Abstract
Local anesthetics play a pivotal role in modern medical practice, providing effective pain management during various surgical, diagnostic, and therapeutic procedures. This review delves into the mechanisms of action, pharmacokinetics, clinical applications, and safety profiles of local anesthetic agents. By blocking voltage-gated sodium channels, these agents impede nerve signal propagation, resulting in reversible loss of sensation in targeted areas. Classic agents like lidocaine, bupivacaine, and ropivacaine are extensively used due to their efficacy and predictable profiles. However, emerging formulations and delivery methods, including liposomal encapsulation and adjuvants like epinephrine, are enhancing the duration and quality of anesthesia while minimizing systemic toxicity. The balance between efficacy and adverse effects is crucial, especially in vulnerable populations such as pediatric and geriatric patients. Furthermore, advancements in understanding the molecular basis of pain perception and nerve physiology are driving the development of novel agents with improved selectivity and duration of action. Local anaesthetics are weak bases and consist of a lipophilic aromatic ring, a link and a hydrophilic amine. The chemistry of the link classifies them as amides or esters. They act by blocking the sodium ionophore, especially in the activated state of the channel, and frequency dependence can be shown. The speed of onset is related to dose and proportion of drug in the unionized lipid-soluble form, which in turn is determined by the pKa and the ambient pH. Local anaesthetic agents, being weak bases, are bound in the plasma to a1-acid glycoproteins, influencing duration of action. Esters undergo hydrolysis by esterases in the plasma. Amides are subject to phase I and II hepatic cytochrome P450 metabolism. The development of the S-enantiomers, levobupivacaine and ropivacaine, has not been without some controversy with regards to therapeutic benefits when assessed by clinical potency models such as the minimum
Keywords
Local anesthetics bupivacaine, levobupivacaine, lidocaine, local anaesthetics, ropivacaine, Medicine
Introduction
The evolution of local anaesthesia began in 1884 in retreat from the toxicity and frequently poor conditions of general anaesthesia. Koller,1 an ambitious young Viennese ophthalmologist, discovered that cocaine instilled into his own conjunctival fornix produced localized insensitivity to touch and injury. Within a year Knapp2 had injected cocaine behind the eye to perform an enucleation. These events marked the origins of modern regional anaesthetic practice. Since then there has been a vast expansion in our knowledge about these drugs and in techniques of administration, although the agents themselves have changed comparatively little. Notably, cocaine, despite its well-documented toxicity is still in use today. However, although modern local anaesthetic agents are safer than their predecessors, they may still provoke toxic reactions, even in experienced hands and using the correct dose. Safe regional anaesthesia requires the practitioner to have a thorough understanding of the agents used, in particular, dose and concentration required, speed of onset and duration of action. They must be capable of recognizing impending toxicity, and have the equipment and current knowledge and skills to manage these events.
Physiology of nerve conduction
The physiology of nerve conduction involves a complex interplay of electrical and biochemical processes that enable the transmission of signals along nerve fibers. Nerve conduction occurs primarily through the propagation of action potentials, which are rapid changes in membrane potential that travel along the length of the nerve fiber. At rest, the membrane of a nerve cell (neuron) maintains a negative internal charge relative to the extracellular fluid, primarily due to the uneven distribution of ions across the cell membrane. This resting membrane potential is maintained by the activity of ion channels, pumps, and transporters. When a nerve impulse, or action potential, is initiated, typically by a stimulus such as pressure, temperature change, or a chemical signal, it triggers a depolarization of the membrane. This depolarization results in the opening of voltage-gated sodium channels in the membrane. Sodium ions rush into the cell, causing a rapid increase in membrane potential known as the upstroke of the action potential. As the action potential propagates along the nerve fiber, adjacent regions of the membrane are depolarized, leading to the sequential opening of voltage-gated sodium channels along the length of the nerve. This process creates a self-propagating wave of depolarization that travels along the nerve fiber.
After the action potential has passed, potassium channels open, allowing potassium ions to leave the cell, leading to repolarization of the membrane and restoration of the resting membrane potential. This repolarization phase is essential for resetting the nerve fiber and preparing it for subsequent action potentials. The speed of nerve conduction is influenced by factors such as the diameter of the nerve fiber (larger fibers conduct signals more rapidly), the presence of myelin sheaths (which insulate and speed up conduction in vertebrate neurons), and the temperature and metabolic state of the nerve.
Chemistry
Local anaesthetics have a common chemical structure, consisting of a lipophilic aromatic ring, a link and a hydrophilic amine group (Figure 1); most are tertiary amines. They can be classified into two groups based on the nature of the link: amides [-NH-CO-] and esters [-O-CO-]. The amide group is the most commonly used clinically and includes lidocaine, prilocaine, (levo-) bupivacaine and ropivacaine. The ester group includes cocaine, procaine, chloroprocaine and amethocaine. Being weak bases they are made soluble for injection by formulating them as strong conjugate acidic hydrochloride salts (pH 3-6).
