunin AA, Poroikov VV, Harutyunyan HS, Yenkoyan KB, Kalueff AV. Towards novel potential molecular targets for antidepressant and antipsychotic pharmacotherapies. International Journal of Molecular Sciences. 2023 May 30;24(11):9482.
- Freeman-Daniels E, Beck SG, Kirby LG. Cellular correlates of anxiety in CA1 hippocampal pyramidal cells of 5-HT 1A receptor knockout mice. Psychopharmacology. 2011 Feb;213:453-63.
- Duman RS, Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012 Sep 5;367(1601):2475-84.
- Papi D, Patra P, Salar S, Rabha SK, Rastogi H. TARGETING GLUTAMATERGIC TRANSMISSION IN NEUROPSYCHIATRIC DISORDERS: CURRENT OPPORTUNITIES AND CHALLENGES. Biochemical & Cellular Archives. 2024 Apr 1;24(1).
- Marazziti D, Carlini M, Dell'Osso L. Treatment strategies of obsessive-compulsive disorder and panic disorder/agoraphobia. Current topics in medicinal chemistry. 2012 Feb 1;12(4):238-53.
- Chatzittofis A. Pharmacological Treatment of Anxiety Disorders: Current and Novel Targets Check for updates. Anxiety Disorders and Related Conditions: Conceptualization and Treatment from Psychodynamic and Cognitive Behavioral Perspectives.:87.
- Amiel JM, Mathew SJ. Glutamate and anxiety disorders. Current psychiatry reports. 2007 Aug;9(4):278-83.
- Li CT, Yang KC, Lin WC. Glutamatergic dysfunction and glutamatergic compounds for major psychiatric disorders: evidence from clinical neuroimaging studies. Frontiers in psychiatry. 2019 Jan 24;9:767.
- Pilc A, Chaki S, Nowak G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochemical pharmacology. 2008 Mar 1;75(5):997-1006.
- Freudenberg F, Celikel T, Reif A. The role of ?-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in depression: central mediators of pathophysiology and antidepressant activity?. Neuroscience & Biobehavioral Reviews. 2015 May 1;52:193-206.
- Pomierny-Chamio?o L, Rup K, Pomierny B, Niedzielska E, Kalivas PW, Filip M. Metabotropic glutamatergic receptors and their ligands in drug addiction. Pharmacology & therapeutics. 2014 Jun 1;142(3):281-305.
- Garakani A, Murrough JW, Freire RC, Thom RP, Larkin K, Buono FD, Iosifescu DV. Pharmacotherapy of anxiety disorders: current and emerging treatment options. Frontiers in psychiatry. 2020 Dec 23;11:595584.
- Réus GZ, Abelaira HM, Coutellier LD, Manosso LM, Pavlovic ZM. Role of Glutamatergic Neurotransmission in the Pathophysiology of Stress-Related Disorders and Chronic Stress Response. InGlutamate and Neuropsychiatric Disorders: Current and Emerging Treatments 2022 Apr 11 (pp. 65-112). Cham: Springer International Publishing.
- Trudeau F, Gagnon S, Massicotte G. Hippocampal synaptic plasticity and glutamate receptor regulation: influences of diabetes mellitus. European journal of pharmacology. 2004 Apr 19;490(1-3):177-86.
- Rubio-Casillas A, Fernández-Guasti A. The dose makes the poison: from glutamate-mediated neurogenesis to neuronal atrophy and depression. Reviews in the Neurosciences. 2016 Aug 1;27(6):599-622.
- Cestari V, Rossi-Arnaud C, Saraulli D, Costanzi M. The MAP (K) of fear: from memory consolidation to memory extinction. Brain research bulletin. 2014 Jun 1;105:8-16.
- Costanzi M, Cannas S, Saraulli D, Rossi-Arnaud C, Cestari V. Extinction after retrieval: effects on the associative and nonassociative components of remote contextual fear memory. Learning & Memory. 2011 Aug 1;18(8):508-18.
