Abstract
A,B-unsaturated carbonyl compounds are compounds consisting of two aromatic rings connected by a three-carbon ?,?-unsaturated carbonyl system. Reactive ?,?-unsaturated carbonyl function in unsaturated carbonyl compound and its derivatives is the reason for its pharmacological activities Unsaturated carbonyl compound s have been reported to present various biological activities such as anti-inflammatory, antioxidant, antitubercular, antibacterial, antiviral, insecticidal, antiprotozoal, anticancer antidiabetic, etc. This review highlights the synthesis methods and pharmacological activities of ?,?-unsaturated carbonyl compounds.
Keywords
Claisen-schmidt condensation, Friedel-Crafts acylation, Anti-oxidant activity, ?,?-Unsaturated carbonyl compound
Introduction
The term “A,B-Unsaturated carbonyl compound ” was coined by Kostanecki and Tambor (1899). Other names for Unsaturated carbonyl compound s are benzyl acetophenone, Phenyl styryl ketone or benzylidene acetophenon.Flavonoids are a group of compounds that include ?,?-Unsaturated carbonyl compound. Plants produce flavonoids naturally, and they are responsible for the colors of leaves and flowers. Many flavonoids are effective in antioxidant and anti-inflammatory properties. Synthetic unsaturated carbonyl compound s, are composed of two aromatic rings connected by a three-carbon A, B unsaturated carbonyl compound have potential applications in medicine and other fields. Unsaturated carbonyl compound is considered a "privileged structure" due to its unique composition. A,B-Unsaturated carbonyl compound are also present in nature and can be obtained from plant species like Angelica, Glycyrrhiza, Humulus and Scutellaria, which are widely used as traditional folk remedies.It is also recognized for its synthetic utility to prepare pharmacologically-interesting heterocyclic systems like pyrazolines , .pyrimidine etc.

Synthetic Methods for Unsaturated carbonyl compound -
There are mainly four modes for the synthesis of ?,?-Unsaturated carbonyl compound:
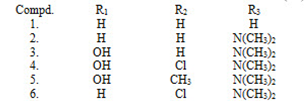
- By the condensation of acetophenones with benzaldehydes (Claisen-schmidt condensation)
- By the condensation of phenols with cinnamoyl chloride by Friede1-Craft acylation reaction.
- Microwave assisted synthesis
- Ultrasound irradiation technique
Condensation of acetophenones with benzaldehydes (Claisen-schmidt condensation):-
The most convenient method for synthesizing unsaturated carbonyl compound s is through the Claisen-Schmidt condensation process. This involves condensing equimolar quantities of acetophenone and benzaldehydes in the presence of aqueous alcoholic alkali. Typically, the concentration of alkali used falls within the range of 10 to 60%. The reaction is carried out either at 50°C for 12 to 15 hours or at room temperature for a week. However, caution must be exercised as the Cannizzaro reaction may also occur, leading to a decrease in the yield of the desired product. To avoid the disproportionation of aldehyde and increase the yield, it is recommended to use benzylidene-diacetate in place of aldehyde
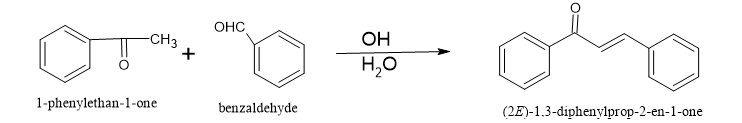
One way to produce ?,?-Unsaturated carbonyl compound s is by condensing phenols with cinnamoyl chloride through the Friedel-Crafts reaction. This method involves acylating a phenol directly to form a C6-C3-C6 unit, where the phenol serves as the A-ring and the acylating agent provides both B-ring carbons and the three-carbon bridge. For example, 2’,4’,6’-trihydroxy-3’,5’-dimethylunsaturated carbonyl compound can be synthesized by performing Friedel-Crafts acylation of 2,4-dimethyl-1,3,5-triolbenzene with 3-phenyl propionyl chloride. The Friedel-Crafts reactions were developed by Charles Friedel and James Crafts in 1877 as to attach substituents to an aromatic ring. There are two main types of Friedel-Crafts reactions: alkylation and acylation reactions.

