Abstract
Hydrogels are three-dimensional polymeric networks that may trap large volumes of fluid. Hydrogels are generated when synthetic and natural polymers cross-link physically or chemically. These items are widely used in a variety of industrial and environmental applications. As expected, natural hydrogels were increasingly supplanted by synthetic forms due to their better water absorption capacity, long service life, and wide range of raw chemical resources. Smart hydrogels with various chemically and structurally responsive moieties have good reaction properties under a variety of environmental circumstances, including pH, temperature, light, electric field, magnetic field, and biological and chemical stimuli. These smart hydrogels are attracting attention from researchers for a variety of applications, including biomedical, industrial, agricultural, electrical, medicinal, and sanitary items. This study covers the most recent discoveries in the field of hydrogel synthesis, focusing on their unique properties and various elements of their responsive behaviour.
Keywords
Hydrogel, Industrial, Application.
Introduction
Hydrogels are a type of soft material used in DDSs that is made up of polymeric chains that are either physically or chemically crosslinked to produce 3-D polymeric networks (PNs) that swell due to high water volume encapsulation 1. HDG architecture may absorb almost a thousand times its dried weight in biological fluids or water. However, whereas HDG design can cause swelling when in contact with aqueous solutions, it also prevents disintegration 2. Furthermore, the water-absorbing capacity of HDGs rises with increasing functional hydrophilic groups connected to the polymeric chains. Their porosity also allows medications to be loaded into the gel matrix and then released at a rate determined by the small molecule or macromolecule's diffusion coefficient across the gel network 3. HDGs are widely used in cellular therapies, cancer research, stem-cell bioengineering, TE, and implanted devices. The incorporation of nanoparticles (NP) or nanomaterials (NMs) into HDGs is a novel approach for creating multifunctional HDG nanoarchitectures with improved characteristics. Various nanoparticulates (NPs) have been used to derive polymeric network hydrogels (PNHDGs) made of polymeric nanomaterials, carbon derivatives, metals, etc 4. Smart-responsive hydrogels have recently developed as sophisticated biomaterials with innovative applications. Smart hydrogels can respond to a variety of physical (thermo-, light, electric field, magnetic field, etc.), chemical (pH, ions, biomolecules, etc.), and biological stimuli by integrating functional units or components. These responses cause changes in polymer chains and networks, resulting in volume contraction/expansion, colour changes, phase transitions, and more 5,6. Thermosensitive hydrogels are a type of smart hydrogel that changes shape from sol to gel at specified temperatures. In general, thermosensitive hydrogels are created from natural and derived thermosensitive polymers by typical synthesis processes such as crosslinking and polymerization. It has intriguing qualities for drug delivery, including mechanical stability, biocompatibility, thermos-transition behaviour, and ease of formulation. Hydrogels can be made in a variety of ''traditional'' chemical processes. These include one-step procedures such as polymerization and parallel cross-linking of multifunctional monomers, as well as multi-step procedures involving the synthesis of polymer molecules with reactive groups and their subsequent cross-linking, possibly also by reacting polymers with appropriate cross-linking agents. Polymer engineers may design and synthesise polymer networks with molecular-scale control over structure, such as cross-linking density, and customised attributes, such as biodegradation, mechanical strength, and chemical and biological responsiveness to stimuli 7.
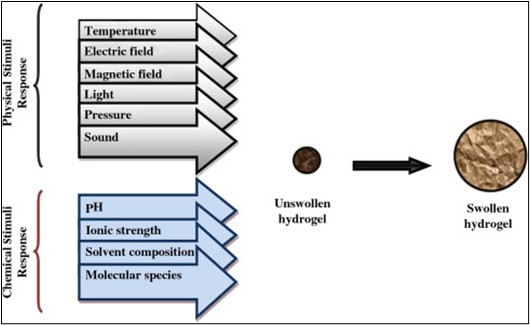
Fig. 1. Stimuli response swelling hydrogel.
Classification of hydrogel:
Classification based on source
Hydrogels can be classified into two groups based on their natural or synthetic origins :
Classification according to polymeric composition
The method of preparation leads to formations of some important classes of hydrogels. These can be exemplified by the following:
- Homopolymeric hydrogels are polymer networks made from a single monomer species, serving as the fundamental structural unit of every polymer network 7.