- Rosen JB, Schulkin J. Hyperexcitability: From normal fear to pathological anxiety and trauma. Frontiers in systems neuroscience. 2022 Aug 4;16:727054.
- Joyce MR, Holton KF. Neurotoxicity in Gulf War Illness and the potential role of glutamate. Neurotoxicology. 2020 Sep 1;80:60-70.
- Hanson JL, Nacewicz BM. Amygdala allostasis and early life adversity: considering excitotoxicity and inescapability in the sequelae of stress. Frontiers in human neuroscience. 2021 Jun 1;15:624705.
- Zhuo M. Cortical kainate receptors and behavioral anxiety. Molecular Brain. 2017 Dec;10:1-7.
- Ko S, Zhao MG, Toyoda H, Qiu CS, Zhuo M. Altered behavioral responses to noxious stimuli and fear in glutamate receptor 5 (GluR5)-or GluR6-deficient mice. Journal of Neuroscience. 2005 Jan 26;25(4):977-84.
- Popoli M, Ieraci A, Musazzi L. The role of the glutamate system in posttraumatic stress disorder and glutamate-based treatments. InGlutamate and Neuropsychiatric Disorders: Current and Emerging Treatments 2022 Apr 11 (pp. 163-193). Cham: Springer International Publishing.
- Roshan-Milani S, Seyyedabadi B, Saboory E, Parsamanesh N, Mehranfard N. Prenatal stress and increased susceptibility to anxiety-like behaviors: role of neuroinflammation and balance between GABAergic and glutamatergic transmission. Stress. 2021 Sep 3;24(5):481-95.
- D'Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proceedings of the National Academy of Sciences. 2007 Feb 6;104(6):1995-2000.
- Chen HH, Liao PF, Chan MH. mGluR5 positive modulators both potentiate activation and restore inhibition in NMDA receptors by PKC dependent pathway. Journal of Biomedical Science. 2011 Dec;18:1-9.
- Galici R, Jones CK, Hemstapat K, Nong Y, Echemendia NG, Williams LC, De Paulis T, Conn PJ. Biphenyl-indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic-and anxiolytic-like effects in mice. Journal of Pharmacology and Experimental Therapeutics. 2006 Jul 1;318(1):173-85.
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annual review of pharmacology and toxicology. 2010 Feb 10;50(1):295-322.
- Miladinovic T, Nashed MG, Singh G. Overview of glutamatergic dysregulation in central pathologies. Biomolecules. 2015 Nov 11;5(4):3112-41.
- Onaolapo AY, Onaolapo OJ. Glutamate and depression: Reflecting a deepening knowledge of the gut and brain effects of a ubiquitous molecule. World journal of psychiatry. 2021 Jul 7;11(7):297.
- Burket JA, Deutsch SI. Metabotropic functions of the NMDA receptor and an evolving rationale for exploring NR2A-selective positive allosteric modulators for the treatment of autism spectrum disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2019 Mar 2;90:142-60.
- Lee MT, Peng WH, Kan HW, Wu CC, Wang DW, Ho YC. Neurobiology of depression: chronic stress alters the glutamatergic system in the brain—focusing on AMPA receptor. Biomedicines. 2022 Apr 27;10(5):1005.
- Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders.
- Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing research reviews. 2005 May 1;4(2):271-87.
- Licznerski P, Duman RS. Remodeling of axo-spinous synapses in the pathophysiology and treatment of depression. Neuroscience. 2013 Oct 22;251:33-50.
- Spedding M, Chattarji S, Spedding C, Jay TM. Brain circuits at risk in psychiatric diseases and pharmacological pathways. Therapies. 2021 Mar 1;76(2):75-86.
- Zuckerman-Levin N, Tiosano D, Eisenhofer G, Bornstein S, Hochberg ZE. The importance of adrenocortical glucocorticoids for adrenomedullary and physiological response to stress: a study in isolated glucocorticoid deficiency. The Journal of clinical endocrinology & Metabolism. 2001 Dec 1;86(12):5920-4.
