The recent developments in floating drug delivery systems (FDDS) includes the uniform distribution of multiple unit dosage forms along the GIT that could result in more reproducible drug absorption and reduced risk of local irritation; this gave birth to oral controlled drug delivery and led to development of Gastro-retentive floating microspheres. Microballoons (MB), a multiple unit dosage forms possessing a spherical cavity enclosed within a hard polymer shell have been develops as a dosage form characterize by excellent buoyancy in the stomach. This gastrointestinal transit-controlled preparation is design to float on surface of gastric juice, which has a specific gravity less than 1. Microballoons, loaded with drug in their outer polymer shells, prepare by the emulsion solvent diffusion method using enteric acrylic polymers; dissolve in a mixture of dichloromethane and ethanol. Dichloromethane evaporation appears to be especially related to cavity formation in microspheres. Microballoons incorporating a drug dispersed or dissolved throughout particle matrix have the potential for controlled release of drugs and floats continuously over the surface of acidic dissolution media containing surfactant for > 12 h in vitro. As the microballoons floats over the gastric contents, the drug is released slowly at the desired rate, which results in increased gastro-retention time and reduces fluctuation in plasma drug concentration.
Floating controlled drug delivery system, Microballoons, Emulsion solvent diffusion method, Enteric acrylic polymers..
Gastric emptying of dosage forms is an extremely variable process and ability to prolong and control the emptying time is a valuable asset for dosage forms, which reside in the stomach for a longer period of time than conventional dosage forms. Several difficulties are faced in designing controlled release systems for better absorption and enhanced bioavailability. One of such difficulties is the inability to confine the dosage form in the desired area of the gastrointestinal tract. Drug absorption from the gastrointestinal tract is a complex procedure and is subject to many variables. It is widely acknowledged that the extent of gastrointestinal tract drug absorption is related to contact time with the small intestinal mucosa.1 Drugs that are easily absorbed from the gastrointestinal tract (GIT) and have a short half-life are eliminated quickly from the blood circulation and require frequent dosing. To avoid this problem, the oral controlled release (CR) formulations have been developed in an attempt to release the drug slowly into the GIT and maintain a constant drug concentration in the serum for a longer period of time. Such oral drug delivery devices have a restriction due to the gastric retention time (GRT), a physiological limitation. Therefore, prolonged gastric retention is important in achieving control over the GRT because this helps to retain the CR system in the stomach for a longer time in a predictable manner.2 There are several drugs reported to be useful for floating drug delivery system that are enlist in (Table 1).
Drugs that have poor bioavailability because of site-specific absorption from the upper part of the gastrointestinal tract are potential candidates to be formulated as floating drug delivery systems, thereby maximizing their absorption1. Retention of drug delivery systems in the stomach prolongs overall gastrointestinal transit time and improves the oral bioavailability of the drugs that are having site-specific absorption from the stomach or upper part of the small intestine.8 A. Jaykrishnan25 developed microballoons of piroxicam capable of floating on simulated gastric and intestinal fluid. Data obtained in this study demonstrated that FDF of piroxicam in microballoons was capable of sustained delivery of the drug for longer periods with increased bioavailability.
M.N. Gambhire et al.8 reported the improved bioavailability of diltiazem hydrochloride undergoes an extensive biotransformation, mainly through cytochrome P-450 CYP3A, which results in less than 4% of its oral dose being excreted unchanged in urine. Bioavailability of DTZ is ~30% to 40% owing to an important first pass metabolism of its oral dose being excreted unchanged in urine. On the other hand Asha patel et al. 26 improved the bioavailability and patient compliance of metformin hydrochloride by developing floating microspheres, which may be used in clinic for prolonged drug release in stomach for at least 8 hrs. There are several commercial products available based
on the research activity of floating drug delivery. (Table 2)
Factors Affecting Gastric Retention
Various attempts have been made to retain the dosage form in the stomach as a way of increasing the retention time. These attempts include introducing floating dosage forms (gas-generating systems and swelling or expanding systems), mucoadhesive systems, high-density systems, modified shape systems, gastric-emptying delaying devices and co-administration of gastric-emptying delaying drugs. Among these, the floating dosage forms have been used most commonly.32 However; most of these approaches are influenced by a number of factors that affect their efficacy as a gastroretentive system.33-35
Density – GRT is a function of dosage form buoyancy that is dependent on the density.