- Depending on the monomer type and polymerization procedure, homopolymers can have a cross-linked skeletal structure.
- Copolymeric hydrogels consist of two or more monomer species and at least one hydrophilic component organised in a random, block, or alternating pattern along the polymer network.
- Multipolymer Interpenetrating Polymeric Hydrogel (IPN) is a type of hydrogel composed of two separate cross-linked synthetic or natural polymer components arranged in a network. SemiIPN hydrogel consists of two components: a cross-linked polymer and a non-cross-linked polymer 8.
Classification based on configuration :
The classification of hydrogels depends on their physical structure and chemical composition can be classified as follows:
- Amorphous (non-crystalline).
- Semicrystalline: A complex mixture of amorphous and crystalline phases.
- Crystalline.
Classification based on type of cross-linking:
Hydrogels can be classified into two types based on the chemical or physical composition of their cross-link junctions. Chemically cross-linked networks contain permanent junctions, whereas physical networks have transient junctions that originate from either polymer chain entanglements or physical interactions such as ionic, hydrogen, or hydrophobic contacts 8.
Classification based on physical appearance
Hydrogels might appear as a matrix, film, or microsphere depending on the polymerization technique used during the preparation process.
Classification according to network electrical charge:
Depending on whether an electrical charge is present on the crosslinked chains, hydrogels can be divided into four groups:
- Nonionic (neutral).
- Ionic (including anionic or cationic).
- Amphoteric electrolyte (ampholytic) containing both acidic and basic groups.
- Zwitterionic (polybetaines) containing both anionic and cationic groups in each structural repeating unit
Natural polymers that produce hydrogels include proteins like collagen and gelatine, as well as polysaccharides like starch, alginate, and agarose. Chemical polymerization technologies have traditionally been used to produce synthetic polymers that create hydrogels.
Hydrogel product sensitive to environmental conditions:
Three-dimensional crosslinked hydrophilic polymer networks can reversibly swell or de-swell in water while maintaining a significant amount of liquid. Hydrogels can be made to shrink or expand in response to changes in their external environment. They may perform dramatic volume transition in response to a variety of physical and chemical stimuli. Physical stimuli include temperature, electric or magnetic field, light, pressure, and sound, while chemical stimuli include pH, solvent composition, ionic strength, and molecular species 9. The extent of swelling or de-swelling caused by changes in the hydrogel's external environment can be so severe that the phenomena is known as volume collapse or phase transition. Synthetic hydrogels have been the subject of substantial research for the past four decades and are still a very active topic of study today.
Properties of Hydrogel :
Hydrophilic gels known as hydrogels have received a lot of interest for their usage in pharmaceutical and biomedical engineering.
- Swelling properties :
A modest change in environmental conditions can cause rapid and reversible alterations in hydrogel. Changes in environmental parameters such as electric signal, pH, temperature, and the presence of enzymes or other ionic species may cause a change in the physical texture of the hydrogel.
- Mechanical properties :
The degree of crosslinking can be changed to achieve the desired mechanical property of the hydrogel. A stronger hydrogel can be produced by increasing the degree of crosslinking, but doing so reduces the hydrogels' percentage of elongation and results in a more brittle structure 7.
- Polymers used in hydrogels:
Hydrogels are made from polymers, both synthetic and natural. Natural polymers include hyaluronic acid, gelatin, and chitosan. Acryolic acid (AA), vinyl acetate (VAc), and hydroxy ethylmethacryate (HEMA) are examples of synthetic monomers and polymers.
- Biocompatible properties :
- The capacity of a substance to function in a particular application with a suitable host reaction is known as biocompatibility. Fundamentally, biocompatibility is composed of two components: (a) Biofunctionality, or the capacity of a material to carry out the particular function for which it is designed.
- Bio-safety, which includes the lack of mutagenesis, cytotoxicity, and/or carcinogenesis, as well as a suitable host response that is both systemic and local (the surrounding tissue)10 .
- Swelling properties :
A modest change in environmental conditions can cause rapid and reversible alterations in hydrogel. Changes in environmental parameters such as electrical signal, pH, temperature, and the presence of enzymes or other ionic species may result in a change in the physical texture of the hydrogel 11.