- Kemppainen RJ, Behrend E. Adrenal physiology. Veterinary Clinics: Small Animal Practice. 1997 Mar 1;27(2):173-86.
- Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Experimental gerontology. 1999 Sep 1;34(6):721-32.
- Virgin Jr CE, Ha TP, Packan DR, Tombaugh GC, Yang SH, Homer HC, Sapolsky RM. Glucocorticoids inhibit glucose transport and glutamate uptake in hippocampal astrocytes: implications for glucocorticoid neurotoxicity. Journal of neurochemistry. 1991 Oct;57(4):1422-8.
- Murai, R., Noda, Y., Matsui, K., Kamei, H., Mouri, A., Matsuba, K., Nitta, A., Furukawa, H. and Nabeshima, T., 2007. Hypofunctional glutamatergic neurotransmission in the prefrontal cortex is involved in the emotional deficit induced by repeated treatment with phencyclidine in mice: Implications for abnormalities of glutamate release and NMDA–CaMKII signaling. Behavioural brain research, 180(2), pp.152-160.
- Son H, Baek JH, Go BS, Jung DH, Sontakke SB, Chung HJ, Lee DH, Roh GS, Kang SS, Cho GJ, Choi WS. Glutamine has antidepressive effects through increments of glutamate and glutamine levels and glutamatergic activity in the medial prefrontal cortex. Neuropharmacology. 2018 Dec 1;143:143-52.
- Réus GZ, Abelaira HM, Coutellier LD, Manosso LM, Pavlovic ZM. Role of Glutamatergic Neurotransmission in the Pathophysiology of Stress-Related Disorders and Chronic Stress Response. InGlutamate and Neuropsychiatric Disorders: Current and Emerging Treatments 2022 Apr 11 (pp. 65-112). Cham: Springer International Publishing.
- Zhang XM, Zhu J. Kainic acid-induced neurotoxicity: targeting glial responses and glia-derived cytokines. Current neuropharmacology. 2011 Jun 1;9(2):388-98.
- Wang Y, Ma Y, Cheng W, Jiang H, Zhang X, Li M, Ren J, Zhang X, Li X. Sexual differences in long-term effects of prenatal chronic mild stress on anxiety-like behavior and stress-induced regional glutamate receptor expression in rat offspring. International Journal of Developmental Neuroscience. 2015 Apr 1;41:80-91.
- Chantong B, Kratschmar DV, Lister A, Odermatt A. Inhibition of metabotropic glutamate receptor 5 induces cellular stress through pertussis toxin-sensitive G i-proteins in murine BV-2 microglia cells. Journal of neuroinflammation. 2014 Dec;11:1-6.
- Schloesser RJ, Jimenez DV, Hardy NF, Paredes D, Catlow BJ, Manji HK, McKay RD, Martinowich K. Atrophy of pyramidal neurons and increased stress-induced glutamate levels in CA3 following chronic suppression of adult neurogenesis. Brain Structure and Function. 2014 May;219:1139-48.
- Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing research reviews. 2005 May 1;4(2):271-87.
- Pêgo JM, Morgado P, Cerqueira JJ, Almeida OF, Sousa N. Corticotrophin-releasing factor mediated anxiety correlates with synaptic changes in bed nucleus of the stria terminalis. Influence of Stress in the Structure and Function of the Amygdala. 2007 Nov:117.
- Linsambarth S, Moraga-Amaro R, Quintana-Donoso D, Rojas S, Stehberg J. The amygdala and anxiety. The Amygdale—Where Emotion Shape Perception, Learning and Memories, Neuroscience InTech. 2017 Jul 5:139-71.
- Anthony TE, Dee N, Bernard A, Lerchner W, Heintz N, Anderson DJ. Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit. Cell. 2014 Jan 30;156(3):522-36.
