Abstract
PCOS, also known as polycystic ovarian syndrome, is a prevalent infertility condition that impacts a noteworthy segment of the worldwide populations. With a prevalence of 8–13%, depending on the criteria applied and population investigated, it is the most frequent endocrinopathy affecting women of reproductive age and the primary cause of anovulatory infertility in women. Although polycystic ovaries have been described since 1721, Stein and Leventhal were the first to report the condition. Polycystic ovarian disease (PCOD) is the term used to describe the condition when polycystic ovaries are discovered in healthy women. Formal diagnostic criteria were not proposed or widely used until a PCOS meeting sponsored by the National Institutes of Health (NIH) in the early 1990s. Numerous researchers conducted numerous experiments and attempted to explain the pathophysiology of PCOS.
Keywords
Polycystic, Ovary, Syndrome, PCOS
Introduction
PCOS, or polycystic ovary syndrome, is a common endocrine disorder that affects mostly women who are of reproductive age., polycystic ovarian syndrome, or PCOS, is a prevalent endocrine condition in females. An estimated 5–10% of people globally are thought to have PCOS Menstrual dysfunction and hyperandrogenism are the two main symptoms of polycystic ovary syndrome (PCOS) in adolescents..[4] It is a diverse disorder with a wide range of clinical phenotypes that are influenced by factors such as body weight, lifestyle, age, genotype, ethnicity, and environment, all of which have varying effects on the long- and early-term hazards.[7] The diagnosis of this disorder is based on abnormalities of the reproductive system, hyperandrogenism, and prolonged anovulation after primary diseases of the ovaries, adrenal, and pituitary glands have been ruled out, however the exact cause of the syndrome is still unknown.[ 8]
STATISTICAL ANALYSIS
When ovarian failure develops before the age of 40 and there is no obvious genetic defect, it is known as premature ovarian failure, or POF. Amenorrhea and enhanced gonadotrophins with a reduced oestrogen concentration are its defining characteristics. Although the precise etiology is not known, it has been connected to various autoimmune diseases and might be a component of the polyendocrine syndrome. While gonadotrophins and their receptors have been the target of antibodies, LH and FSH receptor alterations have also been reported. X chromosome chromosomal abnormalities and iatrogenic factors, such as those that occur after chemotherapy or surgery, are other recognized causes.Nineteen Both POF and PCOS can exhibit oligo/anovulation, hence POF needs to be ruled out in the patient's workup in this situation. The question of whether these ovulatory disorders—despite the existence of ovulatory dysfunction or PCOM—should be seen as having a presentation akin to PCOS in the absence of androgen excess is a contentious issue, as will be explored below. [9] In 1990, the National Institute of Child Health and Human Development of the United States (US) National Institutes of Health (NIH) sponsored an expert conference that marked the first attempt to define PCOS. Following a survey of all participants, primarily from the US, about how they perceived the features of PCOS, they came to the conclusion that PCOS was an androgen-excess disorder of exclusion with implications for the ovaries. Accordingly, ovulatory dysfunction and clinical and/or biochemical hypoandrogenism coexist to constitute PCOS, according to the 1990 NIH criteria. [9] The software utilized for data collection and analysis was BM SPSS Statistics 23.0. By substituting mean values for interval data in place of missing values, the dataset was cleaned up or adjusted for outliers. The study employed various methods to compare participants based on PCOS, including demographic, anthropometric, clinical, and biochemical parameter variables and symptoms. Using independent t-tests for continuous variables and ?2 tests for categorical variables, participants with and without PCOS were compared. For continuous variables, mean ± standard deviation was used, while frequency (%) was used for categorical variables. A P-value of less than 0.05 was deemed statistically noteworthy. Odds ratios were used to illustrate the relationship between categorical variables (ORs). ).[8] The married, infertile woman with shiny, white-surfaced ovaries the size of pigeon eggs was described in 1721 by the Italian physician, naturalist, and medical scientist Vallisneri.. Another report dates back to 1844, when Chereau and Rokitansky reported hydrops follicle and fibrous and sclerotic lesions in the ovaries that had a degenerative nature. Hyperthecosis was first described by Bulius and Kretschmar. Lawson Tait first proposed the need for a bilateral oophorectomy in 1879 in order to treat ovaries with symptomatic cystic degeneration. [6]. When combined with other symptoms of the syndrome, the Androgen Excess Society (AES) released a statement in 2006 that included criteria aimed at making hyperandrogenism a sine qua non diagnostic condition. .[6]