- Utilization of hydrogel products :
Wichterle and Lim developed the first synthetic hydrogels in 1954, and hydrogel technologies can now be used in a variety of applications, including hygienic products , agriculture12, drug delivery systems, sealing, coal dewatering, artificial snow, food additives, pharmaceuticals 10, biomedical applications tissue engineering and regenerative medicines, diagnostics, wound dressing, separation of biomolecules or cells, barrier materials to regulate biological adhesions, and biosensors 13. Furthermore, the ever-expanding range of functional monomers and macromeres broadens their applications. They were utilised in early agricultural water absorbents made from biopolymers by grafting hydrophilic monomers onto starch and other polysaccharides. Acrylic acid and its salts are the primary constituents of hydrogel products used in hygiene applications. Acrylamide is a major component used in the manufacturing of agricultural hydrogel products.
Advantages of hydrogels :
-
- Their high water content allows for elasticity similar to natural tissue.
- Controlled release of medications or nutrients.
- They are biocompatible, biodegradable, and injectable.
- Hydrogels have good transport qualities and are easily customisable.
- Environmentally sensitive hydrogels monitor changes in pH, temperature, or metabolite concentrations and release their load accordingly.
Disadvantages of hydrogels :
-
- High costs.
- Low mechanical strength.
- Can be difficult to manage.
- Difficult to load with medications or nutrients.
- Non-adherent maggots may require supplementary dressings and can produce feeling when moved 14.
Technologies adopted in hydrogel preparation
Generally speaking, natural or synthetic polymers can be used to create hydrogels. In comparison to natural polymers, synthetic polymers are chemically stronger and hydrophobic by nature. Although their mechanical strength slows down the pace of degradation, it also contributes to their durability. The best design should achieve a balance between these two diametrically opposed qualities 7. Additionally, if the natural polymers have the right functional groups or have been functionalized with radically polymerizable groups, it can be used to construct hydrogels based on such polymers. Simply put, a hydrogel is an elastic network made of hydrophilic polymers that have been cross-linked in some way. Therefore, a hydrogel can be made using any method that can be used to make a cross-linked polymer. Hydrogels are often created by copolymerization/cross-linking free-radical polymerizations, which include reacting hydrophilic monomers with multifunctional cross-linkers 11. Hydrogels are created by cross-linking water-soluble linear polymers, both synthetic and natural. There are several methods as following :
- Chemical reactions are used to link polymer strands.
- Producing main-chain free radicals with the ability to recombine as cross-link junctions through the use of ionising radiation.
- Physical interactions, including creation of crystallites, electrostatics, and entanglements.
The hydrogel mass must be cleaned to remove any contaminants left from the preparation process. These include unreacted monomers, initiators, cross-linkers, and undesired products resulting from side reactions. Other studies have looked at the synthesis of hydrogels from acrylamide, acrylic acid, and its salts using inverse suspension polymerization15 and diluted Solution polymerization. Fewer investigations have been conducted on highly concentrated solution polymerization of acrylic monomers, which are typically patented. Chen synthesised acrylic acid-sodium acrylate superabsorbent using intense (43.6 wt%) solution polymerization with potassium persulphate as a thermal initiator16.
Hydrogels are often made from polar monomers. They can be classified as natural polymer hydrogels, synthetic polymer hydrogels, or mixtures of the two.
Bulk Polymerization :
Many vinyl monomers may be suitable for the synthesis of hydrogels. Bulk hydrogels can be made from one or more types of monomers. The large range of monomers allows one to create hydrogels with the necessary physical qualities for a specific application. A tiny amount of crosslinking agent is typically added to any hydrogel formulation. Typically, radiation, ultraviolet, or chemical catalysts are used to commence the polymerization reaction. The type of monomers and solvents utilised determines the appropriate initiator. Polymerized hydrogels can be made in a number of shapes, including films and membranes, rods, particles, and emulsions.
Bulk polymerization is the simplest approach, requiring only monomers and monomer-soluble initiators. The high monomer concentration causes a high rate and degree of polymerization. However, the viscosity of the reaction grows significantly since the conversion produces heat during polymerization. These issues can be avoided by keeping the reaction at low conversions 17. The bulk polymerization of monomers to form a homogenous hydrogel results in a glassy, transparent polymer matrix that is extremely rigid. When immersed in water, the glassy matrix expands to become soft and flexible.
