Abstract
Acetaminophen, generally known as paracetamol, is one of the most commonly used over-the-counter analgesics and antipyretics globally. It is frequently administered for the relief of pain and fever in both adults and children. However, calculating the proper dosage of paracetamol can be difficult due to a variety of factors such as age, weight, underlying medical conditions, and concurrent drugs. This presentation seeks to offer a comprehensive evaluation of the optimal paracetamol dosage using the most recent research and guidelines available till September 2021. The review investigates paracetamol's pharmacokinetics and mechanism of action, focusing on its therapeutic effects as well as potential side effects. It discusses the significance of precisely estimating the optimum dose to provide maximum efficacy while reducing the danger of harm.
Keywords
Acetaminophen, Paracetamol, case study, Adverse Drug Reaction
Introduction
Paracetamol is an analgesic and fever reducer. It is used to treat a wide range of symptoms, including headaches, muscle aches, toothaches, colds, and flu. Paracetamol is available over the counter in a variety of forms, including tablets, capsules, liquids, and suppositories. Adults should take 1,000 milligrams (mg) of paracetamol every 4 to 6 hours, with a maximum daily dose of 4,000 mg. For children, the dose is determined by their weight and age. It is critical to follow the label’s instructions attentively and not exceed the suggested dose. If you take too much paracetamol, it can be hazardous. Paracetamol should not be taken by persons who are allergic to it or have liver or kidney problems. It should also not be taken by pregnant or lactating women unless prescribed by a doctor. If you are on other medications, consult your doctor before using paracetamol. Some drugs may interact with paracetamol, increasing the risk of unwanted effects. When used exactly as prescribed, paracetamol is both safe and effective. However, it is critical to be informed of the potential side effects and hazards before using it.
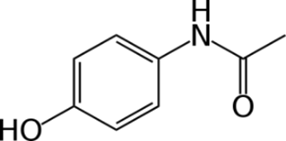
Fig 1. Chemical Structure of Paracetamol
Paracetamol (Panadol, Calpol, Alvedon) is an analgesic and antipyretic medication used to alleviate mild to moderate pain and fever. It is widely used as a component in cold and flu treatments, although it can also be used alone. Paracetamol is the same as acetaminophen (Tylenol). The drug’s name is Paracetamol, as assigned by the International Nonproprietary Name (INN) generic name system. In Europe, Australia, New Zealand, and India, the medicine is referred to as paracetamol. Acetaminophen is the generic name assigned under the United States Adopted Names (USAN) system.
In the United States, Canada, and Japan, the drug is known as acetaminophen. Typically, the INN and USAN generic names for a medicine are identical and do not differ across nations. Paracetamol was developed in 1878, but it wasn’t extensively utilized until the 1950s. Today, paracetamol is one of the most often used pain relievers in the world. This medicine is available in both branded and generic versions. Paracetamol is a popular pain reliever and fever reducer, available both over the counter and on prescription. It has a distinct clinical pharmacological profile, including strong analgesic and Antipyretic effects with no or little anti-inflammatory efficacy, as well as modest gastrointestinal, renal, and vascular adverse effects. Paracetamol has long been suggested as the first-line medication in pain management guidelines. It is often used to treat headaches, muscle pain, menstrual cramps, toothaches, and the common cold. Paracetamol is available in pills, capsules, liquid solutions, and suppositories. The proper dosage of paracetamol varies according to age, weight, and the severity of the ailment being treated. To obtain detailed dose directions, see the product package or visit a healthcare practitioner.
Here is a summary of the history of adverse medication events related with paracetamol use:
- Early Recognition: In the late 1960s and early 1970s, reports surfaced associating high dosages of paracetamol to liver damage. This acknowledgment raised awareness and prompted more inquiries into the drug’s possible hazards.
- Regulatory actions: Regulatory bodies, including the FDA and EMA, have taken action to address the dangers of paracetamol based on developing evidence. These included establishing maximum daily doses and reinforcing warning labels.
- Hepatotoxicity: Paracetamol overdose, especially when used in excess of the prescribed daily dosage or in combination with alcohol, can result in severe liver damage. The most common adverse medication reaction associated with paracetamol is hepatotoxicity. It is primarily caused by the development of a poisonous metabolite known as N-acetyl-p-benzoquinone imine (NAPQI), which depletes glutathione and induces oxidative stress in the liver.
