Abstract
Pyridopyrimidines are a versatile class of fused nitrogen containing heterocyclic compounds that exhibit a broad spectrum of biological activities, making them a focus of extensive pharmacological research. These molecules consists of pyridine ring fused with a pyrimidine ring, forming a rigid structure that can interact with various biologic targets. The ongoing structural modifications and synthesis of novel pyridopyrimidine analogues continue to expand their scope making them promising scaffolds and also recent advancements in its synthetic methodologies have facilitated the design of structurally diverse pyridopyrimidine derivatives. This study reviews the synthesis of pyridopyrimidine along with various biological activities of pyridopyrimidines, including their roles as anti cancer, anti histaminic, antimicrobial, antiviral, and analgesic agents. Beginning with an introduction to the structural diversity and synthetic strategies for pyridopyrimidine derivatives , the review highlights their broad spectrum of pharmacological effects
Keywords
Pyridopyrimidines, Anti cancer, Anti bacterial, Anti viral, Anti histamine, Analgesic.
Introduction
1.1 Docking (1,2,3)
Molecular docking is a computational modelling technique that makes it easier to forecast a molecule's preferred binding orientation when two molecules combine to form a stable complex. The tremendous increase in computer power and availability, as well as the increasing accessibility of databases of tiny molecules and proteins, have made it much easier. In order to comprehend and forecast molecular recognition, automated molecular docking software looks at probable binding modes structurally and predicts binding affinity energetically. In drug discovery, molecular docking has many applications and uses, such as structure-activity studies, lead optimisation, virtual screening for possible leads, generating binding hypotheses to help mutagenesis study predictions, and supporting substrate fitting in x-ray crystallography.
1.2 Pyridopyrimidine (4)
Pyridopyrimidines are compounds which consist of a pyridine fused to a pyrimidine ring.

Figure 1: Pyridopyrimidine structure
1.3 Synthesis (5)
Two component reaction
The synthetic strategy entails the synthesis of b-dicarbonyl derivatives via reaction of Aryl aldehydes with cyanoacetylpyrimidinone in the presence of DMF, under heating and nano catalytic conditions. The bicyclic products were synthesized in good yields (84–96%) due to the role of the nano catalyst and depending on the type of structured substituents on the benzene ring of the aldehyde.

Physical properties
|
IUPAC name
|
Pyrido[3,2-d]pyrimidine
|
|
Molecular formula
|
C7H5N3
|
|
Molecular weight
|
131.138 g/mol
|
|
Molar refractivity
|
38.36 ± 0.3 cm3
|
|
Density
|
1.270 ± 0.06 g/cm3
|
Pharmacological activities

Figure 1: Pharmacological activities of pyridopyrimidine
2. Biological Activities
2.1 Anti Viral Activity (6,7)
Viruses are the most diverse pathogens on earth, capable of infecting all forms of life, be it a human, an animal, plants, or microorganisms and are the most common viral pathogens too. Antiviral agents are drugs that are intended to prevent viral propagation and growth. These days, anti-viral therapy has been very useful in the management of viral diseases including but not limited to; HIV/AIDS, Hepatitis of B and C strands, influenza viruses, and herpes viruses and the quickly spreading SARS-CoV-2 which is causing COVD-19. Hepatitis C virus (HVC) is a very frequent pathogen to contract that ultimately can end with severe cirrhosis, cancer of the hepatocytes (HCC) or liver failure. There is a huge necessity for new types of HCV medications, alongside those already existing medications. Consequently, a group of pyrido [2,3-d] pyrimidines (compound 194) described by Krueger et al. were derived and screened for their cytotoxicity against Huh-7 cells (curented epithelial-like tumorigenic cells) and in genotype 1a and 1b HCV replicon assays. Despite the fact that compound 194, which has isopropyl as R1 and OH as R2, improved potency, its metabolic stability and oral pharmacokinetics were inadequate. Later investigations delved into compound 195, which has amides with an extra ring A attached, which greatly increased its effectiveness and pharmacokinetics.