- Gray JM, Vecchiarelli HA, Morena M, Lee TT, Hermanson DJ, Kim AB, McLaughlin RJ, Hassan KI, Kühne C, Wotjak CT, Deussing JM. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. Journal of Neuroscience. 2015 Mar 4;35(9):3879-92.
- Graybeal C, Kiselycznyk C, Holmes A. Stress-induced deficits in cognition and emotionality: a role for glutamate. Behavioral Neurogenetics. 2012:189-207.
- Islam R. Molecular Pathways Responsible for NMDA Receptormediated Behavioural Plasticity. University of Toronto (Canada); 2017.
- Hillman BG. Studies of GluN2C-containing NMDA receptors in schizophrenia-like behaviors and fear learning; Relevance to the glutamate hypofunction hypothesis of schizophrenia. Creighton University; 2012.
- André JM, Leach PT, Gould TJ. Nicotine ameliorates NMDA receptor antagonist-induced deficits in contextual fear conditioning through high-affinity nicotinic acetylcholine receptors in the hippocampus. Neuropharmacology. 2011 Mar 1;60(4):617-25.
- Maksimovic M. Behavioural and pharmacological characterization of a mouse model for psychotic disorders–focus on glutamatergic transmission.
- Brockway ET. Ventral hippocampal regulation of contextual fear and extinction memory (Doctoral dissertation).
- Sawyer EJ. The Effects of BMS-204352, an Activator of Voltage-Gated Potassium Channels, in the Infralimbic Cortex of the Fmr1 Knockout Mouse, an Animal Model of Fragile X Syndrome (Doctoral dissertation, Tulane University).
- Rodgers RJ, Harvest H, Hassall C, Kaddour LA. D-cycloserine enhances memory consolidation in the plus-maze retest paradigm. Behavioral neuroscience. 2011 Feb;125(1):106.
- Lungwitz EA. Development, validation, and characterization of a novel preclinical animal model of social familiarity-induced anxiolysis. Indiana University-Purdue University Indianapolis; 2018.
- Wadenberg ML, Hicks PB. The conditioned avoidance response test re-evaluated: is it a sensitive test for the detection of potentially atypical antipsychotics?. Neuroscience & Biobehavioral Reviews. 1999 Oct 1;23(6):851-62.
- Bermudo-Soriano CR, Perez-Rodriguez MM, Vaquero-Lorenzo C, Baca-Garcia E. New perspectives in glutamate and anxiety. Pharmacology Biochemistry and Behavior. 2012 Feb 1;100(4):752-74.
- Cortese BM, Phan KL. The role of glutamate in anxiety and related disorders. CNS spectrums. 2005 Oct;10(10):820-30.
- Choksey D. Adolescent refractory anxiety disorders rising: Gut microbiota-associated metabolites to the rescue?. Journal of Student Research. 2024 Feb 29;13(1).
- Anarghou H, Malqui H, Ihbour S, Laaroussi M, Essaidi O, Fetoui H, Bouhrim M, Najimi M, Chigr F. Impact of glyphosate-based herbicide exposure through maternal milk on offspring’s antioxidant status, neurodevelopment, and behavior. Naunyn-Schmiedeberg's Archives of Pharmacology. 2024 Mar 11:1-9.
- Chen CM, Wu CC, Kim Y, Hsu WY, Tsai YC, Chiu SL. Enhancing social behavior in an autism spectrum disorder mouse model: investigating the underlying mechanisms of Lactiplantibacillus plantarum intervention. Gut Microbes. 2024 Dec 31;16(1):2359501.
- Miranda O, Fan P, Qi X, Wang H, Brannock MD, Kosten TR, Ryan ND, Kirisci L, Wang L. DeepBiomarker2: Prediction of alcohol and substance use disorder risk in post-traumatic stress disorder patients using electronic medical records and multiple social determinants of health. Journal of Personalized Medicine. 2024 Jan 14;14(1):94.