Size – Dosage form units with a diameter of more than 7.5mm are reported to have an increased GRT compared with those with a diameter of 9.9mm.
Shape of dosage form – Tetrahedron and ring- shaped devices with a flexural modulus of 48and 22.5 kilo pounds per square inch (KSI) are reported to have better GRT ? 90% to 100% retention at 24 hours compared with other shapes.
Single or multiple unit formulation – Multiple unit formulations show a more predictable release profile and insignificant impairing of performance due to failure of units, allow co-administration of units with different release profiles or containing incompatible substances and permit a larger margin of safety against dosage form failure compared with single unit dosage forms; Fed or unfed state – Under fasting conditions, the GI motility is characterized by periods of strong motor activity or the migrating myoelectric complex (MMC) that occurs every 1.5 to 2 hours. The MMC sweeps undigested material from the stomach and, if the timing of administration of the formulation coincides with that of the MMC, the GRT of the unit can be expected to be very short. However, in the fed state, MMC is delayed and GRT is considerably longer. Nature of meal – Feeding of indigestible polymers or fatty acid salts can change the motility pattern of the stomach to a fed state, thus decreasing the gastric emptying rate and prolonging drug release. Caloric content – GRT can be increased by four to 10 hours with a meal that is high in proteins and fats. Frequency of feed – GRT can increase by over 400 minutes when successive meals are given compared with a single meal due to the low frequency of MMC;
Gender – Mean ambulatory GRT in males (3.4±0.6 hours) is less compared with their age and race- matched female counterparts (4.6±1.2 hours), regardless of the weight, height and body surface). Age – elderly people, especially those over 70, have a significantly longer GRT. Posture – GRT can vary between supine and upright ambulatory states of the patient.
Concomitant drug administration – Anticholinergics like atropine and propantheline, opiates like codeine and
prokinetic agents like metoclopramide and cisapride; and Biological factors – diabetes and Crohn’s disease, etc. Oth et al15 reported that the mean GRT of a bilayer floating capsule of misoprostal was 199±69 min after a single light meal (breakfast). However, after a succession of meals, the data showed a remarkable prolongation of the mean GRT, to 618±208 min. In another study, Iannuccelli et al.36 reported that in the fed state after a single meal, all the floating units had a floating time (FT) of about 5 h and a GRT prolonged by about 2 h over the control. However, after a succession of meals, most of the floating units showed a FT of about 6 h and a GRT prolonged by about 9 h over the control, though a certain variability of the data owing to mixing with heavy solid food ingested after the dosing was observed. Garg and Sharma32 reported that tetrahedron- and ring-shaped devices have a better gastric residence time as compared with other shapes. The diameter of the dosage unit is also equally important as a formulation parameter. Dosage forms having a diameter of more than 7.5 mm show a better gastric residence time compared with one having 9.9 mm. It has been demonstrated using radiolabeled technique that there is a difference between gastric emptying times of a liquid, digestible solid, and indigestible solid. It was suggested that the emptying of large (>1 mm) indigestible objects from stomach was dependent upon interdigestive migrating myoelectric complex. When liquid and digestible solids are present in the stomach, it contracts ~3 to 4 times per minute leading to the movement of the contents through partially opened pylorus. Indigestible solids larger than the pyloric opening are propelled back and several phases of myoelectric activity take place when the pyloric opening increases in size during the housekeeping wave and allows the sweeping of the indigestible solids. Studies have shown that the gastric residence time (GRT) can be significantly increased under the fed conditions since the MMC is delayed.37 While considering the role of specific gravity in GRT, the potential of food in modifying GRT should not be overlooked. One of the earlier in-vivo evaluations of FDDS by Muller-Lissner and et al.38 demonstrated that a GRT of 4–10 h could be achieved after a fat and protein test meal.