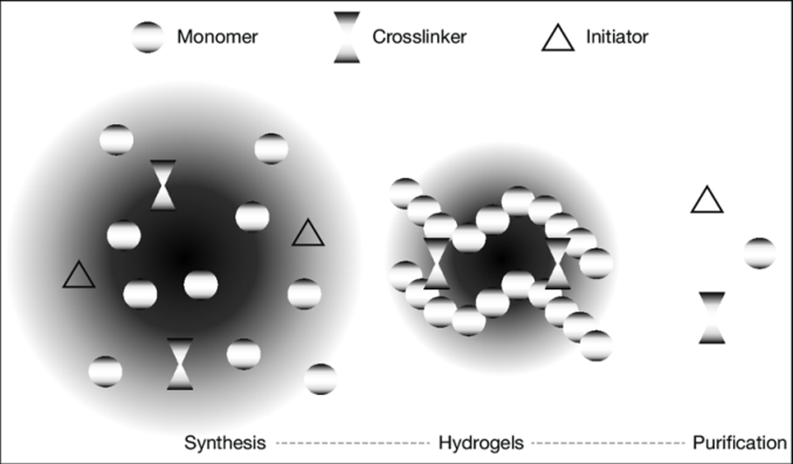
Fig no. 2. Schematic diagram of hydrogel preparation.
Solution polymerization/cross-linking:
Ionic or neutral monomers are combined with the multifunctional crosslinking agent in solution copolymerization/cross-linking operations. Thermal initiators such as UV radiation or redox initiator systems are used to start the polymerization process. The main benefit of solution polymerization over bulk polymerization is the presence of a solvent that acts as a heat sink. To get rid of the initiator, soluble and extractable polymer, oligomers, cross-linking agent, and other contaminants, the produced hydrogels must be cleaned with distilled water. When the amount of water during polymerization is more than the water content corresponding to the equilibrium swelling, phase separation takes place and the heterogeneous hydrogel is generated.
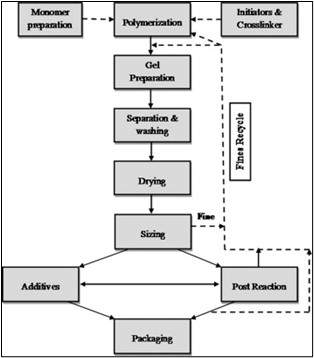
Fig no.3 Hydrogel preparation block diagram (solution polymerization/cross-linking procedure)
Suspension polymerization or inverse-suspension polymerization:
Dispersion polymerization is a desirable approach since the products are obtained in the form of powder or microspheres (beads), eliminating the requirement for grinding. Because the water-in-oil (W/O) technique is preferred over the more usual oil-in-water (O/W), the polymerization is referred to as "inverse suspension." This approach distributes the monomers and initiator in the hydrocarbon phase as a homogeneous mixture. The viscosity of the monomer solution, agitation speed, rotor design, and dispersant type primarily determine resin particle size and form 14. Several extensive discussions of hetero-phase polymerizations have previously been published. The dispersion is thermodynamically unstable, necessitating constant agitation and the addition of a low hydrophilic-lipophilic-balance (HLB) suspending agent.
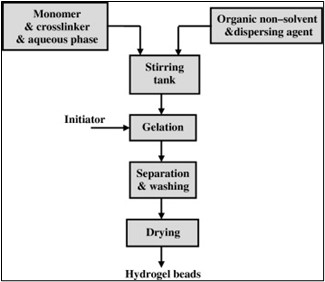
Fig no. 4. Suspension polymerization process.
Grafting to a support:
In general, hydrogels produced from bulk polymerization have a fragile structure. A hydrogel's mechanical characteristics can be improved by grafting or surface coating it onto a stronger support. This process involves generating free radicals on a stronger support surface and then polymerizing monomers directly onto it, resulting in a chain of monomers that are covalently bound to the support. A number of polymeric supports have been used to synthesise hydrogels using grafting procedures 7. Ionising high energy radiation, such as gamma rays and electron beams, has been used as an initiator to manufacture hydrogels of unsaturated chemicals. Irradiation of an aqueous polymer solution causes the production of radicals on the polymer chains. Furthermore, radiolysis of water molecules produces hydroxyl radicals, which attack polymer chains, resulting in the creation of macro-radicals. Recombination of macroradicals on distinct chains forms covalent bonds, resulting in a cross-linked structure. Polymers crosslinked by the radiation technique include polyvinyl alcohol, polyethylene glycol, and polyacrylic acid 14.