- Li HJ, Yu X, Liu X, Xu J, Chen J, Cheng T, Chung S, Shu Y, Shao Z. Calneuron 1 reveals the pivotal roles in schizophrenia via perturbing human forebrain development and causing hallucination-like behavior in mice. bioRxiv. 2024:2024-04.
- Hocaoglu Ç?. Role of Glutamatergic Modulators in the Treatment of Obsessive Compulsive and Related Disorders. Psikiyatride Güncel Yakla??mlar. 2024;16(3).
- Castillo-Vazquez SK, Massieu L, Rincón-Heredia R, García-delaTorre P, Quiroz-Baez R, Gomez-Verjan JC, Rivero-Segura NA. Glutamatergic neurotransmission in aging and neurodegenerative diseases: A potential target to improve cognitive impairment in aging. Archives of Medical Research. 2024 Sep 1;55(6):103039.
- Barut EN, Engin S, Yasar YK, Sezen SF. Riluzole, a neuroprotective agent, preserves erectile function following bilateral cavernous nerve injury in male rats. International journal of impotence research. 2024 May;36(3):275-82.
- Leo A, Bosco F, Guarnieri L, De Sarro C, Rania V, Gallelli L, Citraro R, De Sarro G. Cenobamate enhances the anticonvulsant effect of other antiseizure medications in the DBA/2 mouse model of reflex epilepsy. European Journal of Pharmacology. 2024 Jan 5;962:176222.
- Giri PM, Banerjee A, Ghosal A, Layek B. Neuroinflammation in Neurodegenerative Disorders: Current Knowledge and Therapeutic Implications. International Journal of Molecular Sciences. 2024 Apr 3;25(7):3995.
- O’Connor RM, Cryan JF. 16 Role of metabotropic glutamate receptors in CNS disorders. G protein-coupled receptors: structure, signaling, and physiology. 2010 Sep 30:321.
- Grillon C, Ernst M. A way forward for anxiolytic drug development: Testing candidate anxiolytics with anxiety-potentiated startle in healthy humans. Neuroscience & Biobehavioral Reviews. 2020 Dec 1;119:348-54.
- Cortese BM, Phan KL. The role of glutamate in anxiety and related disorders. CNS spectrums. 2005 Oct;10(10):820-30.
- Hashimoto K, Malchow B, Falkai P, Schmitt A. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. European archives of psychiatry and clinical neuroscience. 2013 Aug;263:367-77.
- Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain research reviews. 2009 Oct 1;61(2):105-23.
- Kalinichev M, Rouillier M, Girard F, Royer-Urios I, Bournique B, Finn T, Charvin D, Campo B, Le Poul E, Mutel V, Poli S. ADX71743, a potent and selective negative allosteric modulator of metabotropic glutamate receptor 7: in vitro and in vivo characterization. Journal of Pharmacology and Experimental Therapeutics. 2013 Mar 1;344(3):624-36.
- V Golubeva A, D Moloney R, M O'Connor R, G Dinan T, F Cryan J. Metabotropic glutamate receptors in central nervous system diseases. Current drug targets. 2016 Apr 1;17(5):538-616.
- A Jaso B, J Niciu M, D Iadarola N, Lally N, M Richards E, Park M, D Ballard E, C Nugent A, Machado-Vieira R, A Zarate C. Therapeutic modulation of glutamate receptors in major depressive disorder. Current Neuropharmacology. 2017 Jan 1;15(1):57-70.
- Murrough JW, Yaqubi S, Sayed S, Charney DS. Emerging drugs for the treatment of anxiety. Expert opinion on emerging drugs. 2015 Jul 3;20(3):393-406.
- Bergink V, Westenberg HG. Metabotropic glutamate II receptor agonists in panic disorder: a double blind clinical trial with LY354740. International clinical psychopharmacology. 2005 Nov 1;20(6):291-3.