Lidocaine + HCl = Lidocaine H++Cl-
MECHANISM OF ACTION
Local anaesthetics work by blocking the inward Na+ current at the sodium ionophore during depolarization, which prevents propagation of the axonal action potential. However, research indicates that their mechanism of action is more complicated than this, with calcium, potassium and G-protein regulated channels also being blocked by local anaesthetics. When delivered by injection, the local anaesthetic is predominantly in the acidic ionized form (pH 3-6). This dissociates in the relatively alkaline perineural tissues (pH 7.4) to lipid-soluble free base. This crosses the axolemma and re-ionizes in the acidic axoplasm to the active moiety, which blocks the sodium ionophore from within the cell or from the membrane lipid bilayer. Therefore the non-ionized form promotes delivery into the axon and the ammonium or ionized state provides activity. When the nerve is stimulated, the sodium ionophore alters structurally. This initiates its cycle through four functional states: resting, activated, inactivated and deactivated (Figure 2). The complex ionophore can be considered to have two functioning gates, an outer m gate and an inner h gate. In its resting state, the outer m gate is closed and the inner h gate is open. On nerve stimulation, the ionophore enters its active state whereby opening of the outer m gate results in the rapid influx of sodium ions. As the membrane potential increases (to around 20 mV) this triggers the closure of the inner h gate and the ionophore enters an inactive state. The deactivated state is formed as a result of closure of the outer m gate once the membrane potential reaches -60 mV. Whilst in an inactivated or deactivated state, the nerve is resistant to further stimulation. Local anaesthetic block is more readily achieved when the ionophore is in the activated than in the inactivated state and least when in the deactivated or resting state (state-dependent block). When the sodium channel is closed, as is the case in the deactivated or resting states, the local anaesthetic can only gain access via the membrane as free base. However, when the sodium channel is open, as is the case in the activated, and to a lesser extent in the inactivated state, the ionized local anaesthetic can also gain access to the nerve via the channel. In addition to this, the ionized form may enter from outside if the ionophore is repeatedly activated or opened. This gives rise to frequency-dependent (or phasic) block. The onset of block with agents that have more ionized molecules outside the membrane can therefore be accelerated by stimulation. Thus, frequency-dependent block is better demonstrated for bupivacaine than for lidocaine. Different local anaesthetics also have varying channel affinity. Lidocaine binds and dissociates rapidly from the channel, whereas bupivacaine binds rapidly but dissociates more slowly.

Fig 1: Reproduced from Anaesthesia and Intensive Care Medicine
This has little effect on neuronal block but assumes greater importance when referring to effects on cardiac toxicity. The S-enantiomer of bupivacaine dissociates more quickly thus reducing cardiotoxicity. The speed of onset of block is related to the concentration of molecules of local anaesthetic that are in the free base or non ionized state. This depends on the initial dose, the dissociation constant (pKa) and the pH of the tissues. By convention, dissociation constants are applied to the acidic forms and this some times causes confusion as inversions of the Hendersone Hasselbalch Equation (1) are often used depending on whether an acid or base is involved
pH =pka+ log ([base]/acid]) ………..1
for an acid :pH= pka+log(ioinized]/[non-ionized]) ………..2
for a base :pH=pka+log ([non-ionized]/[ionized]) ………..3
It is simpler to use equation 1 or a modification for all substances and consider only the acidic version:
pH =pka+ log ([dissociated]/[associated]) ………..4
When looking at the pKa for ‘bases’, it is then helpful consider them in the acidic ionized form. Equations 1 and 4 can be further modified to:
Pka-pH=log ([acid]/[base]) ………..5
Pka-pH=log ([associated]/[dissociated]) ………..6
Using either equation, and given that physiological pH is 7.4, it can be seen that more molecules of bupivacaine (B) (86%) remain in the associated (ionized) form compared with lidocaine (L) (72%), and therefore onset is slower.(table 1)
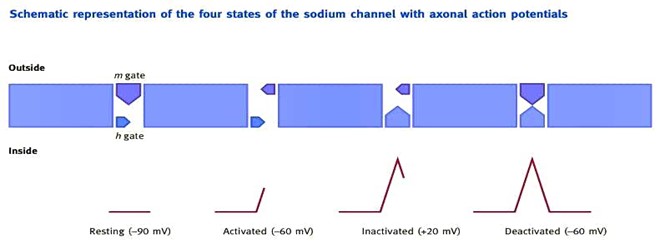
Figure 2 The sequence of activation of the sodium ionophore is shown. Reproduced from Anaesthesia and Intensive Care Medicine
Physicochemical characteristics and suggested dosing maxima
Table 1

0.8=log([BH+]/[B]) Versus 0.4 =log(LH+/[L])
The speed of onset of local anaesthesia can be accelerated by alkalinization or carbonation of the injectate. The addition of bicarbonate causes the strong conjugate acidic ammonium ions to dissociate and increase free base concentrations. The ingress of carbon dioxide into the axoplasm renders the interior even more acidic, encouraging re-ionization – a process referred to as diffusion trapping. Similarly, the acidic environment of an abscess decreases the proportion of local anaesthetic in the free base state, which may explain the resistance to conduction block in such situations. However, it is useful to put the role of pKa and pH in perspective. From Table 1 it can be seen that chloroprocaine (used in the USA) has a high pKa consistent with a slow onset of block. However, this agent is used when a fast onset is required for epidural anaesthesia, such as for emergency Caesarean section. As a 3% w/v solution, larger doses can be given because of its low potential for systemic toxicity due to ester hydrolysis. Therefore, dosing is more important than pKa. Potencies of local anaesthetic agents are related to lipid solubility and are quantified as octanol partition coefficients (Table 1). In addition, for agents that are enantiomers (enantiomorphs, optical isomers), s-enantiomers may be more potent than r-enantiomers. However, these apparent differences in potencies are inconsistent and may be related to differences in vasoconstrictive and pharmacokinetic properties.
Pharmacokinetics
Local anaesthetic agents are weak bases, and thus become bound in the plasma to ?1-acid glycoproteins. Esters undergo rapid ester hydrolysis by plasma pseudocholinesterases (and other esterases) and therefore have a lower potential for systemic toxicity, but a higher incidence of allergic reactions than the amides, owing to the formation of para-amino benzoic acid as a metabolite of hydrolysis Amides undergo phases I and II hepatic cytochrome P450 metabolism. Phase I includes hydroxylation, N-dealkylation and methylation. Phase II includes conjugation with amino acids such as glycine. Local anaesthetics are extracted as they pass through the acidic lung tissue. This effect of ion trapping is also seen during pregnancy, as the low fetal pH results in ionization and therefore trapping of the local anaesthetic.