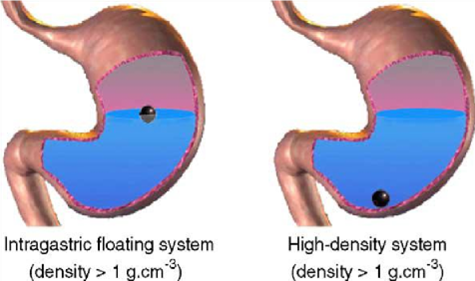
Approaches to Design Gastro-retentive Dosage Forms Several approaches have been developed in order to prolong the residence time of dosage forms in the stomach. One technique involves the preparation of a device that remains buoyant in stomach contents due to a density lower than that of the gastric fluids. An intragastric flotation system can prolong gastric residence time (GRT) of dosage forms, resulting in better drug absorption at the proximal small intestine as well as in the stomach. Extension of GRT can also provide sustained pharmacological action.39 Over the last three decades, various attempts have been done to retain the dosage form in the stomach as a way of increasing retention time.
High-density systems
Gastric contents have a density close to water (¨1.004 g/cm3). When the patient is upright small high-density pellets sink to the bottom of the stomach(Fig 1) Highdensity formulations include coated pellets, which have a density greater than that of the stomach contents (1.004 g/ cm ). This is accomplished by coating the drug with a heavy inert material such as barium sulfate, zinc oxide, titanium dioxide, iron powder, etc. Other delayed gastric emptying approaches of interest include sham feeding of indigestible polymers or fatty acid salts that change the motility pattern of the stomach to a fed state, thereby decreasing the gastric emptying rate and permitting considerable prolongation of drug release.40-45
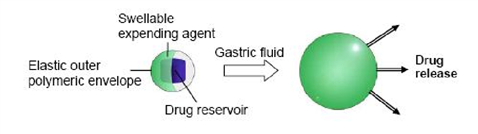
Swelling systems
This type of system capable of swelling to a size that prevents their passage through the pylorus; as a result, the dosage form is retained in the stomach for a longer period of time. The swelling is usually results from osmotic absorption of water. The dosage form is small enough to be swallowed, and swells in gastric liquids. 34, 46 In 1980s, Mamajek and Moyer 47 patented a drug reservoir, surrounded by a swellable expanding agent. The whole system was coated by an elastic outer polymeric membrane (Fig. 2), which was permeable to both the drug and body fluids and could control drug release. The device gradually decreased in volume and rigidity as a result of depletion of drug and expanding agent and/or bioerosion of the polymer
envelope, enabling its elimination.
Bio/mucoadhesive systems
Bio/mucoadhesive systems bind to the gastric epithelial cell surface, or mucin, and extend the GRT by increasing the intimacy and duration of contact between the dosage form and the biological membrane. The epithelial adhesive properties of mucin have been applied in the development of Gastro retentive drug delivery systems. 48-49 The basis of mucoadhesion is that a dosage form can stick to the mucosal surface by different mechanisms. Different theories are invoked to explain these mechanisms. Firstly, the electronic theory proposes attractive electrostatic forces between the glycoprotein mucin network and the bioadhesive material. Secondly, the adsorption theory suggests that bioadhesion is due to secondary forces such as Van der Waals forces and hydrogen bonding. The wetting theory is based on the ability of bioadhesive polymers to spread and develop intimate contact with the mucus layers, and finally, the diffusion theory proposes physical entanglement of mucin strands and the flexible polymer chains, or an interpenetration of mucin strands into the porous structure of the polymer substrate.50-52
Floating systems
Floating systems was firstly described by Davis (1968), as low-density systems that have sufficient buoyancy to float over the gastric contents and remain in the stomach for a prolonged period. While the system floats over the gastric contents, the drug is released slowly at the desired rate, which results in increased gastro-retention time and reduces fluctuation in plasma drug concentration. 