- Wu LJ, Ko SW, Toyoda H, Zhao MG, Xu H, Vadakkan KI, Ren M, Knifed E, Shum F, Quan J, Zhang XH. Increased anxiety-like behavior and enhanced synaptic efficacy in the amygdala of GluR5 knockout mice. PLoS One. 2007 Jan 24;2(1):e167.
- Bauminger H, Gaisler-Salomon I. Beyond NMDA receptors: Homeostasis at the glutamate tripartite synapse and its contributions to cognitive dysfunction in schizophrenia. International Journal of Molecular Sciences. 2022 Aug 3;23(15):8617.
- Nasir M, Trujillo D, Levine J, Dwyer JB, Rupp ZW, Bloch MH. Glutamate systems in DSM-5 anxiety disorders: their role and a review of glutamate and GABA psychopharmacology. Frontiers in psychiatry. 2020 Nov 19;11:548505.
- Simon AB, Gorman JM. Advances in the treatment of anxiety: targeting glutamate. NeuroRx. 2006 Jan 1;3(1):57-68.
- Pilc A, Chaki S, Nowak G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochemical pharmacology. 2008 Mar 1;75(5):997-1006.
- Escobar AP, Wendland JR, Chávez AE, Moya PR. The neuronal glutamate transporter EAAT3 in obsessive-compulsive disorder. Frontiers in Pharmacology. 2019 Nov 15;10:1362.
- Adwas AA, Jbireal JM, Azab AE. Anxiety: Insights into signs, symptoms, etiology, pathophysiology, and treatment. East African Scholars Journal of Medical Sciences. 2019 Oct;2(10):580-91.
- Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011 Jun 1;60(7-8):1017-41.
- Bandelow B, Baldwin D, Abelli M, Altamura C, Dell’Osso B, Domschke K, Fineberg NA, Grünblatt E, Jarema M, Maron E, Nutt D. Biological markers for anxiety disorders, OCD and PTSD–a consensus statement. Part I: neuroimaging and genetics. The World Journal of Biological Psychiatry. 2016 Jul 3;17(5):321-65.
- Robbins TW, Murphy ER. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends in pharmacological sciences. 2006 Mar 1;27(3):141-8.
- Adwas AA, Jbireal JM, Azab AE. Anxiety: Insights into signs, symptoms, etiology, pathophysiology, and treatment. East African Scholars Journal of Medical Sciences. 2019 Oct;2(10):580-91.
- Kent JM, Mathew SJ, Gorman JM. Molecular targets in the treatment of anxiety. Biological psychiatry. 2002 Nov 15;52(10):1008-30.
- Koskinen MK, Hovatta I. Genetic insights into the neurobiology of anxiety. Trends in Neurosciences. 2023 Apr 1;46(4):318-31.
- Schumacher J, Kristensen AS, Wendland JR, Nöthen MM, Mors O, McMahon FJ. The genetics of panic disorder. Journal of medical genetics. 2011 Jun 1;48(6):361-8.
- Finn CT, Smoller JW. The genetics of panic disorder. Current Psychiatry Reports. 2001 Apr;3(2):131-7.
- Philibert RA, Crowe R, Ryu GY, Yoon JG, Secrest D, Sandhu H, Madan A. Transcriptional profiling of lymphoblast lines from subjects with panic disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007 Jul 5;144(5):674-82.
- Weissman MM. Family genetic studies of panic disorder. Journal of psychiatric research. 1993 Jan 1;27:69-78.
- Trzesniak C, Araújo D, Crippa JA. Magnetic resonance spectroscopy in anxiety disorders. Acta Neuropsychiatrica. 2008 Apr;20(2):56-71.
- Maddock RJ, Buonocore MH. MR spectroscopic studies of the brain in psychiatric disorders. Brain imaging in behavioral neuroscience. 2012:199-251.