Choices of local anaesthetic
Cocaine Cocaine is an ester of benzoic acid and is found naturally in the leaves of Erythroxylon cola. It has been used medically and recreationally for many hundreds of years. In addition to its local anaesthetic actions on nerve membranes, it is also able to block the re-uptake of nor-epinephrine at sympathetic neurones, potentiating the effects of catecholamines. It is used as a topical anaesthetic, usually for procedures involving the nasal mucosa. It was traditionally combined with epinephrine and bicarbonate as Moffet’s solution to provide a solution with very intense vasoconstrictor proper ties. In overdose, it produces hypertension, tachy arrhythmias, tachypnoea, nausea and a myriad of central nervous system (CNS) effects. Toxicity is enhanced by its slower metabolism compared to other ester local anaesthetics. Lidocaine (Lignocaine, Xylocaine) Introduced in 1947, lidocaine is a tertiary amide derivative of diethylamino-acetic acid. In concentrations of 0.5–4%, it is capable of delivering a profound block with a rapid speed of onset due to its low pKa. Lidocaine contains a dimethylbenzene moiety termed xylene. The amide link confers the term xylidine or xylidide. The major metabolite of N-dealkylation of one of the -C2H5 groups is mono ethylglycine xylidide. Hepatic amidases result in various xylidine metabolites. Drugs that decrease hepatic blood flow (e.g. propranolol), decrease metabolism and clearance. Lidocaine is also used as a class 1b anti-arrhythmic agent. When administered spinally at high concentrations, reports of transient radicular irritation suggest that it is neurotoxic. Repeated injection may reveal tachyphylaxis. Prilocaine Prilocaine is a secondary amide analogue of lidocaine with a similarly rapid onset but longer duration. Due to rapid tissue uptake and bio transformation, prilocaine plasma levels fall rapidly making it a safe local anaesthetic, so it is a popular choice for intra-venous regional anaesthesia. Toxicity may be manifest by the usual signs and symptoms of local anaesthetic overdose. In addition, ortho-toluidine one of the breakdown products of prilocaine metabolism is able to convert ferrous iron to ferric iron in haemoglobin, causing methaemoglobinaemia. Treatment is with high concentrations of oxygen and intra-venous methylene blue (1mg/kg). Neonates are particularly at risk be cause their red cells are deficient in methaemoglobin reductase. Prilocaine is also found in eutectic mixture of local anaesthetics (EMLA). This oil/water emulsion contains 2.5% prilocaine and 2.5% lidocaine. Both constituents have a melting point considerably higher than body temperature, yet combined as a eutectic mixture they have a lower melting point (181C) than either substance separately. At room temperature EMLA becomes a cream. It is able to penetrate the skin easily so is used extensively in paediatrics to reduce the pain of venepuncture. Caution must be exercised when using large amounts as methaemoglobinaemia has been described. Broken or inflamed skin should be avoided as absorption may greatly exceed that intended. An aqueous cream preparation of 4% tetracaine (Ame top, Smith & Nephew Healthcare Ltd.) performs similarly to EMLA, penetrating skin rapidly, but can cause severe skin reddening.
Bupivacaine Introduced in 1963, Bupivacaine is the butyl (C4H9) derivative of N-alkyl pipecoloxylidine, and is therefore part of the same homologous series as mepivacaine (methyl: CH3) and ropivacaine (propyl: C3H7). All pipecoloxylidine derivatives have an asymmetric carbon atom that confers the property of chirality or enantiomerism. The usual preparation of bupivacaine is as a racemate. Following N-dealkylation, the pipecoloxylidine metabolite still has some activity. Ropivacaine Ropivacaine is the propyl derivative of N-alkyl pipecoloxylidine. Ropivacaine (or propivacaine) is the S-enantiomer of the propyl (C3H7) derivative of N-alkyl pipecoloxylidine. Metabolites include 3-hydroxyropivacaine (40%) and pipecoloxylidine (5%). The octanol partition coefficient for ropivacaine (115) suggests lower potency when compared with bupivacaine (346). Howev er, ropivacaine may exert more vasoconstrictor activity to offset this. At lower concentrations, it may be motor sparing. Its reduced lipid solubility resulting in less penetration of the A? fibres may, in part, explain this. Levobupivacaine is the s-enantiomer of racemic bupivacaine. The proportion of plasma-bound levobupivacaine is greater (95%) than racemate (93%). Chirocaine is bound by Directive 91/507 of the European Community (EC), which states that the %w/v should be expressed in terms of the agent (free base) alone and not the hydrochloride salt. Therefore, levobupivacaine, by virtue of being expressed as free base, has 13% more molecules than the equivalent %w/v of the racemate bupivacaine (Marcain, AstraZeneca Pharmaceuticals), which predates the Directive. This difference in expressed formulation should be considered when comparing this agent with other local anaesthetics. Articaine Articaine, synthesized in 1969 and is widely used in dental anaesthesia it has been shown to be a useful agent for ophthalmic anaesthesia in concentrations of 2–4%. Its molecular structure includes an additional ester linkage which provides a good safety profile due to its rapid metabolism by esterases (elimination half-life less than 20 minutes), therefore allowing re-administration during the procedure. It has the same pKa and toxicity as lidocaine. The benzene ring is replaced with a thiophene ring allowing faster and better penetration of the cell membrane and bone. Concerns have been raised regarding persistent paraesthesia, due to possible neurotoxicity.