Polymerization by irradiation:
High energy radiation such as electron beams and gamma rays have been utilised as an initiator to prepare unsaturated compound hydrogels14. Radicals are created on the polymer chains as a result of radiation applied to an aqueous polymer solution. Additionally, hydroxyl radicals are created when water molecules undergo radiolysis. These radicals attack polymer chains and eventually become macro-radicals. Ultimately, a cross-linked structure is created as a result of the macro-radicals recombining on various chains to generate covalent bonds. The radiation approach can be used to crosslink polymers such as acrylic acid, ethylene glycol, and vinyl alcohol.
Physical cross-linking:
It is the most popular and straightforward method for creating hydrogels by the physical cross-linking of polymers. This physical cross-linking involves ion interactions include hydrophobic association, polyelectrolyte complexation, and hydrogen bonding. The following are the several techniques utilised in the preparation of physically cross-linked hydrogels:
- Heating/cooling a polymer solution-
Cooling hot gelatin or carrageenan solutions produces physically cross-linked gels. Gel formation is caused by the association of helices, helix formation, and the development of junctional zones. Some examples include polyethylene glycol-polylactic acid hydrogel and polyethylene oxide-polypropylene oxide.
Complex coacervate gels are formed by combining polyanions and polycations. The essential premise of this approach is that polymers with opposing charges cling together, forming soluble and insoluble complexes depending on the concentration and pH of the corresponding solutions. One example is combining polycationic chitosan with polyanionic xanthan.
Cross linking between polymers occurs when di-or trivalent counter ions are added to an ionic polymer. The idea behind this technique is to gel a polyelectrolyte solution (Na+alginate-) with a multivalent ion that has opposite charges (Ca2+ + 2Cl-). Additional instances include chitosan-polylysine, chitosan-glycerol phosphate salt, and hydrogels made of chitosan and dextran.
A hydrogen bond is produced by the combination of an electron-deficient hydrogen atom and a functional group with a high electron density. Hydrogels, for example, can be formed when PA and PNVP create hydrogen bonds. The molar ratios of each polymer, polymer concentration, solvent type, solution temperature, and polymer shape all have an impact on hydrogels.
This procedure involves the employment of a crosslinking agent to join two polymer chains, as well as the grafting of monomers onto the polymer backbone. Natural and synthetic polymers can be cross-linked by reacting their functional groups (such as OH, COOH, and NH2) with cross-linkers like aldehyde (e.g., glutaraldehyde, adipic acid dihydrazide). IPN is the process of polymerizing a monomer within another solid polymer to create an interpenetrating network structure.
Hydrogels and their application in controlled drug release:
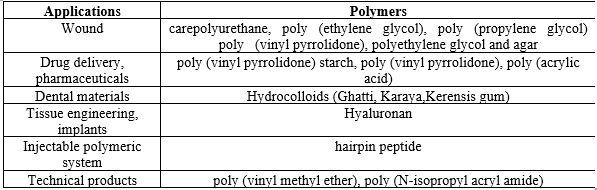
Table.1: Pharmaceutical Applications of hydrogels types of polymers
Hydrogel technical features:
The functional features of an ideal hydrogel material can be listed as follows:
- The maximum absorbency under load (AUL).
- The lowest price and the greatest absorption capability in saline.
- The lowest soluble concentration and residual monomer.
- Maximum durability and stability in the swelling environment and during storage.
- The best biodegradability is achieved without the generation of hazardous species during decomposition.
- Colourless, odourless, completely non-toxic, and photostable 18.
Applications of hydrogels in drug delivery:
Hydrogels have received a lot of attention as promising candidates for bioadhesive devices, controlled release devices, and therapeutic agent-targetable devices. They can be used for drug delivery in a variety of ways, including oral, rectal, ocular, epidermal, and subcutaneous applications.