- Therapeutic Misadventure: Adverse responses to paracetamol can also occur when patients accidentally exceed the recommended dosage or consume many paracetamol-containing medicines concurrently without recognizing it. This inadvertent overdose can result in liver damage.
- Recognizing Symptoms: Paracetamol toxicity symptoms may not appear quickly and may take several days to show. Initially, patients may experience nonspecific symptoms such as nausea, vomiting, and malaise. As liver damage worsens, symptoms of hepatotoxicity such as jaundice, stomach discomfort, and increased liver enzymes may occur.
- Regulatory Measures: Over time, regulatory bodies have monitored paracetamol’s safety and introduced additional safeguards to reduce the likelihood of adverse reactions. These include further decreasing the maximum recommended daily dose and giving better directions on dosing and duration of use.
An adverse medication reaction.
An adverse drug reaction (ADR) is defined as “an appreciably harmful or unpleasant reaction resulting from an intervention related to the use of a medicinal product; adverse effects usually predict hazard from future administration and warrant prevention, specific treatment, dosage regimen modification, or product withdrawal.” Adverse drug reactions (ADRs) are unplanned, adverse occurrences associated with the use of medications that occur as a cause and during a considerable proportion of unscheduled hospital admissions. Since 2012, the definition has expanded to encompass reactions caused by error, misuse, or abuse, as well as suspected reactions to unlicensed or off-label drugs, in addition to the authorized use of a medicinal product in regular doses.
A thorough medication history can help a prescriber comprehend the patient's previous experiences with drug therapy, notably detecting previous ADRs that may preclude re-exposure to the medicine. Preventing ADRs requires avoiding therapy in patient cohorts with a higher vulnerability or administering treatment under a therapeutic strategy that decreases the probability of an adverse effect (for example, co-administration of other medications, monitoring blood test results). Spontaneous reporting (via the Yellow Card Scheme in the UK) of suspected ADRs is a vital aspect of pharmacovigilance, however ADRs are significantly underreported across healthcare settings and sectors. If in doubt, send a report. [2 ]Seminal studies conducted in the late twentieth and early twenty-first centuries in the United States and the United Kingdom established that ADRs are a prevalent occurrence in clinical practice, including as a cause of unscheduled hospital admissions, happening during hospitalization, and presenting after discharge. The prevalence of ADRs has remained largely stable throughout time, with data indicating that 5% to 10% of patients may experience an ADR at admission, during hospitalization, or after discharge, despite numerous prevention measures. The incidence of occurrences is inextricably linked to the method employed to identify them, and the vast majority of ADRs do not produce substantial systemic symptoms. Antiplatelet, anticoagulant, cytotoxic, immunosuppressant, diuretic, antidiabetic, and antibiotic medications have all been linked to ADR-related hospitalizations. Fatal ADRs are frequently caused by hemorrhage, with an antithrombotic/anticoagulant combined with a nonsteroidal anti-inflammatory medicine (NSAID) being the most common probable cause.
Types of Adverse Drug Reactions:
- Dose-related
- Allergic
- Idiosyncratic
Dose-related adverse medication reactions are an exaggeration of the medicine’s therapeutic effects. A person using a blood pressure medicine, for example, may feel dizzy or light-headed if the treatment lowers blood pressure too quickly. A diabetic may experience weakness, sweating, nausea, and palpitations if insulin or another antidiabetic treatment lowers blood sugar levels too much. This type of adverse medication reaction is usually anticipated, but it might also be inevitable. It can happen if a drug dose is excessively high, if the person is extremely sensitive to the medicine, or if another drug delays the metabolism of the first drug, increasing its level in the blood. Dose-related reactions might be severe or mild, although they are rather common.
Allergic medication reactions are not dose-related but do require prior exposure to the substance. Allergic reactions occur when the body’s immune system responds inappropriately to a substance (also known as sensitization). After a person has been sensitized, subsequent exposures to the substance cause one of several types of allergic reactions. Doctors may use skin tests to predict allergic medication reactions.
Idiosyncratic adverse medication responses arise from processes that are currently unknown. This form of adverse medication reaction is quite unpredictable. Rashes, jaundice, anemia, a reduction in white blood cell count, kidney damage, and nerve injury that may impair vision or hearing are some of the adverse medication reactions seen.