Microwave assisted synthesis:-
The Claisen–Schmidt condensation stays the most common method in homogeneous phase or in interfacial solidliquid conditions using barium hydroxide catalyst (C-200). Microwave irradiation technique is now a well-known technique in organic synthesis. This method facile more advantages over other conventional methods, because it reduces the reaction time, by-products, evaporation of solvents, and most importantly it results enhanced yields (Gupta and Mahajan 2019). A solvent-free, molecular iodine impregnated alumina catalyzed microwave irradiation reaction of acetophenones with aldehydes was reported.
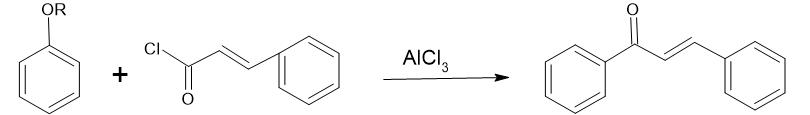
Friedel-Crafts acylation:-
Highly substituted unsaturated carbonyl compound s were prepared by using this technique but, it is very infrequently using method. Shotter et al. (1978) were synthesized unsaturated carbonyl compound s using Friedel crafts acylation in the presence of Lewis acid catalyst. Acylation of aromatic ethers with cinnamoyl chloride in the presence of strong Lewis acid catalyst AlCl3 yields the unsaturated carbonyl compound s.
Ultrasound irradiation technique: -
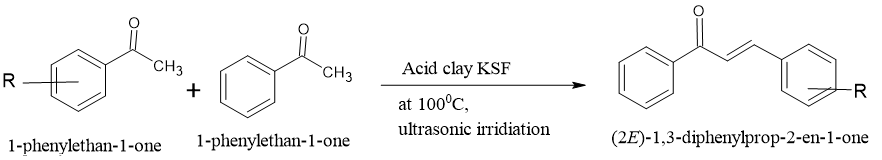
Ultrasound irradiation is a useful technique for synthesizing chemicals, similar to microwave-assisted synthesis. This approach offers quick reaction times and high yields. The technique works by activating catalytic sites using ultrasonic waves, which increase the vibrational state of the lattice and induce chemical reactions. Heterogeneous catalysts such as K2CO3, basic Al2O3, Ba(OH)2, KF-Al2O3, and amino-grafted zeolite can be used for the synthesis of unsaturated carbonyl compound s using ultrasound irradiation. Researchers have reported high yields of unsaturated carbonyl compound s using K2CO3 as a catalyst. Similarly, they found that acidic clay (KSF) under ultrasonic irradiation technique produces unsaturated carbonyl compound s with outstanding yields (96-80%) without solvents. In another experiment, authors prepared different unsaturated carbonyl compound s using KOH/EtOH (52-97% yield without catalyst) and KF/MeOH (83-98% yield with the presence of Al2O3) under ultrasonic irradiation at moderate temperature conditions.
Pharmacological activities:-
?,?-unsaturated carbonyl compound as an antimicrobial agent:-
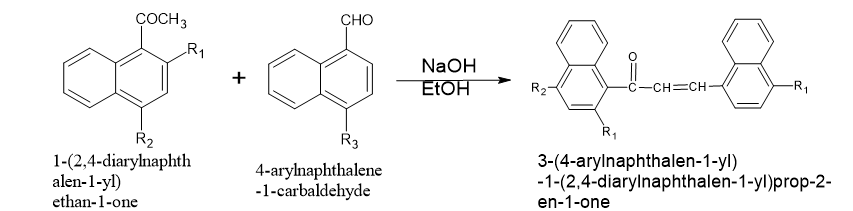
?,?-unsaturated carbonyl compound s with antimicrobial activity were synthesized by condensing 1-acetylnaphthalene or substituted 1-acetylnaphthalenes with 1-naphthaldehyde or 4-dimethylamino-1-naphthaldehyde in ethanolic NaOH solutions.
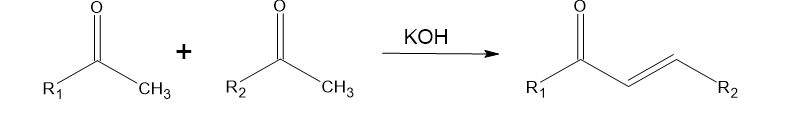
A series of ?,?-unsaturated carbonyl compounds having antimicrobial activity was prepared by Claisen-Schmidt condensation of acetophenones with aromatic aldehydes in the presence of aqueous solution of potassium hydroxide and ethanol at room temperature.