Figure:1 PCOS prevalence based on various diagnostic criteria
ETIOLOGY
Genetic factors are strongly implicated in PCOS, although the exact etiology is unknown. For instance, a significant proportion of mothers and siblings of PCOS-affected women share the same morphological features on ultrasound. Further more, evidence points to the autosomal transmission of the implicated genetic sequences. It's possible that a gene, or set of genes, prevents follicular maturation and makes the ovary vulnerable to insulin-stimulated androgen secretion. This genetic predisposition in men may manifest as premature baldness.Although many women's syndrome does not fully manifest until later in their reproductive years, the symptoms usually start during puberty.[11]
PATHOGENESIS
The pathogenesis of PCOS is poorly understood, but the primary defect may be insulin resistance leading to hyperinsulinaemia. In the ovary, the cardinal feature is functional hyperandrogenism . Circulating concentrations of insulin and luteinising hormone (LH) are generally raised. [1]

Figure:2 The cardinal feature is functional ovarian hyperandrogenism
SYMPTOMS AND SIGNS
Dermatological Features
High levels of androgens typically lead to various dermatological symptoms. These include hirsutism (coarse and dark hair on the body areas where men typically grow hair—e.g., the face, abdomen, chest, and back), acne, and balding/alopecia. In adolescents, some of the dermatological symptoms may be caused by puberty rather than PCOS.[20]

Figure:3 dermatological features Hirsutism, Balding ,Acne ,Skin discoloration (acanthosis nigricans),Oily Skin
Menstrual Disorders
- Amenorrhea
- Oligomenorrhea
- Menorrhagia
Menstrual disorders can range from heavy bleeding (menorrhagia) to complete absence of menstruation (amenorrhea), or menstruation delayed for 35 days or longer (oligomenorrhea). 91% of women with irregular menstrual cycles will develop PCOS. Reports of infertility are 15 times more common in those with PCOS.[20]
Polycystic Ovaries
PCOS may be associated with excessive follicles, which are defined as 25 or more follicles measuring 2 mm to 10 mm in a single transvaginal ultrasound view. There may also be an ovary larger than 10 mL, or increased ovarian volume.[20]

Recurrent miscarriage
Recurrent miscarriages and early pregnancy after in vitro fertilization (IVF) cycles have been linked to polycystic ovaries. It was suggested that the primary cause of the reproductive losses was hypersecretion of LH. Nonetheless, there have been contradictory reports, sparking heated discussion. For instance, Thomas et al. (1989) discovered no correlation between LH levels and the results of IVF, but other studies have found no rise in LH in women who experience PCO and recurrent miscarriages. Some authors have recommended using gonadotropin releasing hormone (GnRH) analogs to suppress elevated LH levels in women attempting to conceive. More research is required to assess this strategy,
even though a trial involving 106 women with PCO and elevated LH levels who received GnRH analogs did not demonstrate an improvement in pregnancy outcomes.[11]
Metabolic symptomatology
Insulin resistance and obesity, which are prevalent in 30–50% of PCOS-affected women, are the metabolic components of the disease. Approximately 80% of obese women and 30–40% of women with PCOS who are of normal weight have the ensuing hyperinsulinemia. Compared to women with PCO, whose fat distribution is primarily in the lower body segment, women with PCO typically have higher insulin resistance and an increased waist:hip ratio (Bringer et al., 1993). Acanthosis nigricans, a feathering pigmented area of tissue in the neck and axillary regions, is another condition that some women may present with. this is now recognized as a non-specific marker of moderate to severe insulin resistance Hypersecretion of insulin results in ovarian secretion of androgens, leading to hirsutism and menstrual disturbance.[11]