34 The density of a dosage form also affects the gastric emptying rate. A buoyant dosage form having a density of less than that of the gastric fluids floats. Since it is away from the pyloric sphincter, the dosage unit is retained in the stomach for a prolonged period.1 Several formulation parameters can affect the GRT. More reliable gastric emptying patterns are observed for multiparticulate formulations as compared with single unit formulations, which suffer from “all or none concept.” As the units of multiparticulate systems are distributed freely throughout the gastrointestinal tract, their transport is affected to a lesser extent by the transit time of food compared with single unit formulation.53 Single-unit formulations are associated with problems such as sticking together or being obstructed in the gastrointestinal tract, which may have a potential danger of producing irritation.1 The purpose of designing multiple-unit dosage form is to develop a reliable formulation that has all the advantages of a single-unit form and also is devoid of any of the abovementioned disadvantages of single-unit formulations. In pursuit of this endeavor many multiple-unit floatable dosage forms have been designed. Microspheres have high loading capacity and many polymers have been used such as albumin, gelatin, starch, polymethacrylate, polyacrylamine, and polyalkylcyanoacrylate. Spherical polymeric microsponges also referred to as “microballoons,” have been prepared. Microspheres have a characteristic internal hollow structure and show an excellent in vitro floatability.54 In Carbon dioxide–generating multiple-unit oral formulations several devices with features that extend, unfold, or are inflated by carbon dioxide generated in the devices after administration have been described in the recent patent literature. These dosage forms are excluded from the passage of the pyloric sphincter if a diameter of ~12 to 18 mm in their expanded state is exceeded.55 Therefore, a multiple-unit flotation system that can be distributed widely throughout the gastrointestinal tract, affording the possibility of longer lasting and more reliable release of drugs, has been sought.39 Iannuccelli et al. 56, 57 reported that an air-included multiple-unit compartment system showed excellent buoyancy in vitro and prolonged GRT relative to the controls in vivo under fed state. However, in the fasted state, intragastric buoyancy of the devices did not influence GRT. Furthermore, Kawashima et al. 58, 21 developed microballoons (hollow microspheres) in order to prolong GRT of the dosage form. This gastrointestinal transit-controlled preparation is designed to float on gastric juice with a specific density of less than 1.
Classification of Floating Drug Delivery Systems (FDDS)
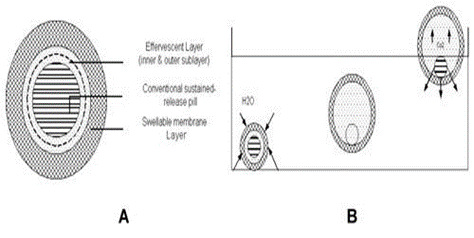
Effervescent Floating Dosage Forms
These are matrix types of systems prepared with the help of swellable polymers such as methylcellulose and chitosan and various effervescent compounds, e.g., sodium bicarbonate, tartaric acid, and citric acid. The matrices are fabricated so that upon arrival in the stomach, carbon dioxide is liberated by the acidity of the gastric contents and is entrapped in the gellified hydrocolloid. This produces an upward motion of the dosage form and maintains its buoyancy. A decrease in specific gravity causes the dosage form to float on the chime.59 Ichikawa et al55 developed a new multiple type of floating dosage system composed of effervescent layers and swellable membrane layers coated on sustained release pills. The inner layer of effervescent agents containing sodium bicarbonate and tartaric acid was divided into 2 sublayers to avoid direct contact between the 2 agents. These sublayers were surrounded by a swellable polymer membrane containing polyvinyl acetate and purified shellac. When this system was immersed in the buffer at 37ºC, it settled down and the solution permeated into the effervescent layer through the outer swellable membrane. CO2 was generated by the neutralization reaction between the 2 effervescent agents, producing swollen pills (like balloons) with a density less than 1.0 g/mL. It was found that the system had good floating ability independent of pH and viscosity and the drug (Para-amino benzoic acid) released in a sustained manner. (Fig.3) Talwar et al.7 developed a once-daily formulation for oral administration of ciprofloxacin. The formulation was composed of 69.9% ciprofloxacin base, 0.34% sodium alginate, 1.03% xanthum gum, 13.7% sodium bicarbonate, and 12.1% cross-linked poly vinyl pyrrolidine. The viscolysing agent initially and the gel-forming polymer later formed a hydrated gel matrix that entrapped the gas, causing the tablet to float and be retained in the stomach or upper part of the small intestine (spatial control). The hydrated gel matrix created a tortuous diffusion path for the drug, resulting in sustained release of the drug.