- Swanberg KM, Campos L, Abdallah CG, Juchem C. Proton Magnetic Resonance Spectroscopy in Post-Traumatic Stress Disorder—Updated Systematic Review and Meta-Analysis. Chronic Stress. 2022 Oct;6:24705470221128004.
- Ito H, Mori K, Harada M, Hisaoka S, Toda Y, Mori T, Goji A, Abe Y, Miyazaki M, Kagami S. A proton magnetic resonance spectroscopic study in autism spectrum disorder using a 3-tesla clinical magnetic resonance imaging (MRI) system: the anterior cingulate cortex and the left cerebellum. Journal of Child Neurology. 2017 Jul;32(8):731-9.
- Stan AD, Schirda CV, Bertocci MA, Bebko GM, Kronhaus DM, Aslam HA, LaBarbara EJ, Tanase C, Lockovich JC, Pollock MH, Stiffler RS. Glutamate and GABA contributions to medial prefrontal cortical activity to emotion: implications for mood disorders. Psychiatry Research: Neuroimaging. 2014 Sep 30;223(3):253-60.
- Chitty KM, Lagopoulos J, Lee RS, Hickie IB, Hermens DF. A systematic review and meta-analysis of proton magnetic resonance spectroscopy and mismatch negativity in bipolar disorder. European Neuropsychopharmacology. 2013 Nov 1;23(11):1348-63.
- Stanley JA. In vivo magnetic resonance spectroscopy and its application to neuropsychiatric disorders. The Canadian Journal of Psychiatry. 2002 May;47(4):315-26.
- Zhang JM. Human Brain Glutamate, Glutamine, ?-Aminobutyric Acid Proton Magnetic Resonance Spectral Quantification with the Fast Pade Transform. University of California, Los Angeles; 2013.
- Burger A. Investigating neuroinflammation in schizophrenia: a proton magnetic resonance spectroscopy (1H-MRS) and cytokine study.
- Demchenko I, Tassone VK, Kennedy SH, Dunlop K, Bhat V. Intrinsic connectivity networks of glutamate-mediated antidepressant response: a neuroimaging review. Frontiers in Psychiatry. 2022 May 26;13:864902.
- Iqbal J, Huang GD, Xue YX, Yang M, Jia XJ. The neural circuits and molecular mechanisms underlying fear dysregulation in posttraumatic stress disorder. Frontiers in Neuroscience. 2023 Dec 5;17:1281401.
- Contoreggi C. Corticotropin releasing hormone and imaging, rethinking the stress axis. Nuclear medicine and biology. 2015 Apr 1;42(4):323-39.
- Licata SC, Renshaw PF. Neurochemistry of drug action: insights from proton magnetic resonance spectroscopic imaging and their relevance to addiction. Annals of the New York Academy of Sciences. 2010 Feb;1187(1):148-71.
- Wang Q, Zhang Z, Dong F, Chen L, Zheng L, Guo X, Li J. Anterior insula GABA levels correlate with emotional aspects of empathy: a proton magnetic resonance spectroscopy study. PloS one. 2014 Nov 24;9(11):e113845.
- Fisher E, Gillam J, Upthegrove R, Aldred S, Wood SJ. Role of magnetic resonance spectroscopy in cerebral glutathione quantification for youth mental health.
- Zahid U. Antipsychotics, glutamate, and the brain (Doctoral dissertation, King's College London).
- Gonsalves MA, White TL, Barredo J, DeMayo MM, DeLuca E, Harris AD, Carpenter LL. Cortical glutamate, Glx, and total N-acetylaspartate: potential biomarkers of repetitive transcranial magnetic stimulation treatment response and outcomes in major depression. Translational Psychiatry. 2024 Jan 6;14(1):5.
- Sava S, Yurgelun-Todd DA. Functional magnetic resonance in psychiatry. Topics in Magnetic Resonance Imaging. 2008 Apr 1;19(2):71-9.
- Meyerhoff DJ, Durazzo TC. Proton magnetic resonance spectroscopy in alcohol use disorders: a potential new endophenotype?. Alcoholism: Clinical and Experimental Research. 2008 Jul;32(7):1146-58.