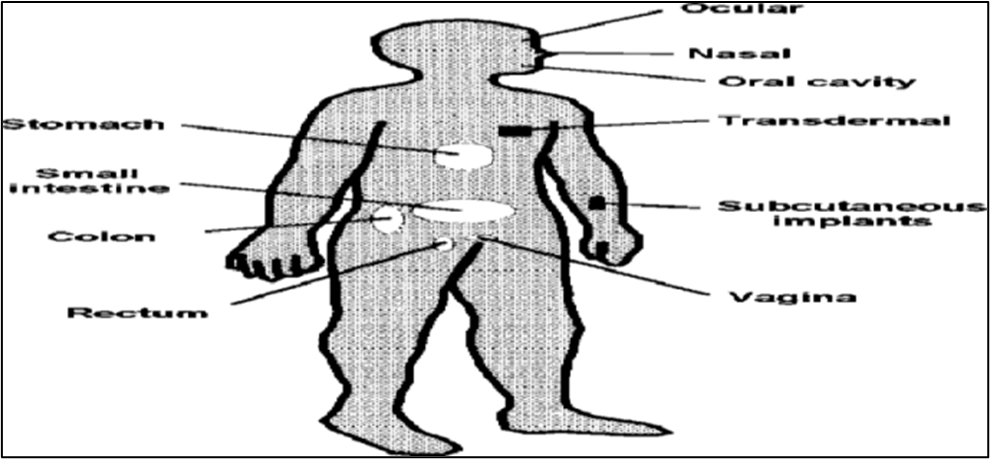
Fig no. 5. Tissue locations applicable for hydrogel-based drug delivery systems
- Drug delivery in the oral cavity :
Drug is embedded in hydrogels and delivered to the oral cavity for local therapy of mouth disorders such as stomatitis, fungal infections, periodontal disease, viral infections, and oral cavity malignancies.
- Drug delivery in the GI tract :
The GI tract is the most popular route of medication delivery due to its ease of administration for compliance therapy and wide surface area for systemic absorption. Hydrogel-based devices, similar to buccal delivery, can be created to deliver medications to particular areas in the GI tract. For example, stomach-specific antibiotic medication delivery systems are used to treat Helicobacter pylori infection in peptic ulcer disease.
- Protein drug delivery:
Interleukins are currently administered as hydrogels rather than injections. These hydrogels have demonstrated improved patient compliance. The hydrogels generate an in-situ polymeric network that slowly releases proteins. These are biodegradable and biocompatible too 19.
- Wound healing :
Hydrogels' cross-linked nature allows them to hold water and drugs. Because of their propensity to keep water, they can hold and maintain wound exudates. When applied, gelatin and sodium alginate-based hydrogels can cover and protect wounds against bacterial infection.
- Hydrogels for brain :
The blood-brain barrier, like other barriers in the human body, presents a problem for medication delivery, with 98% of newly synthesized medicines failing to cross it. As a result, there are few medicines available for medication delivery to the central nervous system. Camptothecin, a long-term sustained-release medication, is filled with PLGA microspheres, as shown in rats. These microspheres extend the survival time of rats with malignant gliomas.
- Transdermal Delivery :
Different hydrogel-based drug delivery devices are developed to administer medication via the skin. Swollen hydrogels have applications in wound dressing as controlled release tools. Formulations based on hydrogel are being investigated for transdermal iontophoresis in order to achieve improved product penetration, specifically for hormones and nicotine.
Characterization of hydrogels:
In general, the morphology, swelling property, chemical structure, and elasticity of hydrogels are what define them. The following are crucial characteristics for hydrogel characterization :
-
- pH:
With a digital pH meter, the pH of hydrogels is determined. Before using a pH meter, it must be calibrated.
-
- Scanning Electron Microscopy (SEM):
SEM can reveal details about the composition, surface topography, and other characteristics of the material, including electrical conductivity. In a SEM, the magnification can be adjusted from roughly 10 to 500,000 times across a range of up to 6 orders of magnitude 20.
-
- Fourier Transform Infrared Spectroscopy:
It is an effective method for determining a substance's chemical structure. It is predicated on the idea that a substance's fundamental constituents, or chemical bonds, can be stimulated and absorb infrared light at frequencies that are distinctive of chemical bonds.