?,?-unsaturated carbonyl compound as antifungal activity:-
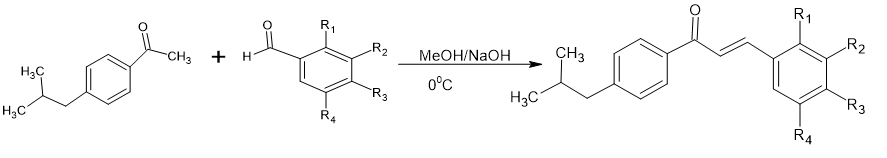
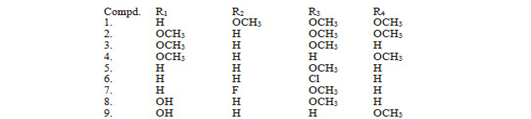
A series of unsaturated carbonyl compound s was synthesized by reacting 1-(4-isobutylphenyl) ethanone with different substituted aldehyde by Clasien-Schimidt condensation having antifungal activity.
Anti-inflammatory:-
A new series of quinolinyl unsaturated carbonyl compound derivatives was synthesized by the reaction of quinolinyl and chloroquinolinyl acetophenones with substituted aromatic aldehydes as and inflammatory agent.
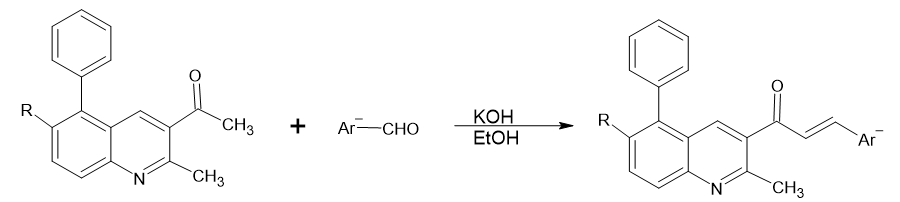
R = H, Cl; Ar = C6H5, p-C6H4NO2, p-C6H4Cl, p- C6H4OH, p- C6H4OCH3, p- C6H4CH3, p- C6H4N(CH3)2, p- C6H4N(C2H5)2, furyl, thiophene
?,?-unsaturated carbonyl compound as Anti-inflammatory agent:-
Fluorinated unsaturated carbonyl compound derivatives with potent anti-inflammatory activity were synthesized by Claisen-Schmidt condensation method followed by reaction with SOCl2/ETOH.
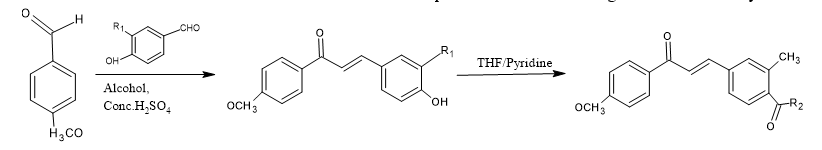
?,?-unsaturated carbonyl compound as Antioxidant agent:-
Jian-Zhang Wu and his co-workers have synthesized a series of ?,?-unsaturated carbonyl compound derivatives having antioxidant activity.
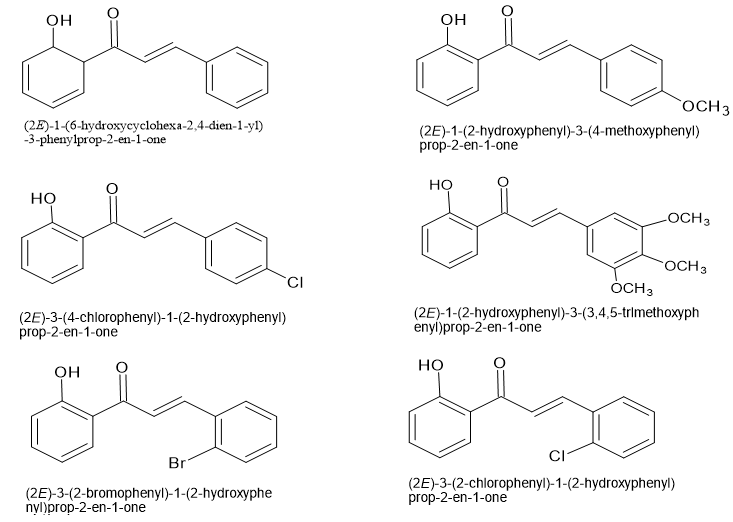
?,?-unsaturated carbonyl compound as Ant diabetic agent: -
?,?-unsaturated carbonyl compound and their corresponding 2-pyrazoline derivatives were synthesized and evaluated for their anti-diabetic activity.

CONCLUSION:-
Several ?,?-unsaturated carbonyl compounds have been shown to interfere with various pharmacological activities .The pharmacological evaluation includes in vitro and in vivo tests to assess their potential applications in medicine. The results show significant variations in the biological activities of different ?-ketoesters and highlight their potential as lead compounds for the development of novel drugs. The study offers a comprehensive overview of various synthetic methods and provides a foundation for further research and development of ?-ketoesters as potential therapeutic agents in various fields of medicine.Several ?,?-unsaturated carbonyl compounds have been shown to interfere with various pharmacological activities . These mechanisms have been discussed in this study. To be more precise, some of these substances produce reactive oxygen species, reduce the potential of the mitochondrial membrane,Induce apoptosis, and so on.
REFERENCES:-
- Kachroo M,Panda R,Yadav Y, synthesis and biological activities of some new pyrimidine derivatives from Unsaturated carbonyl compound s. Pharma.Chem.2014;6(2):352-9.9.
- Ahmed M.,Younis O.,Orabai E. A.,Sayed A.M.,Kamal El-Dean A. M.,HAssanien R,Davis R.L.,Tsutsumi O.,And Tolba M. S.,Synthesis of novel as biocomptiable chromophores with aggregation-induced emission sensitive to molecular aggreaion.ACS Omega.2020;5,29988-30000.
- Dansena H. Dhondgade HJ, Chandarkar K. Pharmacological pontentials of pyrimidine derivative:A review .Asian J PharmaClin Res.2015;8(4):171-7
- Mahapatra, D.K;Bharti S.K; ASati, V. Unsaturated carbonyl compound Scaffolds as anti-infective agents :Structural and molecular target perspectives .Eur.j.Med.Chem,2015;101:496-524.


 Wadje Suresh Subhash *
Wadje Suresh Subhash *
 Md Rayees Ahmad
Md Rayees Ahmad














 10.5281/zenodo.10986394
10.5281/zenodo.10986394