- Chesna EA. Influence of genetic variation on social behaviors and frontal cortex differences in generalized anxiety disorder (Doctoral dissertation, Sam Houston State University).
- Wijnen BF, Ten Have M, De Graaf R, van der Hoek HJ, Lokkerbol J, Smit F. The economic burden of mental disorders: results from the Netherlands mental health survey and incidence study-2. The European Journal of Health Economics. 2024 Aug;25(6):925-34.
- Lee Y, Magnus A, Chatterton ML, Mihalopoulos C. The economic burden of pharmaceuticals in people diagnosed with depression, anxiety-related disorder and substance use in Australia. Value in Health. 2015 Nov 1;18(7):A409-10.
- Kertz SJ, Woodruff-Borden J. Human and economic burden of GAD, subthreshold GAD, and worry in a primary care sample. Journal of Clinical Psychology in Medical Settings. 2011 Sep;18:281-90.
- Molina MR, Spessato B, Jansen K, Pinheiro R, Silva R, Souza LD. Prevalence of comorbidities between mood and anxiety disorders: associated factors in a population sample of young adults in southern Brazil. Cadernos de saude publica. 2014 Nov;30(11):2413-22.
- Yapici Eser H, Kacar AS, Kilciksiz CM, Yalcinay-Inan M, Ongur D. Prevalence and associated features of anxiety disorder comorbidity in bipolar disorder: a meta-analysis and meta-regression study. Frontiers in psychiatry. 2018 Jun 27;9:229.
- Regier DA, Rae DS, Narrow WE, Kaelber CT, Schatzberg AF. Prevalence of anxiety disorders and their comorbidity with mood and addictive disorders. The British journal of psychiatry. 1998 Jul;173(S34):24-8.
- Egeberg A, Andersen YM, Gislason GH, Skov L, Thyssen JP. Prevalence of comorbidity and associated risk factors in adults with atopic dermatitis. Allergy. 2017 May;72(5):783-91.
- Creswell C, Cartwright-Hatton S. Family treatment of child anxiety: Outcomes, limitations and future directions. Clinical child and family psychology review. 2007 Sep;10:232-52.
- Kessler RC, Soukup J, Davis RB, Foster DF, Wilkey SA, Van Rompay MI, Eisenberg DM. The use of complementary and alternative therapies to treat anxiety and depression in the United States. American Journal of Psychiatry. 2001 Feb 1;158(2):289-94.
135. Mewton L, Smith J, Rossouw P, Andrews G. Current perspectives on Internet-delivered cognitive behavioral therapy for adults with anxiety and related disorders. Psychology research and behavior management. 2014 Jan 30:37-46


 Arnab Roy*
Arnab Roy*
 Prof. (Dr.) K. Rajeswar Dutt
Prof. (Dr.) K. Rajeswar Dutt
 Ankita Singh
Ankita Singh
 Mahesh Kumar Yadav
Mahesh Kumar Yadav
 Kristy Kumari
Kristy Kumari
 Vishal Kumar
Vishal Kumar
 Vicky Kumar
Vicky Kumar
 Rishu Raj
Rishu Raj
 Chandan Kumar
Chandan Kumar
 Megha Chattaraj
Megha Chattaraj
 Priyanka Daniel
Priyanka Daniel
 Manish Kumar Singh
Manish Kumar Singh
 Dhananjay Sahu
Dhananjay Sahu
 Md. Danish Gayas Ansari
Md. Danish Gayas Ansari
 Adarsh Kumar Singh
Adarsh Kumar Singh
 Faiz Alam
Faiz Alam
 Sandeep Kumar
Sandeep Kumar
 Hasnain Ansari
Hasnain Ansari
 Meezan Alam
Meezan Alam


 10.5281/zenodo.13854617
10.5281/zenodo.13854617