Some adverse drug reactions are unrelated to the medication’s therapeutic impact yet are frequently foreseeable because the underlying mechanisms are well recognized. For example, stomach discomfort and bleeding frequently occur in patients who routinely use aspirin or other nonsteroidal anti-inflammatory drugs. These drugs inhibit the formation of prostaglandins, which protect the digestive tract from stomach acid.
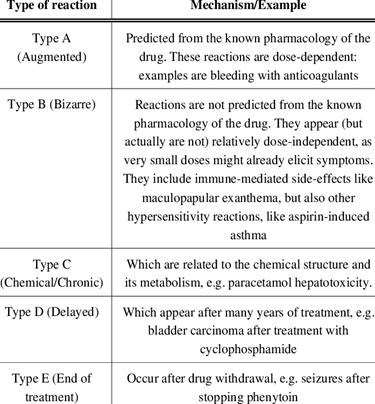
Fig 2. Overview of types of ADR’s
CRITERIA FOR IDENTIFYING ADRs:
ADR identified by physicians will be considered and will be included in the study.
ANALYSIS OF ADRs:
Nature and description of ADDRs reported.
CAUSALITY ASSESSMENT OF ADR BASED ON ALGORITHM:
The degree of association of an adverse of an adverse reaction with a drug is done with the help of Naranjo’s algorithm.
SEVERITY OF ADRS:
After the causality assessment has been done, the severity of the ADR is analyses using adapted Hart wig severity scale.
Naranjo’s algorithm – ADR probability scale
The Naranjo Algorithm, or Adverse Drug Reaction Probability Scale, is a method by which to assess whether there is a causal relationship between an identified untoward clinical event and a drug using a simple questionnaire to assign probability scores.
Adverse Drug Reaction Probability Scale
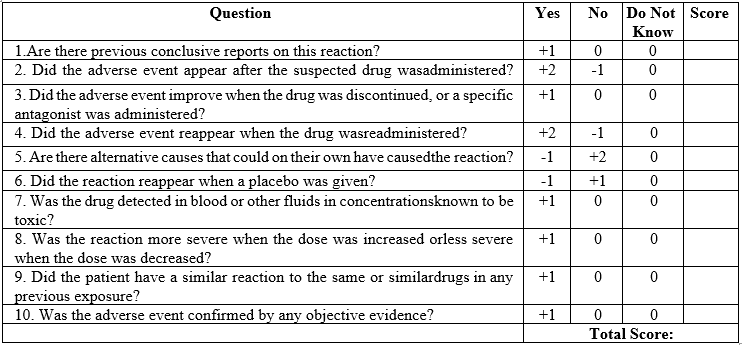
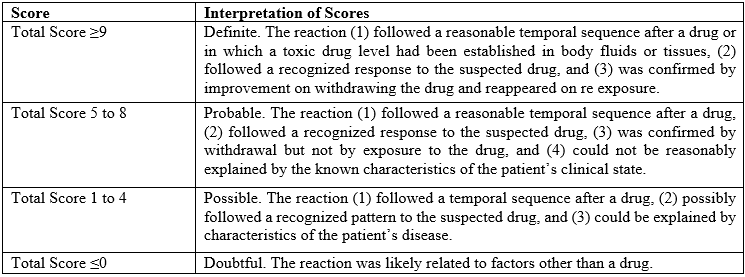
The Scale is classified as:
- Mild: A reaction that does not required treatment or hospital stay.
- Moderate: A reaction that requires treatment and prolongs hospitalization by at least one day. 3.Severe: A reaction that is potentially life threatening or contributes to the death of patient is permanently disabling requires intensive medical care or results in a congenital anomaly cancer or unintentional overdose.
Preventability of ADRs:
Complete preventability of ADR is not possible, but some of the ADR can be preventable if that ADR can give at least one answer of Schumock and Thornton Scale.
Predictability of ADRs:
Patients who have had the drug on previous occasion(s): If the drug was previously well- tolerated at the same dose and route of administration, the ADR is NOT PREDICTABLE; there was a history of allergy or previous reaction to the drug, the ADR is PREDICTABLE. Patients who have never had the drug previously: Incidence of the ADR reported in product information or other literature determines its predictability.
Observation
Following the eighth dose of paracetamol IV (1gm/1000ml), the patient developed red rashes on their upper limbs. Potassium chloride injection was a concurrent medication that was terminated as soon as rashes appeared. For the response, injection Avil was administered.