Topical agents
Topical agents may be used to provide surface anaesthesia of mucous membranes, the cornea or the skin.Topical anaesthetics must cross tissue barriers to have effect. The drug must have a low pKa, so that more of the drug is in the non-ionized form, or be used in concentrations higher than those used for injection. The development of a eutectic mixture of local anaesthetics for topical use has been welcomed, particularly in paediatric practice. This 50:50 mixture of solid lidocaine and prilocaine as lipid-soluble free bases has the lowest melting point of any combination. This defines the eutectic point and hence the eutectic mixture. Methaemoglobinaemia has been reported following extensive application and systemic absorption of prilocaine in infants. Amethocaine is also available for topical application as 4% tetracaine, aqueous cream preparation (Ametop, Smith & Nephew Healthcare Ltd). It penetrates the skin quickly but can cause marked skin irritation. A combination of lidocaine and epi nephrine (Iontocaine) has recently been introduced, and provides dermal analgesia by iontophoresis, whereby an electric current delivers charged drug ions to the skin (Phoresor Iontophoretic Drug Delivery System, IOMED Inc.). All except benzocaine are used in ophthalmology where they produce excellent topical anaesthesia. Clinical differences are stinging when dropped onto the eye (pro xymetacaine least, tetracaine worst, oxybuprocaine in between), and corneal toxicity, which is worst with tetracaine.
Stereochemistry
The pipecoloxylidines contain a chiral carbon and can exist as two enantiomers, R (+) and S (-). The prefixes R (rectus) and S (sinister) refer, respectively, to the clockwise or anticlockwise arrangement of atoms or molecules around the asymmetric car bon atom based on an arbitrary hierarchical series of sequence rules. Evidence from animal and human models suggested that among certain chiral local anaesthetics, the S- was less toxic than the R-enantiomer or the racemate. This led to the development of the S-enantiomer of the propyl derivative: S-propivacaine (ropivacaine: Naropin). Two human toxicology studies showed that 12-25% more ropivacaine could be tolerated before the onset of central nervous system (CNS) toxicity. This perceived advantage for ropivacaine was based on the assumption of equipotency with bupivacaine. However, studies using the minimum local analgesic concentration (MLAC) model, have found that ropivacaine is 40% less potent than bupivacaine.The human toxicology and MLAC efficacy studies have been formally compared to derive therapeutic indexes and the relative therapeutic ratio in humans for both local anaesthetics. The relative therapeutic ratio (ropivacaine: bupivacaine) for clinical CNS toxicity of 0.75 was found to be significantly in favour of bupivacaine. Therefore, the perceived benefits for ropivacaine in relation to toxicity, motor block sparing and differential sensory blockade need to be re-evaluated and may be explained by potency issues. The most recent development has been that of S-bupivacaine (levobupivacaine: Chirocaine). A human toxicology study found that the median tolerated dose of levobupivacaine for the onset of CNS symptoms was 5% greater than the racemate. Levobupivacaine also caused significantly fewer reductions in cardiovascular performance as assessed by stroke index, acceleration index and ejection fraction. Some of these effects may represent less vasodilatation or reductions in preload because of the significant augmentation of diastolic pressure with the race mate. An efficacy MLAC study showed that the potency of lev obupivacaine was 98% that of the racemate on a %w/v basis (87% in molar terms). Comparing therapeutic indexes, the relative therapeutic ratio (levobupivacaine: racemic bupivacaine) for CNS toxicity is 1.03 and therefore marginally in favour of levobupivacaine. There is a spectrum of potencies (low, intermediate, high) for ropivacaine, levobupivacaine and racemic bupivacaine with regard to analgesia, motor block and toxicity. Therefore, while ropivacaine and levobupivacaine may appear to widen sensory motor separation, the relative ratios of effects suggest that differences are more apparent than real and pharmacologically they may not exist! However, the profiles of apparent differences in separation should still be clinically useful for the lower potency pipecoloxylidines and may be explained by differences in potency.
Toxicity
Systemic toxic reactions follow absorption of an inappropriately high dose, or accidental intra vascular injection of an appropriate dose. The magnitude of the effect will depend on the toxicity of the drug, the dose administered, the speed and site of administration and pH of the blood. Methods to reduce the incidence of these events include careful techniques of needle placement, aspiration prior to every slow injection, the use of a test dose, use of a less toxic local anaesthetic, awareness of maximum doses in different settings, and the addition of other agents (opioids, clonidine, hyaluronidase, bicarbonate, epinephrine) to reduce the amount of local anaesthetic required. Inevitably, toxic reactions do occur. Early signs involve the CNS with peri-oral and tongue paraesthesia, a metallic taste, dizziness, slurred speech, diplopia, tinnitus, confusion and restless ness, progressing on to muscle twitching, convulsions and coma. Treatment is aimed at maintaining oxygenation, fluids, pressors, inotropes and the use of anticonvulsants where appropriate. As plasma levels increase impending cardiovascular toxicity may be heralded by development of a bradycardia, with a long PR interval and widened QRS complex. Progressive blockade of the cardiac conduction tissue leads to varying degrees of block, the appearance of multi-focal ectopic beats, reenterant arrhythmias, tachycardia and ventricular fibrillation. Isolated organ preparations have demonstrated that some of these disturbances arise from drug actions in the brain as well in the heart.16 It is highly unlikely that a serious cardiac adverse event will occur in isolation without preceding CNS toxicity. Similar to CNS toxicity, treatment is supportive, relying on the use of oxygen, fluids, vasopressors, inotropes and anti-arrhythmics where needed. Amio darone and bretylium may be useful in this setting, and there is evidence suggesting lipid emulsion infusions and clonidine may prove useful. Circulatory arrest due to bupivacaine toxicity is often refractory to treatment, and as depression of neurological function induced by the local anaesthetic may have a neuro-protective role resuscitation should be prolonged. Recently, the administrations of lipid containing solutions, e.g., intra-lipid have proved effective in treating toxic reactions with bupivacaine especially where cardiovascular problems occur. Treatment protocols have been produced and ideally should be available wherever these drugs are used