Non-Effervescent Floating Dosage Forms
Non-effervescent floating dosage forms use a gel forming or swellable cellulose type of hydrocolloids, polysaccharides, and matrix-forming polymers like polycarbonate, polyacrylate, polymethacrylate, and polystyrene. The formulation method includes a simple approach of thoroughly mixing the drug and the gel-forming hydrocolloid. After oral administration this dosage form swells in contact with gastric fluids and attains a bulk density of < 1>1 Hoo-kyun choi et al60 developed microballoons by solvent diffusion technique. Eudragit S 100 in dichloromethane and ethanol was found to form Microballoons that floated on water and simulated gastric fluid as evidenced by the scanning electron microscopy. High drug loading was achieved and drug loaded microspheres were able to float on gastric and intestinal fluid. Yasunori Sato et al.39 prepared riboflavin-containing microballoons for floating controlled drug delivery system. Microballoons afforded significantly high urinary excretion of riboflavin relative to that observed for non-floating in the fasted and the fed conditions. Kawashima et al 23 prepared multiple-unit hollow microspheres by emulsion solvent diffusion technique. Drug and acrylic polymer were dissolved in an ethanoldichloromethane mixture, and poured into an aqueous solution of PVA with stirring to form emulsion droplets. The rate of drug release in micro balloons was controlled by changing the polymer-to-drug ratio. Microballoons were floatable in vitro for 12 hours when immersed in aqueous media. Radiographical studies proved that microballoons orally administered to humans were dispersed in the upper part of stomach and retained there for 3 hours against peristaltic movements.
Development and Evaluation of Microballoons
Formulation development
Microballoons are in strict sense, spherical empty particles without core. These microspheres are characteristically free flowing powders consisting of proteins or synthetic polymers, ideally having a size less than 200 micrometer. Solid biodegradable microspheres incorporating a drug dispersed or dissolved throughout particle matrix have the potential for controlled release of drugs.61 Generally, techniques used to prepare microballoons involve simple solvent evaporation or solvent diffusion/ evaporation methods. Polycarbonate, Eudragit S, cellulose acetate, calcium alginate, agar and low methoxylated pectin are commonly used as polymers. Buoyancy and drug release are dependent on quantity of polymer, the plasticizer–polymer ratio and the solvent used.62 The polymer is dissolved in an organic solvent and the drug is either dissolved or dispersed in the polymer solution. The solution containing the drug is then emulsified into an aqueous phase containing polyvinyl alcohol to form oil in water emulsion. After the formation of a stable emulsion, the organic solvent is evaporated either by increasing the temperature under pressure or by continuous stirring.63 The solvent removal leads to polymer precipitation at the o/w interface of droplets, forming cavity and thus making them hollow to impart the floating
properties.64-65 In-vitro and in-vivo evaluation of Microballoons Various parameters that need to be evaluated in multiparticulate drug delivery systems, include floating duration, differential scanning calorimetry (DSC), particle size analysis, flow properties, surface morphology, and mechanical properties are also performed.1 The particle size is determined by optical microscopy; true density is determined by liquid displacement method; tapped density and compressibility index are calculated by measuring the change in volume using a bulk density apparatus; angle of repose is determined by fixed funnel method. The hollow nature of microspheres is confirmed by scanning electron microscopy.66-68
Particle size
The release profiles are also dependent on the size of microspheres. The rate of drug release decreases with increasing microsphere size. Therefore, size distribution plays a very important role in determining the release characteristics of the microspheres. Various methods for determining microsphere size includes sieve methods, light/optical microscopy, resistance blockage techniques (Coulter analysis), laser light scattering method, sedimentation method and for particles less than 1 ?m, photon correlation spectroscopy.61
Rawat M, Saraf S, Saraf S.69 determined the particle size of microspheres by optical microscopy using a compound microscope (Erma, Tokyo, Japan). A small amount of dry microspheres suspended in purified water (10 mL). The suspension was ultrasonicated for 5 seconds. A small drop of suspension thus obtained was placed on a clean glass slide. The slide containing microspheres was mounted on the stage of the microscope and 300 particles were measured using a calibrated ocular micrometer. The process was repeated for each batch prepared.