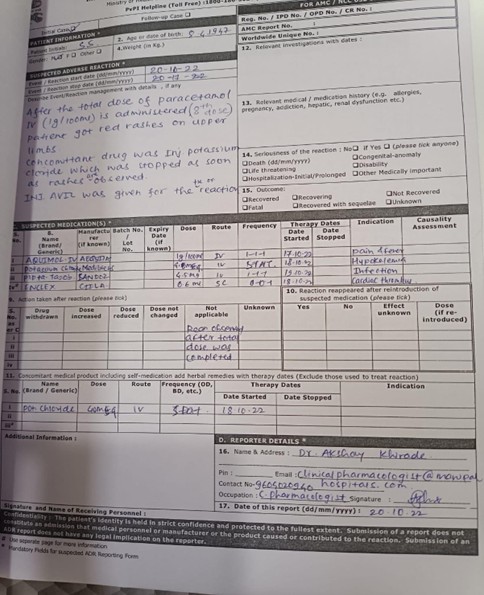
Fig 3. Adverse Drug Reaction Report
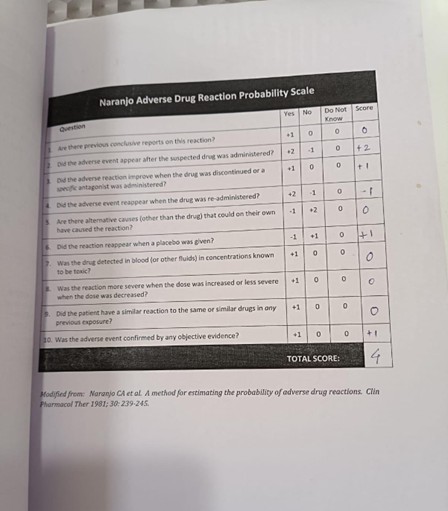
Fig 4. Naranjo’s scale
Patient Demographics
After the total dose of paracetamol IV (1gm/1000ml) is administered (8th dose) patient got red rashes on upper limbs. Concomitant drug was injection Potassium Chloride which was stopped as soon as rashes are observed. Injection Avil was given for the reaction.
Concomitant
Medications Potassium Chloride 40mg Suspected Medications
Brand Name – Potassium Chloride
Manufacturer- Medilife
Dose – 40mg
Route – Intravenous (IV)
Frequency – STAT
Date Started – 18/10/2022
Indication – Hypokalemia
Mechanism of action of Paracetamol
Prostaglandin synthesis inhibition: Cyclooxygenase (COX), an enzyme involved in prostaglandin synthesis, is weakly inhibited by paracetamol. Chemicals called prostaglandins are involved in inflammation and discomfort.
One possible mechanism of action for paracetamol is the activation of descending serotonergic pathways in the brain. The regulation of pain is influenced by these pathways.
Cannabinoid receptor involvement: According to some research, paracetamol may also function by attaching to cannabinoid receptors. Pain alleviation and other benefits are mediated by cannabinoid receptors. The exact mechanism of action of paracetamol is still being investigated. However, it is clear that paracetamol is a safe and effective medication for pain relief and fever reduction.
Here are some more specifics regarding paracetamol’s mode of action:
Prostaglandin synthesis inhibition: The COX enzyme has two isoforms, COX-1 and COX-2, which are both weakly inhibited by paracetamol. Prostaglandins are molecules that contribute to inflammation and pain, and they are produced by COX enzymes. By attaching to an enzyme site distinct from the one that is blocked by nonsteroidal anti-inflammatory medicines (NSAIDs), paracetamol inhibits COX enzymes. The reason paracetamol has less side effects than NSAIDs is due to this differential in binding.