COMPLICATIONS
Metabolic Risk
Metabolic abnormalities are predisposed in PCOS due to the underlying hormonal imbalance that is characterized by increased androgen production and insulin resistance. Obesity, dyslipidemia, and insulin resistance (IR) are the main characteristics of metabolic syndrome. Clinical criteria include high density lipoprotein, blood pressure >130/85 mmHg, triglycerides >150 mg/dl, impaired glucose tolerance, and waist circumference >88 cm. Additionally, more severe hyperinsulemia and hyperactivity of the hypothalamic pituitary-adrenal axis, which result in elevated adrenal androgens and a worsening of the hyperandrogenic state, are linked to superimposed obesity. Condition Severe reproductive phenotypes marked by hyperandrogenism and chronic ovulation are made worse by it. Furthermore, it has a synergistic effect on the development of diabetes, endometrial cancer, and cardio-metabolic risk in women with PCOS.[7]
Cardiovascular Risk:
Due to their increased prevalence, women with PCOS are more likely to have diabetes, dyslipidemia, obesity, impaired glucose tolerance, and other classic cardiovascular disease (CVD) risk factors. By increasing plasminogen activator inhibitor-1 levels and decreasing fibrinolysis, hyperinsulemia plays a role in the prothrombotic state. In addition to these, non-classic CVD risk factors include elevated C-reactive protein (CRP), homocysteine, tumor necrosis factor-?, IL-6, IL-18, and others that are linked to a systemic inflammatory state and impaired fibrinolysis. prevalent in PCOS-afflicted women. Even after controlling for age and BMI, there is anatomical evidence of increased subclinical markers of atherosclerosis in PCOS compared to controls. These markers include endothelial dysfunction, impaired pulse wave velocity, increased carotid intima media wall thickness, presence of carotid plaque, and increased coronary artery calcification.[7]
Oncology Risk
Chronic anovulatory cycles in PCOS women cause unopposed estrogen action on the endometrium. Endometrial hyperplasia results from it, and endometrial cancer could develop from it. Obesity, Type II diabetes, subclinical inflammation, and metabolic syndrome—all of which are strongly associated with PCOS populations and are recognized risk factors for endometrial cancer—further increase this predisposition. As a result, endometrial hyperplasia and cancer are three to four times more common in women with PCOS, regardless of their age.Period cyclicity is ensured by regular drawl bleeding combined with monthly progesterone or oral contraceptive pills. This prevents endometrial buildup, which lowers the risk of endometrial cancer.[7]
Psychological Problems
Due to obesity, acne, and excessive hair, PCOS affects how people feel about their bodies. Reduced quality of life and negative effects on mood and psychological well-being are also consequences of infertility and long-term health-related issues. It follows that women who have PCOS are more likely to experience low self-esteem, depression, anxiety, negative body image, and psychosexual dysfunction. It has been observed that women with PCOS13 have higher rates of depressive symptoms than non-BMI matched controls, with prevalence ranging from 28 to 64% for depression and 34 to 57% for anxiety. Consequently, as part of the diagnosis and treatment of PCOS, all psychosocial issues must be investigated and addressed.[7]
DIAGNOSIS
The fact that PCOS is a metabolic/reproductive disorder was highlighted in 1990 with the publication of stringent National Institutes of Health (NIH) criteria, which require the simultaneous presence of anovulation and hyperandrogenemia for the diagnosis on either a biochemical or clinical level (hirsutism/acne). For a significant number of physicians (especially Europeans), this distinction was necessary for the diagnosis of polycystic ovarian morphology on ultrasound, despite excellent studies that have since clearly demonstrated that this condition may be found in about 20–30% of normally ovulating, but not hyperandrogenemic, women. The three diagnostic criteria. Now that these criteria can be combined in different ways, four distinct PCOS phenotypes have been identified:[5]
Type A:
hyperandrogenism, chronic anovulation and polycystic ovaries.
Type B:
hyperandrogenism and chronic anovulation.