Test for Buoyancy and In-vitro Drug Release
Buoyancy and in vitro drug release are usually carried out in simulated gastric and intestinal fluids maintained at 37oC. In practice floating time is determined by using the USP disintegration apparatus containing 900 ml of 0.1 N HCl as a testing medium maintained at 37oC. Time require to float the HBS dosage form is noted as floating (or floatation) time.34 In-vitro drug release is usually performs to determine the type of drug release before developing in to therapeutic system. A. K. Shrivastsava and et.al 70 studied drug release using a modified USP XXIV (17) dissolution apparatus type I (basket mesh # 120, equals 125 mm) at 100 rpm in distilled water and 0.1 mol L–1 HCl (pH 1.2) as dissolution fluids (900 mL) maintained at 37 ± 0.5 °C. Withdrawn samples (10 mL) were analyzed spectrophotometrically as stated above. The volume was replenished with the same amount of fresh dissolution fluid each time to maintain the sink condition. Ibrahim El-Gibaly71 performed the test for buoyancy of the microparticles by using a water bath shaker with a shaking speed of 100 o.p.m. (oscillations per minute) at 37±/0.5 8C, soaking 50 microparticles in 100 ml of enzyme-free S.G.F. (HCl/NaCl solution containing 0.02% Tween 80; pH 1.2) or enzyme-free S.I.F. (KH2PO4/NaOH buffer containing 0.02% Tween80; pH 7.4).
Dissolution Tests
A dissolution test is an important investigation to determine drug content and is performed using the USP dissolution apparatus. Samples are withdrawn periodically from the dissolution medium replenished with the same volume of fresh medium each time, and then analyzed for their drug contents after an appropriate dilution. 72 S. Ray and et.al26 used one hundreds mg of pure drug for the dissolution studies and microspheres equivalent to 100mg of the pure drug were used. Two ml of the aliquot was withdrawn at predetermined intervals and filtered. The required dilutions were made with 0.1N HCl and the solution was analyzed for the drug content spectrophotometrically
Specific Gravity
The specific gravity of a substance is a comparison of its density to that of gastric fluid. The specific gravity of FDDS can be determined by the displacement method using analytical grade benzene as a displacing medium73. Timmermans and Moes recommended that the initial (dry state) bulk density of the dosage form and changes in the floating strength with time should be characterized prior to in vivo comparison between floating and non-floating units.
74
In vivo Gastric Retentivity
In-vivo visualization is a crucial parameter for evaluating the GI tract retention characterization of the dosage forms. The inclusion of radio opaque material in to a solid dosage forms enable it to visualize by X-ray. Similarly, the inclusion of ?-emitting radio-nuclide in a formulation allows indirect external observation using a ?-camera or scintiscanner. In case of ?-scintigraphy, the ?-rays emitted by the radio-nuclide are focused on a camera, which helps to monitor the location of the dosage forms in the GI tract.72 V. Iannuccelli36 carried out in vivo study by administering to humans floating and control units and monitoring them through a radiological method. Six healthy subjects (one male, five females; mean age 37913 years; mean weight 55±10kg) participated after giving informed consent. During the experiments the subjects remained in a sitting or upright posture. In each subject the position of the floating and control units was monitored by X-ray photographs (Siregraph-B, Siemens, Karlsruhe, Germany) of the gastric region at determined time intervals. Kawashima et.al21 estimated the hollow structure of microspheres made of acrylic resins by measuring particle density (Pp) by a photographic counting method and a liquid displacement method. An image analyzer was used to determine the volume (v) of particles (n) of weight (w): Porosity was measured by € = (1 – Pp /Pt) × 100, where Pt is the true density. Yasunori Sato et al.75 determined the apparent particle density by projective image counting method. Microballoons were placed on a glass plate. Heywood diameter and microballoons number were measured by an image processing and analysis system (Q5001W, Leica, Japan). Subsequently, the apparent particle density was calculated according to following equation.
Apparent Particle Density (w/v) = w/? (?d3 n/6)
Where w = weight of microballoons, v = volume of microballoons, d = Heywood diameter, and n = number of microballoons J. Varshosaz et al.2 studied the Dissolution efficiency (DE) after 8 hr of release test to compare the results of dissolution tests of different formulations. The other dissolution parameter used for comparing the different formulations was mean dissolution time (MDT) that is calculated from the amount of drug released to the total cumulative drug. MDT is a measure of the rate of the dissolution process. Higher the MDT, the slower the release rate.
Applications of Floating Microspheres
Floating drug delivery offers several applications for drugs having poor bioavailability because of the narrow absorption window in the upper part of the gastrointestinal tract. It retains the dosage form at the site of absorption and thus enhances the bioavailability. These are summarized as follows.