One possible mechanism of action for paracetamol is the activation of descending serotonergic pathways in the brain. The regulation of pain is influenced by these pathways. When taking paracetamol, it causes an increase in the serotonin in brain. Cannabinoid receptor involvement: According to some research, paracetamol may also function by attaching to cannabinoid receptors. Pain alleviation and other benefits are mediated by cannabinoid receptors. When taken orally, paracetamol has the potential to bind and activate cannabinoid receptors. Pain alleviation results from its activation after that. Research is still ongoing to determine paracetamol’s precise mode of action. Nonetheless, it is evident that paracetamol is a drug that is both safe and efficient for treating pain and lowering fever. The enzyme called prostaglandin H(2) synthetase (PGHS) is in charge of breaking down arachidonic acid into the unstable PGH(2). The constitutive PGHS-1 and the inducible PGHS-2 are the two main types of this enzyme. There are two locations for PGHS: a cyclooxygenase (COX) and peroxidase (POX) sites. The COX site requires a tyrosine-385 radical to convert arachidonic acid to PGG(2). The formation of a ferryl protoporphyrin IX radical cation from the reducing agent Fe(3+) at the POX site is required for the conversion of tyrosine-385 to its radical state. Paracetamol functions as a reducing cosubstrate at the POX site, limiting the availability of the ferryl protoporphyrin IX radical cation. This action can be mitigated by the presence of hydroperoxide-producing lipoxygenase enzymes within the cell (peroxide tone) or by flooding the POX site with substrate such as PGG(2). Peroxide tone and swamping account for paracetamol’s lack of peripheral analgesic, platelet, and anti-inflammatory effects. Paracetamol’s actions could also be mediated via an active metabolite (p-aminophenol). Fatty acid amide hydrolase converts p-aminophenol to arachidonic acid, resulting in AM404. AM404 acts through cannabinoid receptors. It may also function via PGHS, particularly in brain regions with high levels of fatty acid amide hydrolase.
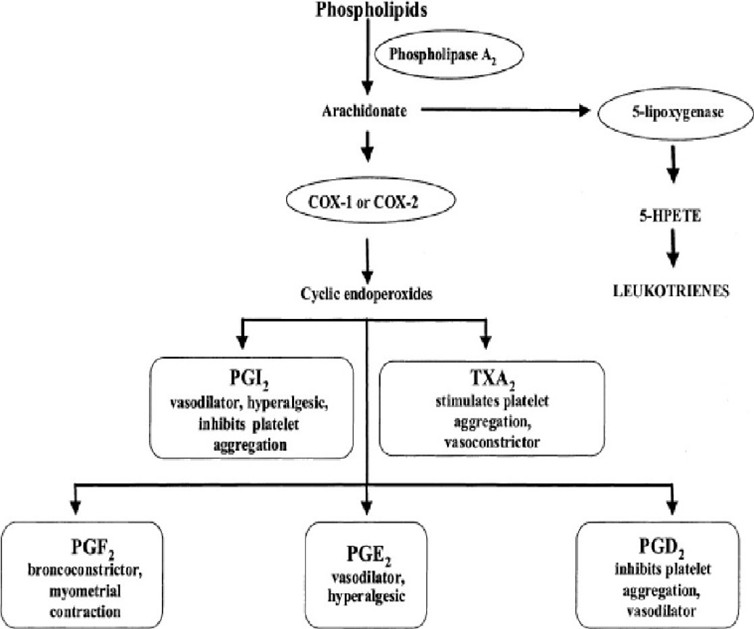
Fig 5. Mechanism of action of Paracetamol
Acetaminophen (paracetamol) is widely thought to be a mild inhibitor of prostaglandin production. However, paracetamol’s in vivo effects are comparable to those of selective cyclooxygenase-2 (COX-2) inhibitors. Paracetamol reduces PG concentrations in vivo, however unlike specific COX-2 inhibitors, it does not reduce rheumatoid arthritis inflammation. It does, however, reduce swelling following oral surgery in people and inhibit inflammation in rats and mice. Paracetamol is a poor inhibitor of PG synthesis of COX-1 and COX-2 in broken cell systems; nevertheless, therapeutic dosages of paracetamol block PG synthesis in intact cells in vitro when arachidonic acid levels are low.
Paracetamol Dose for Pediatrics
Paracetamol is a drug that is widely used in children and is available without a prescription. Paracetamol is most commonly used to relieve pain and lower fevers. Panadol, Dymadon, and Panamax are some of the paracetamol brands available in Australia with children's formulations.
While having a high temperature isn't always a bad thing because it boosts your immune system, it can be extremely painful. This is common with youngsters and can cause sleep disturbances for parents, brothers, and sisters. However, it is vital to understand that, while paracetamol can help patients feel better, it has little effect on the actual course of the underlying sickness.
Paracetamol is one of the most regularly used ‘over-the-counter’ medications, particularly for mild diseases that affect many youngsters. However, it is not often administered in the proper dosage, which might render it ineffective or hazardous. When used properly, paracetamol is an effective drug for making children more comfortable when they have minor illnesses or pain. Understanding and verifying the appropriate doses is critical if parents are to use it safely and successfully. The correct paracetamol dose for a child is determined by their weight. You should always administer the dose specified on the package or bottle based on your child’s weight.