Type C:
hyperandrogenism and polycystic ovaries.
Type D:
chronic anovulation and polycystic ovaries.
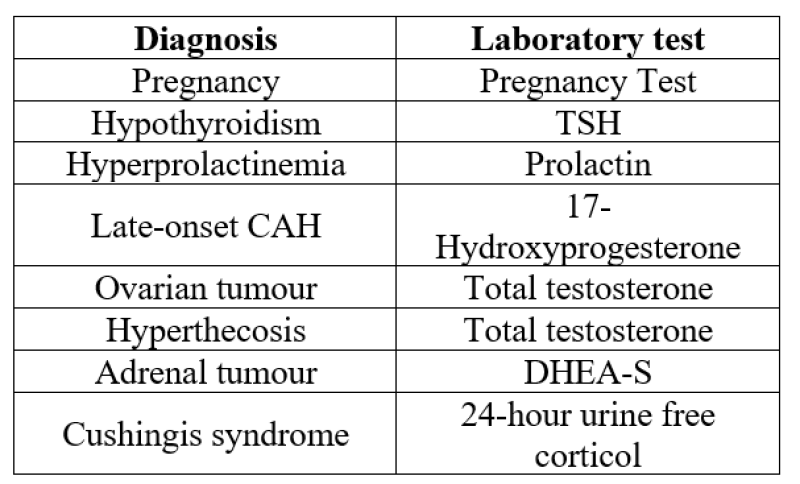
Suggested laboratory and radiographic studies for the diagnosis of PCOS:
Endocrinology:
Testosterone level
Luteinizing hormone level
Follicle-stimulating hormone level
DHEA-sulfate level
17-hydroxyprogesterone level
Prolactin level Urinary:
Human chorionic gonadatropin level
Other tests:
Lipid profile (total cholesterol, LDL* , HDL† , triglycerides)
Oral glucose tolerance test
Pelvic ultrasound Dexamethasone suppression test
LDL = low-density lipoprotein
HDL = high-density lipoprotein
The most popular diagnostic criteria for PCOS at the moment is the 2003 Rotterdam criteria, which is also the definition that the 2018 international PCOS EBG recommends be used to diagnose adult women with PCOS.10. Teenage diagnosis can be especially difficult because normal pubertal physiology and PCOS diagnostic features overlap. Because of this, applying the Rotterdam criteria to teenagers may lead to an overdiagnosis of PCOS, which could have negative long-term health effects. The new PCOS guideline suggests modified diagnostic criteria in adolescents due to a lack of research on an accurate diagnostic approach based on evidence-informed consensus. According to the guideline, clinical HA (severe acne and/or hirsutism) or biochemical HA together with menstrual cycle irregularity that is well-defined based on time post menarche are the two primary criteria for diagnosing PCOS in adolescents. Within eight years of menarche, PCOM is advised not to be used as a diagnostic criterion for PCOS. Teens who fit one of the diagnostic criteria for PCOS may be deemed "at risk" for the condition and benefit from monitoring in addition to symptom management.[5]
TREATMENT
Behavioral Treatment
Many strategies for addressing obesity in the general population were assessed in a recent systematic reviewFew studies explicitly assessed the improvement in mental health outcomes with lifestyle changes, despite the fact that two clinical trials aimed at obese patients with binge eating disorder discovered improvements in depression symptoms with weight loss treatments interventions.[3]
Oral Contraceptive Pills
OCPs are used to treat hirsutism in women with PCOS and to control menstrual cycles . We would anticipate that OCP treatment would improve symptoms of anxiety and depression if hirsutism is a contributing factor to the increased risk of anxiety and/or depression. According to earlier information, women who were randomly assigned to receive OCPs (20 ?g ethinyl estradiol/1 mg norethindrone acetate) in the OWL PCOS study showed a significant improvement in the prevalence of depression and a nonsignificant improvement in the prevalence of anxiety after 16 weeks. There was no improvement in depression or anxiety scores among lean women with PCOS who received OCP.[3]
Insulin Sensitizers
Women with PCOS who have metabolic risk factors and insulin resistance are prescribed metformin. RCTs comparing the use of metformin to either LS or LS in women with PCOS have examined quality of life as an outcome rather than depression or anxiety, and OCPs have examined quality of life as well. Although the quality of life improved for both groups in each of these studies, there were no group differences. It has been demonstrated that the thiazolidinedione family member pioglitazone, another insulin sensitizer, lowers the concentration of inflammatory cytokines in PCOS-affected women as well as insulin resistance and fasting glucose. Pioglitazone is an anti-inflammatory PPAR-gamma agonist that activates peroxisome proliferator induced receptors.[3]