Allergy
The first case of allergy to local anaesthetics was reported in 1920 when Monk described the development of contact dermatitis in the hands of a dentist who was repeatedly exposed to apothesin, which was an amino-ester cogener of procaine. There followed further reports of mild hypersensitivity reactions, but very few patients developed anaphylaxis. The allergenic trigger was found to be para-aminobenzoic acid (PABA), from which all ester local anaesthetics are derived, and which is generated as an intermediate metabolite on ester hydrolysis. Sensitivity to PABA may occur through exposure to ester local anaesthetics, or to cosmetics and foodstuffs that contain preservatives which are antigenically similar. In addition, sulphonamide substances structurally resemble PABA so cross reactivity may occur when sulphonamide sensitive patients are then exposed to ester local anaesthetics. The development and use of amide instead of ester local anaesthetics in the 1940s reduced reporting of allergic reactions. It is now recognized that allergy to amides is extremely rare, with some specialists estimating that less than 1% of reported allergic reactions to amide local anaesthetics represent true immune system mediated re sponses.20 Local anaesthetics are too small (o300Da) to be antigenic, but may bind to plasma or tissue proteins as a hapten that possesses antigenic properties. Despite the rarity of true allergic reactions, it is not unusual to meet patients who report that they are allergic to local anaesthetics. This often follows an adverse reaction in the dental chair following an injection. In this setting, the patient is often highly anxious and predisposed to vasovagal events, exacerbated by the upright position of some dental chairs. Alternatively, they may have received an intra-vascular injection of local anaesthetic containing epinephrine with surprising and unpleasant cardiovascular effects. However, although true allergy to amide local anaesthetics is rare it must be taken seriously and appropriate investigations or referral organized. Allergists may perform skin prick testing for those with a history of mild allergy, or use laboratory in vitro tests (lymphocyte proliferation test) in patients with a history of anaphylaxis
The future
Further understanding of the role of chirality in local anaesthetic pharmacology is required to justify clinical use. Local anaesthetic sparing combinations with other analgesics such as opioids, epinephrine, clonidine, midazolam and ketamine continue to be studied to develop synergistic regimens.4 Research is continuing for novel local anaesthetics. Tonicaine is a longer duration derivative of lidocaine, sameridine has local anaesthetic and opioid receptor agonist properties. Butyl amino-benzoate has a low pKa, is poorly soluble and appears selective for Ad and C fibres, thus producing minimal motor block with a prolonged duration of action. Naturally occurring toxins such as capsaicin, tetrodotoxin and saxitoxin continue to be of interest. Tetrodotoxin and saxitioxin are naturally occurring neurotoxins, found in marine life, which block the sodium ionophore extracellularly. Tetro dotoxin, which has a pyrimidine and saxitoxin, a purine struc ture, are some of the most potent non-protein biological toxins. Their strong and selective affinities for the sodium channel have generated interest for possible roles in pain therapy. Another related class of local anaesthetics being evaluated include the basic esters of phenylcarbamic acid. These are up to 300 times more potent than currently used local anaesthetics. Unlike current agents, their potency increases with the acidity of the sur rounding tissues. The therapeutic role of local anaesthetics is likely to expand as researchers elucidate their anti-inflammatory, neuroprotective, antithrombotic and anti-cancer properties. It has become clear from errors in the development of enantiomers for clinical application that determining the relative potencies of drugs is of prime importance in evaluating claims for perceived advantages in toxicity and blocking characteristics.
LOCAL ANESTHESIA TECHNIQUES IN MEDICINE
Primary Surgical Anesthesia
As in dentistry, infiltration and localized peripheral nerve blocks are the more common techniques for administration of local anesthetics in medicine. Nerve block techniques are often used as the sole anesthetic technique in the medicine for a large variety of surgical procedures, just as in dentistry. Nerve blocks serve as a useful alternative for patients who would otherwise be at a higher risk for complications of general anesthesia because of complex medical conditions. For example, a patient requiring an incision and drainage of an infected ankle fracture who has a compromised cardiac history may be at an increased risk for further cardiopulmonary complications if the surgery is performed under general anesthesia. The placement of a nerve block, with or without sedation, not only lowers the potential risk for additional systemic complications but also provides the patient and surgeon with a comfortable, reliable, high-quality anesthetic experience.