-
- Swelling measurement :
There are currently three approaches available for measuring hydrogel swelling:
Method A :
This method involves using a roller mixer to submerge the dried hydrogel in deionized water for 48 hours at room temperature. Following its swelling, the hydrogel is filtered through a 30 mesh stainless steel net (681 ?m). This is how the swelling is computed. WS-Wd/Wd = Swelling where Ws is the hydrogels' weight when they are inflated and Wdis their weight when they are dry.
Method B:
In a volumetric vial, the dry hydrogel (0.05-0.1g) was dispersed in an appropriate amount of water (25-30 ml) for 48 hours at room temperature. The mixture is then centrifuged to separate the water-bound substance and the unabsorbed water. The free water is removed, and the swelling can be quantified using Method A above.
Method C:
In Method C, the dry gel is submerged in deionized water for 16 hours at room temperature. After swelling, the hydrogel was filtered through a stainless-steel net with a mesh size of 100 (149 ?m). Swelling is computed as follows:- Swelling = C×100/B. Where C is the mass of the hydrogel generated after drying, and B is the weight of the insoluble fraction after the extraction of water 21.
- X-ray diffraction:
Diffraction analysis determines if a material is crystalline or amorphous. It determines whether polymers retain their crystalline structure or are distorted during the procedure of pressurization process. Diffraction analysis is a prominent method for studying the crystalline structure of hydrogels.
- In -Vitro drug release study:
Because hydrogels are swollen polymeric networks with drug molecules occupying their interiors, release studies are conducted to better understand the mechanism of release over time. To determine the equivalency of the medication solutions, the parameters are matched with the standard plot 4.
7. Spreadability study:
The equipment consisted of a wooden block with a scale and two glass slides containing a pan set on a pulley. Excess formulation was sandwiched between two glass slides, and a 100 gram weight was placed on the upper glass slide for 5 minutes to compare the formulation and produce consistent thickness. Weight can be added, and the time it takes to separate the two slides is used to calculate spreadability. S= (m × l) / t where S is spreadibilty, m is weight tied on the upper slide, l is the length of the glass slide, and t is time taken in seconds 22.
-
-
-
- Drug Content Determination:
The drug content of Hydrogel was determined by dissolving one gram of the formulation in 100 ml of methanol, making suitable dilutions, and filtering the resultant solution using a millipore filter (0.45?m). Absorbance was measured at 296 nm with a UV-spectrophotometer (Shimadzu UV 1800). The slope and intercept of the standard calibration curve generated through linear regression analysis were used to calculate drug content 23.
9. Antifungal Activity Studies :
The produced hydrogel formulations were evaluated against a candida albican strain utilizing the agar cup method. Cups with a diameter of 10mm were produced aseptically in savoured dextrose agar after being inoculated with the tested fungal suspension strain (106cfu/ml) by distributing it on the agar surface. Each prepared mixture was put into the cups using a sterile syringe. Each cup's zone of inhibition was observed, and the radius of this zone was quantified 23.
10. Stability Studies:
The hydrogel was packaged in aluminium tubes (5 grams) and tested for stability at 25°C/60% relative humidity (RH) and 40°C/75% RH for three months. Samples were collected at 15-day intervals were analyzed for physical appearance, pH, rheological characteristics, drug content, and drug release 24.
CONCLUSION
Recently, several hydrogel-based networks have been created and adapted to fulfill the needs of various applications. These hydrogels have the advantage of being able to expand when mixed with an aqueous solution. The current review discusses the classification of hydrogels on several basis, their physical and chemical properties, the technical feasibility of their use, the technique of preparation, and their intended use. The study reveals that hydrogels have excellent characteristics and will have numerous potential uses as next-generation biomaterials. This is why hydrogels are referred to as intelligent or smart biomaterials. There are several methods for preparing hydrogels. Some of these are addressed in this article.
REFERENCES
- Das, S., Kumar, V., Tiwari, R., Singh, L. & Singh, S. RECENT ADVANCES IN HYDROGELS FOR BIOMEDICAL APPLICATIONS. Asian J. Pharm. Clin. Res. 11, 62 (2018).