Psychiatric Medications
The effectiveness of psychiatric medications such as tricyclic antidepressants (TCAs), anti-anxiety medications, serotonin-norepinephrine reuptake inhibitors (SNRIs), and selective serotonin reuptake inhibitors (SSRIs) in treating depression or anxiety in women with PCOS has not been studied. Currently, the use of these drugs is based on accepted practices for treating anxiety and depression, however when prescribing them to women with PCOS, side effects like weight gain should be taken into account.[3]
METHOD FOR EVALUATION
Differential diagnoses and screening tests;[4]
CONCLUSION
About 3% to 5% of women who are of reproductive age have polycystic ovarian syndrome.2.5 In women in the same age range, it is the leading cause of infertility. Both our understanding of the complex causes of PCOS and our capacity to address its symptoms have significantly improved. Clinicians must be aware of the possibility of PCOS in female patients of all ages, as this syndrome foreshadows health risks that go beyond infertility and include the cardiovascular system and cancer of the reproductive organs. It is necessary to work toward creating more precise radiologic and/or laboratory diagnostic markers for PCOS.
ACKNOWLEDGEMENT
We are thankful to the principle and management of ali allana college of pharmacy akkalkuwa for providing necessary facilities during completion of this work
REFERENCE
- Schriock E, Martin MC, Jaffe RB. Polycystic ovarian disease. The Western Journal of Medicine. 1985 Apr 1;142(4):519-22.
- Sheehan MT. Polycystic ovarian syndrome: diagnosis and management. Clinical Medicine & Research. 2004 Feb 1;2(1):13-27.
- Cooney LG, Dokras A. Depression and anxiety in polycystic ovary syndrome: etiology and treatment. Current psychiatry reports. 2017 Nov;19:1-0.
- Khan MJ, Ullah A, Basit S. Genetic basis of polycystic ovary syndrome (PCOS): current perspectives. The application of clinical genetics. 2019 Dec 24:249-60.
- Livadas S, Diamanti-Kandarakis E. Polycystic ovary syndrome: definitions, phenotypes and diagnostic approach. Polycystic Ovary Syndrome. 2013;40:1-21.
- Szydlarska D, Machaj M, Jakimiuk A. History of discovery of polycystic ovary syndrome. Advances in Clinical & Experimental Medicine. 2017 May 1;26(3).
- Kachhawa G, Singh A. Long term complications of Polycystic ovary syndrome. Global Journal of Reproductive Medicine. 2017;2(1):7-9.
- Mehreen TS, Ranjani H, Kamalesh R, Ram U, Anjana RM, Mohan V. Prevalence of polycystic ovarian syndrome among adolescents and young women in India. Journal of Diabetology. 2021 Jul 1;12(3):319-25.
- https://www.longdom.org/open-access-pdfs/symptoms-and-treatment-involved-in-polycystic-ovary-syndrome.pdf
- https://orwh.od.nih.gov/sites/orwh/files/docs/PCOS_Booklet_508.pdf
- Gabor T. Kovacs, Robert Norman Polycystic Ovary Syndrome Second Edition March 2007 Published in the United States of America by Cambridge University Press, New York


 Siddiqui Mohammad Fardeen*
Siddiqui Mohammad Fardeen*
 Umar Mohammad Khan
Umar Mohammad Khan
 Thorat Dharmesh Jitendra
Thorat Dharmesh Jitendra





 10.5281/zenodo.10781584
10.5281/zenodo.10781584