Infiltration Anesthesia
Similar to maxillary infiltration techniques used in dentistry, local anesthetics are deliv ered subcutaneously and allowed to diffuse into the surrounding tissue. This method provides adequate anesthesia for the overlying skin and subcutaneous structures. With the use of a longer needle and injecting while simultaneously withdrawing the needle, local anesthetic coverage to a larger area or field is achieved. Infiltration or field blocks are used frequently during carpal tunnel surgeries to anesthetize the nerves of the wrist or during ankle procedures to block the nerves of the ankle. Although useful, field blocks often require larger volumes of local anesthetic, espe cially if vasoconstrictors are not added
Major regional nerve blocks
Regional nerve blocks are conceptually identical to the various nerve blocks used in dentistry, but larger nerves covering broader areas are targeted. For example, by delivering local anesthetic to the main nerve branches innervating the extremities, it is possible to provide adequate anesthesia to allow a patient to undergo an invasive arm or leg surgical procedure without any intraoperative pain. Anesthesiologists almost exclusively provide major regional anesthesia, and have additional equipment at their disposal that helps identify anatomic relationships and direct needle placement. These techniques include the use of electronic nerve stimulators, portable ultra sound machines, and even fluoroscopy. An electronic nerve stimulator emits small electrical stimuli that can elicit motor or sensory responses when delivered in the proximity of a nerve. By attaching the nerve stimulator to a special needle, anesthesiologists watch for a motor response and are then able to amplify that response by directing the needle closer to the nerve. Once the motor response is maximized, the local anesthetic is delivered through the needle, right at the level of the targeted nerve. This procedure helps to eliminate problems associated with missed blocks. Portable ultrasound machines are now used to visualize the anatomic structures so that the needle can be guided directly toward the targeted neural structures. Ultrasound machines are even capable of visualizing vascular structures, which helps reduce the likelihood of an intravascular injection. Similarly, the use of fluoroscopy, a conventional x-ray unit that relays immediate information from both static and moving images, allows for real-time visualization of both soft and hard tissue. With this technique, it is possible to inject medications directly at the level of specific nerve roots as they exit the spinal column with visualization of the needle and surrounding tissue simultaneously.
Specific regional nerve blocks: upper extremities
The main innervations of the upper extremities all arise from levels C5–C8 and T1. These nerves coalesce to form the brachial plexus shortly after exiting the intervertebral foramina. After intermingling within the brachial plexus, they extend distally to provide motor and sensory innervation to the shoulder, upper arm, forearm, and hand. There are several techniques that are used to provide anesthesia via deposition of local anesthetic along the distribution of the brachial plexus nerves. From the proximal aspect of the brachial plexus distally, the techniques include interscalene, supra clavicular, infraclavicular, axillary, and digit blocks (Fig. 1). Selection of the type of upper extremity block depends on what dermatomes or areas of the arm are involved in the surgery. As expected, deposition of local anesthetic at each specific nerve will provide anesthesia to all areas innervated distally from that site of injection. Upper extremity nerve blocks are easily placed; are usually fairly well tolerated by patients, requiring minimal sedation for comfort; and have a fairly low complication rate when performed by trained personnel, such as an anesthesiologist. The Bier block (intravenous regional anesthesia) is a technique that is capable of providing dense anesthesia for a short duration. It can be used for surgical procedures involving the hand or forearm, such as carpal tunnel release. The method involves placing an intravenous catheter in the dorsum of the hand, exsanguinating the limb in a proximal direction from the fingers down to the forearm with an elastic wrap, and then inflating a tourniquet on the upper arm. After removing the elastic wrap, 40 to 50mLof0.5%lidocaineisslowly injected intravascularly through the catheter. After delivery of the anesthetic the catheter is removed; and adequate anesthesia is achieved within 10 minutes. The tourniquet is slowly deflated after completion of the surgical procedure, preventing a rapid surge of lidocaine back into the systemic circulation, thereby reducing the risk of anesthetic overdose and central nervous system symptoms.

Fig. 3. Brachial plexus blocks.
Specific regional nerve blocks: lower extremities
Despite the nerves beingless superficial and often more difficult to locate than those in the upper extremities, regional anesthesia remains a viable option for patients under going surgical procedures on their lower extremities. Surgical procedures involving the calf and thigh may require multiple injections because of the pathways of the nerves innervating the leg. The nerves supplying the leg arise from level L2–S3. Similar to the upper extremity nerves, the femoral (L2–L4), obturator (L2–L4), and lateral femoral (L1–L3) all join together to form the lumbar plexus. By injecting local anesthetic at the level of the lumbar plexus, it is possible to cover the anterior knee, thigh, and medial aspect of the foot. The remaining main nerve, the sciatic nerve (L4–S3), innervates the posterior aspect of the leg. To provide anesthesia to the entire leg, both sites would need coverage (Fig. 2).
NEURAXIAL TECHNIQUES
Neuraxial anesthesia involves the delivery of local anesthetics to the nerves within or immediately exiting the spinal cord (see Fig 2). This anesthesia consists of the nerves that are contained within the subarachnoid space, so-called spinal anesthesia, and those that transverse the epidural space. Depending on the site of delivery, the admin istered drugs will then interact with the spinal nerves within the spinal column or as they exit through the intervertebral foramina
Spinal Technique
The subarachnoid, intrathecal, or spinal space exists between the pia and arachnoid mater within the spinal meninges. It extends superiorly through the foramen magnum and down to level S2 and the filum terminals Thisanatomic space contains the spinal nerves, which terminate around level L1–L2, along with cerebral spinal fluid. Because this is a continuous space, any administered medication can potentially migrate well beyond the site of injection and involve additional levels of the spinal cord, or even the brain. Migration depends not only on the position of the patient but also on the properties of the drug, such as baricity, and volume administered. Spinal blocks are frequently used for shorter surgical procedures involving the lower extremities or perianal region, such as hemorrhoidectomy. The benefits of a spinal block include a quick onset, high reliability, and minimal risk of drug toxicity. By injecting a small bolus of an opioid, such as fentanyl or morphine, with the local anesthetic into the lower lumbar spinal space, the anesthesiologist can completely anesthetize the patient from the waist down and also provide potent analgesia for the