- Khan, B. et al. Recent progress in thermosensitive hydrogels and their applications in drug delivery area. MedComm – Biomater. Appl. 2, e55 (2023).
- Idumah, C. I., Nwuzor, I. C. & Odera, R. S. Recent advances in polymer hydrogel nanoarchitectures and applications. Curr. Res. Green Sustain. Chem. 4, 100143 (2021).
- Chatterjee, A. As A Review on Hydrogels as Drug Delivery in the Pharmaceutical Field.
- Cao, J., Yuan, P., Wu, B., Liu, Y. & Hu, C. Advances in the Research and Application of Smart-Responsive Hydrogels in Disease Treatment. Gels 9, 662 (2023).
- Lavrador, P., Esteves, M. R., Gaspar, V. M. & Mano, J. F. Stimuli?Responsive Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 31, 2005941 (2021).
- Ahmed, E. M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 6, 105–121 (2015).
- Hacker, M. C. & Mikos, A. G. Synthetic Polymers. in Principles of Regenerative Medicine 587–622 (Elsevier, 2011). doi:10.1016/B978-0-12-381422-7.10033-1.
- Shin, J., Braun, P. V. & Lee, W. Fast response photonic crystal pH sensor based on templated photo-polymerized hydrogel inverse opal. Sens. Actuators B Chem. 150, 183–190 (2010).
- Kashyap, N., Kumar, N. & Kumar, M. N. V. R. Hydrogels for Pharmaceutical and Biomedical Applications. Crit. Rev. Ther. Drug Carrier Syst. 22, 107–150 (2005).
- Shantha, K. L. & Harding, D. R. K. Synthesis and evaluation of sucrose?containing polymeric hydrogels for oral drug delivery. J. Appl. Polym. Sci. 84, 2597–2604 (2002).
- Hamidi, M., Azadi, A. & Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 60, 1638–1649 (2008).
- Stamatialis, D. F. et al. Medical applications of membranes: Drug delivery, artificial organs and tissue engineering. J. Membr. Sci. 308, 1–34 (2008).
- Nandini Sahu, Diksha Gupta, & Ujjwal Nautiyal. Hydrogel: Preparation, Characterization and Applications. Asian Pac. J. Nurs. Health Sci. 3, 1–11 (2020).
- Mohana Raju, K. & Padmanabha Raju, M. Synthesis of novel superabsorbing copolymers for agricultural and horticultural applications. Polym. Int. 50, 946–951 (2001).
- Yamasaki, H. & Harada, S. Process for preparation of high water-absorbent polymer beads. (1984).
- Kiatkamjornwong, S. [No title found]. ScienceAsia 33(s1), 039 (2007).
- Chirani, N. et al. History and Applications of Hydrogels. Vol. 4, 13–23 (2015).
- Junginger, H. E., Verhoef, J. C. & Thanou, M. Drug delivery: Mucoadhesive hydrogels. Encycl. Pharm. Technol. 1169–1182 (2007).
- Zohuriaan, J. & Kabiri, K. Superabsorbent Polymer Materials: A Review. Iran. Polym. J. Engl. Ed. 17, (2008).
- Qavi, S., Pourmahdian, S. & Eslami, H. Acrylamide Hydrogels Preparation via Free Radical Crosslinking Copolymerization: Kinetic Study and Morphological Investigation. J. Macromol. Sci. Part A 51, 842–848 (2014).
- Nautiyal, D. U., Devi, A., Kaur, S. & Komal. Hydrogels: a smart drug delivery device. Asian Pac. J. Health Sci. 1, 92–105 (2014).
- Amin, S., Rajabnezhad, S. & Kohli, K. Hydrogels as potential drug delivery systems. Sci Res Essays.
- Helal, D., El-Rhman, D. A., Abdel-Halim, S. & El-Nabarawi, M. FORMULATION AND EVALUATION OF FLUCONAZOLE TOPICAL GEL Research Article. https://www.semanticscholar.org/paper/FORMULATION-AND-EVALUATION-OF-FLUCONAZOLE-TOPICAL-Helal-El-Rhman/0198bc0174273aeb75d57c3ed32a34d34631f324 (2012).


 Spandan vijay sonar *
Spandan vijay sonar *






 10.5281/zenodo.12740530
10.5281/zenodo.12740530