Calculating doses –
The usual dose of paracetamol for children is 10-15 mg per kilogram of weight. In other words, if a child weighs 20 kg it should have 10-15mg x 20, which is 200-300 mg. This dose can be taken once every 4 to 6 hours, up to 4 times in 24 hours if needed. In practice, all children’s paracetamol products have clear instructions on the container or packaging outlining the correct dose of liquid, tablets or suppositories to give. It’s recommended to give the lowest dose that’s effective for the shortest period of time. You should not exceed the recommended dose except on the advice of your doctor. No child should take a total of more than 60 mg per kilogram of their body weight in a day. Caution is needed to never exceed the adult dose of paracetamol (4000 mg/day), which can happen if weight-based dosing is applied to children weighing over 65 kg. Paracetamol dosing errors can lead to acute liver failure in children. Calculation of a paracetamol dose in children should be based on body weight rather than age, and regularly updated as children grow. Caregivers should be given clear instructions on how to measure and administer doses. Paracetamol can be prescribed to infants from birth. However, paracetamol has a narrow therapeutic index and infants and children are at increased risk of overdose. Children aged under five years who are acutely unwell are particularly vulnerable to paracetamol toxicity, which can lead to liver failure and death.
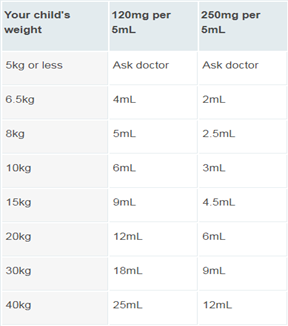
Fig 6. Paracetamol Dose for Pediatrics as per weight
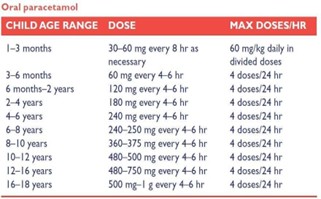
Fig 7. Paracetamol dose for pediatrics as per age
Paracetamol Dose for Geriatrics
Paracetamol is the most often used analgesic in the elderly, and it is typically administered in accordance with empirical dosing standards. However, physiological changes associated with aging can impact paracetamol’s pharmacokinetics and thus its effects. To evaluate the steps required to achieve more evidence-based paracetamol dose regimens in older individuals, we employed the pediatric research decision tree ideas.
A search was conducted to find research on paracetamol pharmacokinetics and safety in older persons (>60 years) or studies that analyzed pharmacokinetics and/or safety in older adults. Following title and abstract filtering, 259 of the 6088 articles discovered were retained. Further abstract and full-text screening yielded 27 articles, 20 of which described pharmacokinetics and seven safety. These investigations found no difference in absorption with age. When compared to younger subjects, robust older subjects had a lower volume of distribution (Vd) (3.9-22.9%), and weak older respondents had an even lower Vd (20.3%). Similar to Vd, age and frailty reduced paracetamol clearance (29-45.7 and 37.5%, respectively) when compared to younger participants. Because of the sparse and varied evidence, it was impossible to draw solid and relevant conclusions about the increased risk of paracetamol safety in older persons. This review is a first step toward bridging knowledge gaps and transitioning to evidence-based paracetamol dose in older adults. The remaining information gaps include safety when utilizing therapeutic dosages, pharmacokinetic alterations in frail older persons, and to what degree changes in paracetamol pharmacokinetics should lead to a change in dosage in feeble and robust older people.