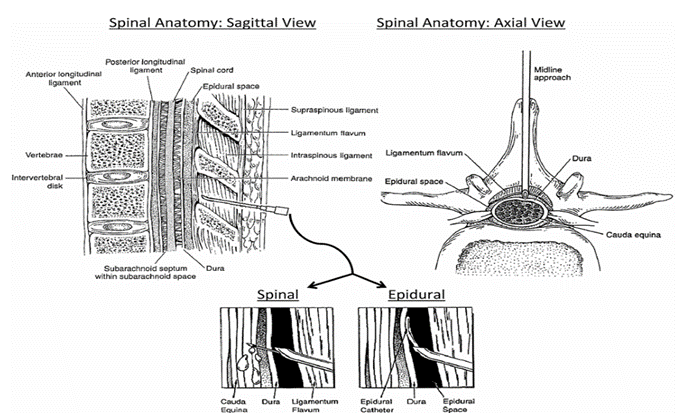
Fig. 4. Neuraxial anesthesia
Epidural Technique
Similar to the spinal block, the epidural technique also works directly on the spinal nerves, though not within the spinal cord. The epidural space extends vertically from the foramen magnum down to the sacral hiatus;however, itliesoutside thespinal meninges. It is bound anteriorly by the posterior longitudinal ligament and posteriorly by the ligamentum flavum. Of importance, the lateral aspect of the epidural space is bound by the intervertebral foramina, which is where the spinal nerves exit the spinal canal. Delivery of local anesthetics creates a band of anesthesia that involves mainly the nerve roots at the levels adjacent to the epidural without blocking all the nerves inferiorly. Because the epidural space is actually a potential space, it has no important contents or structures. As such, it will easily accept not only medications but also the passage of flexible catheters through which drugs can be delivered continuously via a computer-programmed pump, which is the main advantage of this technique. Some of the benefits to epidural anesthesia include titration of the analgesic level, prolonged orcontinuous drug delivery, and flexible dosage schedules. Epidurals are most frequently placed for obstetric anesthesia or during peripheral vascular procedures on the lower extremities. For example, it is common for epidural catheters to be placed to help provide pain control for labor once the mother is dilated past 4 to 5 cm. Should the mother have to undergo a Caesarean section, the epidural can provide the sole anesthetic, allowing the mother to remain conscious during the procedure. An alternative technique would be placement of a spinal block prior to undergoing Caesarean section, but the downsideis theinability to titrate postoperative pain control. However, in selected patients the combination of spinal and epidural techniques can be used when the advantages of both techniques are desired. The use of thoracic epidurals during invasive thoracic surgical procedures has greatly increased. The ability to selectively block the thoracic nerves and improve postoperative pain control following thoracotomies helps patients breathe much easier, preventing potential postoperative complications such as splinting, atelectasis, and pneumonia.
COMBINED GENERAL AND LOCAL ANESTHESIA
As discussed earlier, local anesthetics are versatile drugs that can serve as sole anesthetics if needed. However, it is common for anesthesiologists to combine their use with a general anesthetic technique. When used as a supplement, local anesthetics reduce and even eliminate the perception of the noxious stimuli (ie, pain) produced by the surgical procedure. This multifaceted approach helps reduce general anesthetic requirements, leading to the administration of smaller doses of systemically acting general anesthetic drugs, invariably reducing the risk of potential systemic complications and pharmacologic side effects. An example includes the placement of anupperextremitynerveblockpriortogeneralanesthesiafortherepairofahumeral fracture. This technique is commonly used in dentistry, where regional anesthesia of the oral cavity is usually predictable. The method permits light general anesthesia to be administered, allowing rapid recovery and resulting in fewer adverse effects of systemically administered anesthetics.
POSTOPERATIVE ANALGESIA WITH LOCAL ANESTHETICS
In addition to serving as a sole anesthetic and a powerful adjunct to general anesthesia, local anesthetics are being increasingly used for postoperative analgesia. Local anesthetics are arguably the most effective postoperative analgesic. For example, bupivacaine is often injected subcutaneously along an incision after closure of an abdominal wound, effectively blocking any pain from the incisional site for several hours. This drug provides adequate analgesia that extends well into the immediate postoperative period, decreasing the amount of opioids required by patients in the post anesthesia care unit. High doses of opioids can cause a variety of postoperative complications ranging from nausea, vomiting, urinary retention, respiratory depression, and even respiratory arrest. By decreasing the opioid requirements with the use of local anesthetics, patients are kept comfortable with a lower risk of opioid side effects. In addition to incisional anesthesia, anesthesiologists often use peripheral techniques in combination with a small catheter and pump mechanism to provide continuous delivery of local anesthetic to the surgical area. In addition, the use of an ON-Q Pain Relief System (I-Flow Corp, Lake Forest, CA, USA) allows the patient to ambulate at home while wearing the self-contained pump system (Fig. 3) in a portable fanny pack. This device provides powerful pain control for several days following a surgical procedure without the patient having to remain in the hospital. It not only drastically reduces the opioid requirements for patients but also allows for a more rapid recovery period and/or early rehabilitation for orthopedic surgical patients.

Fig. 5. ON-Q Pain Relief System
SUMMARY
In conclusion, local anesthetic agents play a crucial role in modern medical practice, providing effective pain management and anesthesia for a wide range of procedures. This review has highlighted the diverse mechanisms of action, pharmacokinetic profiles, clinical applications, and potential adverse effects associated with various local anesthetic agents. Local anesthetics are some of the most versatile drugs available to medical and dental professionals. Although local anesthetics are mainly used to alleviate painful stimuli from surgical procedures, they can also be administered during specific medical emergencies. These drugs are capable of being administered by varying arrays of techniques ranging from topical to intravenous routes. Despite the almost exclusive use of amide local
REFERENCE
- Stoelting RK, Miller RD, editors. Basics of anesthesia. 4th edition. New York: Churchill Livingstone; 2000
- Morgan EG, Mikhail MS, Murray MJ, et al, editors. Clinical anesthesiology. 3rd edition. New York: McGraw-Hill; 2002.
- McLeod GA, Munishankar B, Columb MO. An isobolographic analysis of diamorphine and levobupivacaine for epidural analgesia in early la- bour. Br/ Anaesth 2007; 98: 497-502.