Sustained Drug Delivery
Drugs that are easily absorbed from the gastrointestinal tract (GIT) and have a short half-life are eliminated quickly from the blood circulation, so they require frequent dosing. To avoid this drawback, the oral sustained-controlled release formulations have been developed in an attempt to release the drug slowly into the GIT and maintain an effective drug concentration in the serum for longer period of time.76 Chaffman, M., Brogden, R., developed a type of floating microspheres of alginate by the addition of CaCO3 gas forming agent, which combines sustained release and prolonged gastric retention time of the hydrophilic model drug. Diltiazem hydrochloride (DTZ), was selected as the model drug because its high frequency of drug
administration resulted from relatively short biological halflife of 3–4 h.77
Site-Specific Drug Delivery
These systems are particularly advantageous for drugs that are specifically absorbed from stomach or the proximal part of the small intestine, e.g., riboflavin and furosemide.
Microballoons might be able to float in the stomach sufficiently in the fed condition. This phenomenon could prolong the gastric residence time (GRT) of riboflavin and delay arrival at the absorption site; consequently, the sustained pharmacological action could be provided. In addition, Microballoons enabled increased drug absorption rate of riboflavin as the floating Microballoons in the stomach gradually sank and arrived at the absorption site. Therefore, multiple unit floating systems, i.e.,
Microballoons, should be possibly beneficial with respect to site-specific delivery.39
Absorption Enhancement
Drugs that have poor bioavailability because of site-specific absorption from the upper part of the gastrointestinal tract are potential candidates to be formulated as floating drug delivery systems, thereby maximizing their absorption. The bioavailability of cinnarizine was markedly influenced by the gastric acidity exhibiting pH-dependent dissolution behavior. J. Varshosaz et al.2 develops the floating microballoons that increased GRT in the stomach to increase its solubility and improve its bioavailability. Pharmacokinetic advantages As a sustained release system floating microsphere offer various potential advantages evident from several recent publications. Drugs that have poor bioavailability because of their absorption restricted to upper GI tract can be delivered efficiently thereby maximizing their absorption and improving their absolute bioavailability.34 Pharmacokinetic parameters were derived from the plasma concentration versus time plot. The area under the curve (AUC), the peak concentration (Cmax) and the time to attain peak concentration (Tmax ) were obtained from such plots. The elimination rate constants kel for the different dosage forms were determined from the semi-logarithmic plot of plasma concentration versus time. kel was calculated from the terminal linear portion of the curve using linear regression analysis. Elimination half-lives t1 / 2 were calculated by dividing 0.693 by the elimination rate constant. 25 A. Jayakrishnan et al.25 estimate the pharmacokinetic parameters and demonstrated that assessment of the area AUC0–? showed that bioavailability was minimum for the free drug. The bioavailability of microspheres alone was about 1.4 times that of the free drug and it was about 4.8 times for the dosage forms consisting microspheres plus the loading dose.
Limitations
One of the disadvantages of floating systems is that they require a sufficiently high level of fluids in the stomach for the drug delivery buoy to float therein and to work efficiently. However, this limitation can be overcome by coating the dosage form with bioadhesive polymers, thereby enabling them to adhere to the mucous lining of the stomach wall. Alternatively, the dosage form may be with a glass full of water (200–250 ml). Floating systems are not feasible for those drugs that have solubility or stability problems in gastric fluids. Drugs such as nifedipine, which is well absorbed along the entire GI tract and which undergoes significant first-pass metabolism, may not be desirable candidates for FDDS since the slow gastric emptying may lead to reduced systemic bioavailability.78 The drugs recently reported to be entrapped in floating microspheres are enlist in (Table 3).
CONCLUSION
Floating controlled drug delivery systems i.e., Microballoons are potentially beneficial with respect to improving drug bioavailability, resulting in improved pharmacological action. By prolonging the gastric empting time of the dosage forms, this system not only provide controlled release of the drug but also present the drug in the absorbable forms at regions of optimal absorption. The control of gastro intestinal transit could be the focus of the next decade and may result in new therapeutic possibilities with substantial benefits for patients.


 Suhas Patil *
Suhas Patil *
 Dr. A.W Ambekar
Dr. A.W Ambekar
 10.5281/zenodo.14524625
10.5281/zenodo.14524625