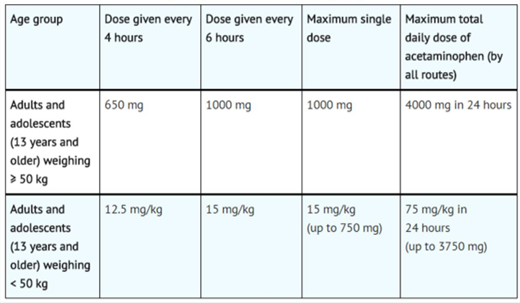
Fig 8. Paracetamol Doses for Adults
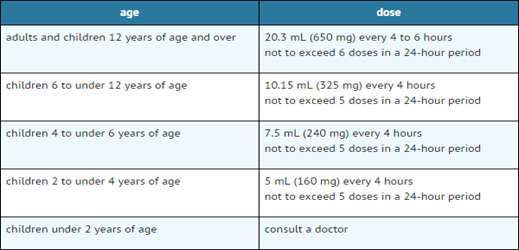
Fig 9. Paracetamol dose as per age group
Types of Paracetamol Formulations
For Adults:
Tablets – 325mg to 500mg
Oral-disintegrating tablets – 80mg to 160mg
Caplets – 325mg, 500mg and 650mg
Extended-release caplets – 650mg
Capsules – 500mg
Gel caps / gel tabs – 500mg
Oral suspension or solution – 160mg or 5ml
Liquid (oral) – 500mg or 5ml
Syrup (oral) – 160mg or 5ml
For Paediatric:
Tablets – 325mg to 500mg
Oral-disintegrating tablets – 80mg to 160mg
Caplets – 325mg, 500mg and 650mg
Extended-release caplets – 650mg
Capsules – 500mg
Gel caps / gel tabs – 500mg
Oral suspension or solution – 160mg or 5ml
Liquid (oral) – 500mg or 5ml
Syrup (oral) – 160mg or 5ml
List of Paracetamol brand names

DISCUSSION
The proper dosage of paracetamol, a common analgesic and antipyretic drug, was discussed in this review paper. A thorough review of the literature and clinical studies that were available led to the discovery of several important conclusions. First off, people are normally advised to take 500–1000 mg of paracetamol every 4-6 hours, with a daily maximum of 4000 mg. But while figuring out the right dose, individual factors like age, weight, and underlying medical issues should be considered.
Second, it is critical to follow the suggested dosage parameters to reduce the risk of paracetamol-related side effects, notably hepatotoxicity. Paracetamol overdose can cause serious liver damage and, in extreme cases, death. As a result, healthcare professionals and individuals should take caution and not exceed the recommended daily dose.
Furthermore, special consideration should be given to specific demographics, such as youngsters, the elderly, and those with liver or kidney abnormalities. These groups may require dosage changes or further monitoring to ensure that paracetamol is used safely and effectively. Furthermore, the research emphasized the necessity of educating healthcare professionals and the general public on proper paracetamol dose. Clear and accurate information, together with appropriate labeling and packaging, can help prevent accidents overdoses and enhance patient safety.
CONCLUSION
Lastly, future study should focus on determining the best paracetamol dosage for specific patient demographics, as well as assessing potential interactions with other drugs. Continued monitoring and surveillance of paracetamol use will help to enhance dose recommendations and reduce dangers. In conclusion, this review paper underlines the importance of following appropriate paracetamol dose guidelines to guarantee safety and efficacy. Understanding the prescribed dosage, taking individual circumstances into account, and raising awareness are all necessary for healthcare professionals and patients to make informed decisions about paracetamol use. Further inquiry is needed.
REFERENCES
- Ulderico Freo, Chiara Ruocco, Alessandra Valerio, Irene Scagnol, Enzo Nisoli. Paracetamol: A Review of Guideline Recommendations, Journal of clinical medicin, 2021 Aug, 10(15), 3420.
- Aronson JK. Ferner RE. Clarification of terminology in drug safety. Drug Safety, 2005, 28, 851– 70.
- Jamie J Coleman, Sarah K Pontefract, Adverse drug reactions. Clinical Medicine, 2016, 16(5), 481-485.
- https://www.msdmanuals.com/home/drugs/adverse-drug-reactions/risk-factors-for-adverse-drug-reactions.
- Brian J Anderson, Paracetamol (Acetaminophen): mechanisms of action. Paediatr Anaesth, 2008, 18(10):915-21.
- KD Tripathi, Essentials of medical pharmacology, Jaypee publication, 5th edition, 2004, page no. 167-184.
- https://bpac.org.nz/2018/paracetamol.aspx
- Paola Mian, Karel Allegaert, Isabel Spriet, Dick Tibboel, Mirko Petrovic. Paracetamol in Older People: Towards Evidence-Based Dosing. Drugs Aging. 2018; 35(7): 603–624.
- https://www.wikidoc.org/index.php/List_of_paracetamol_brand_names


 Atharv S. Gangarde*
Atharv S. Gangarde*
 Kiran H. Bibave
Kiran H. Bibave













 10.5281/zenodo.11521608
10.5281/zenodo.11521608