- Capogna G, Celleno D, Fusco P, Lyons G, Columb M. Relative potencies of bupivacaine and ropivacaine for labour analgesia. Br J Anaesth 1999; 82: 371-3.
- Ruetzler K, Sima B, Mayer L, et al. Lidocaine/tetracaine patch (Rapydan) for topical anaesthesia before arterial access: a double-blind, randomized trial. Br J Anaesth 2012; 109: 790-6.
- Hollmann W, Durieux ME, Graf BH. Novel local anaesthetics and novel indications for local anaesthetics. Curr Opin Anaesthesiol 2001; 14: 741-51.
- Curtis MJ, Pugsley MK. Drugs and the cardiovascular system. In: Page CP, Curtis MJ, Sutter MC, et al, editors. Integrated pharmacology. 2nd edition. New York: Mosby; 2002. p. 369–73.
- Ries CR, Quastel DMJ. Drug use in anesthesia and critical care. In: Page CP, Curtis MJ, Sutter MC, et al, editors. Integrated pharmacology. 2nd edition. New York: Mosby; 2002. p. 356–9.
- Lyons G, Columb M. Up-down sequential allocation and regional anaesthesia. Acta Anaesthesiol Scand 2011; 55: 337-9.
- Faust RJ, Cucchiara RF, Rose SH, et al, editors. Anesthesiology review. 3rd edition. Philadelphia: Churchill Livingstone; 2002.
- Favier JC, Da Conceicao M, Fassassi M, Allanic L, Steiner T, Pitti R. Successful resuscitation of serious bupivacaine intoxication in a patient with pre-existing heart failure. Can J Anaesth 2003;50(1):62–6.
- Fathi AA, Soliman MM. Carticaine versus lidocaine for peribulbar anesthesia in cataract surgery. J Cataract Refract Surg 2002;28(3):513–6.
- Finucane BT. Allergies to local anesthetics—the real truth. Can J Anaesth 2003;50(9):869–74 [in English and in French].
- Koller C. Historical notes on the beginning of local anaesthesia. JAMA 1928;90:1742–3.
- Knapp H. On cocaine and its uses in ophthalmic and general surgery. Arch Ophthalmol 1884;13:402–48.
- Xiong Z, Strichartz GR. Inhibition by local anesthetics of Ca2+ channels in rat anterior pituitary cells. Eur J Pharmacol 1998;363(1):81–90.
- Hollmann MW, Wieczorek KS, Berger A, Durieux ME. Local anesthetic inhibition of G protein-coupled receptor signaling by interference with Galpha(q) protein function. Mol Pharmacol 2001;59(2):294–301.
- Olschewski A, Hempelmann G, Vogel W, Safronov BV. Blockade of Na+ and K+ currents by local anesthetics in the dorsal horn neurons of the spinal cord. Anesthesiology 1998;88(1):172–9.
- Vanhoutte F, Vereecke J, Verbeke N, Carmeliet E. Stereo selective effects of the enantiomers of bupivacaine on the electrophysiological properties of the guinea-pig papillary muscle. Br J Pharmacol 1991;103(1):1275–81.
- Sudoh Y, Cahoon EE, Gerner P, Wang GK. Tricyclic anti depressants as long-acting local anesthetics. Pain 2003;103(1–2):49–55.
- Acalovschi I, Cristea T. Intravenous regional anesthesia with meperidine. Anesth Analg 1995;81(3):539–43.
- Bardsley H, Gristwood R, Baker H, Watson N, Nimmo W. A comparison of the cardiovascular effects of levobupivacaine and rac-bupivacaine following intravenous administration to healthy volunteers. Br J Clin Pharmacol 1998;46(3): 245–9.
- Albright GA. Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology 1979;51(4): 285–7.
- McLeod GA, Burke D. Levobupivacaine. Anaesthesia 2001;56(4):331–41.
- Gristwood RW. Cardiac and CNS toxicity of levobupivacaine: strengths of evidence for advantage over bupivacaine. Drug Saf 2002;25(3):153–63.
- Fathi AA, Soliman MM. Carticaine versus lidocaine for peribulbar anesthesia in cataract surgery. J Cataract Refract Surg 2002;28(3):513–6.
- Gouws P, Galloway P, Jacob J, English W, Allman KG. Comparison of articaine and bupivacaine/lidocaine for sub Tenon’s anaesthesia in cataract extraction. Br J Anaesth 2004;92(2):228–30.
- Weinberg G, Ripper R, Feinstein DL, Hoffman W. Lipid emulsion infusion rescues dogs from bupivacaine induced cardiac toxicity. Reg Anesth Pain Med 2003;28(3): 198–202.
- Favier JC, Da Conceicao M, Fassassi M, Allanic L, Steiner T, Pitti R. Successful resuscitation of serious bupivacaine intoxication in a patient with pre-existing heart failure. Can J Anaesth 2003;50(1):62–6.
- Kanai Y, Katsuki H, Takasaki M. Lidocaine disrupts axonal membrane of rat sciatic nerve in vitro. Anesth Analg 2000;91(4):944–8.
- Reynolds F. Damage to the conus medullaris following spinal anaesthesia. Anaesthesia 2001;56(3):238–47


 Sai S.Shirsath *
Sai S.Shirsath *
 Manasi D.lipte
Manasi D.lipte
 Vaishnavi B.Gaikwad
Vaishnavi B.Gaikwad
 Sambhaji O.Kadam
Sambhaji O.Kadam
 Prajakta D.Wadkar
Prajakta D.Wadkar






 10.5281/zenodo.13825961
10.5281/zenodo.13825961