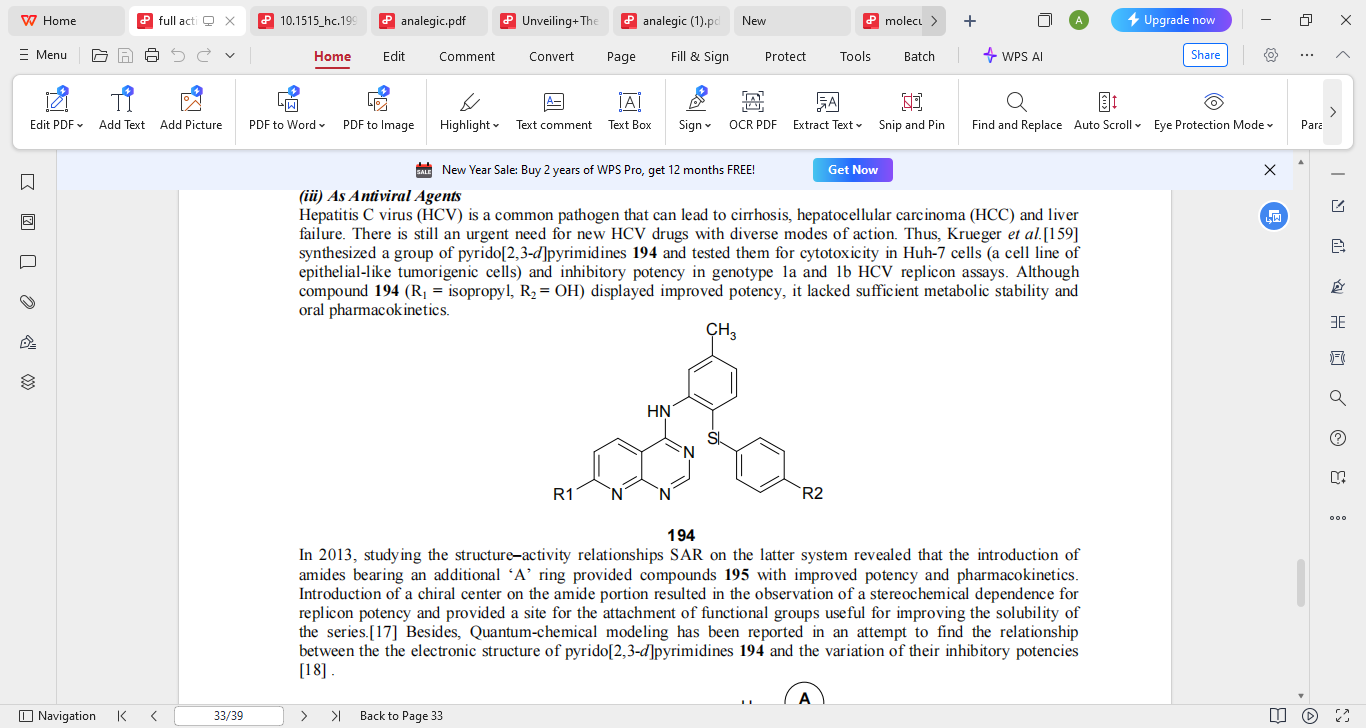
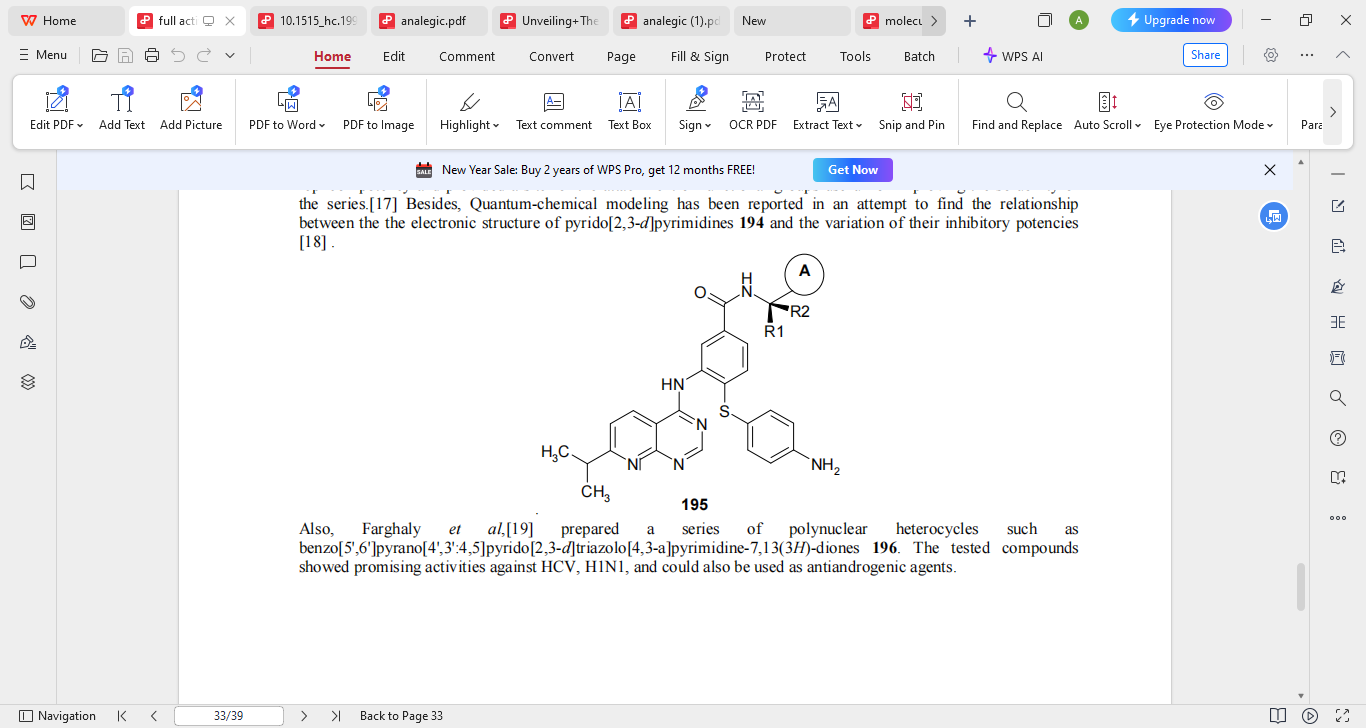
2.2 Anti Cancer Activity (8,9)
Gineinah et al interpolated pyrido[2,3-d]pyrimidine derivatives and subsequently merged this with one or more piperizing or triazolic heterocyclic moieties. Some adjustments were made to the starting molecule 83, the compounds that were studied maintained their biological potency owing to the dominant moiety (pyridopyrimidine). An increase in activity was observed with the addition of N-phenylthiosemicarbazide moiety’s 85 to the fifth position of the pyridopyrimidine and a decrease in activity was observed with the addition of an electron-withdrawing fluoro to compound 86 on the 4th position of the phenyl ring, transferring the ester to acid hydrazide, compound 84 also decreased activity. The cyclization of compounds 87 and 88 along with thiosemicarbazide onto [1,2,4]-triazoles brought forth excellent and strong anti-tumor results making them far more effective than compounds 85 and 86, whether non-cyclized or not. Additionally, compound 89 had less merging than compound 87 and 88 proving the free Sulfanyl group’s (–SH) solemn strength. Compound 90 was found to be relatively stronger in the fusion cancer drug as opposed to 89, showing that the piperazine moiety improved the anti-tumor action reflecting the activity 90 as a cancer drug. Likewise, due to the inclusion of piperazine, compounds 91 and 92 showed good results.

2.3 Anti-Bacterial Activity (10,11)
Khadiga M. has carried out an exploratory study aimed at evaluating the antibacterial potential of biologicaly active polymeric fused polymeric fused pyrido[2,3-d]pyrimidines having khellinone and trimethoxyphenyl moieties. As demonstrated in SCHEME 1, these heterocyclic compounds were obtained with multistep reactions using 2-amino-3-cyanopyridine derivatives as the starting materials.
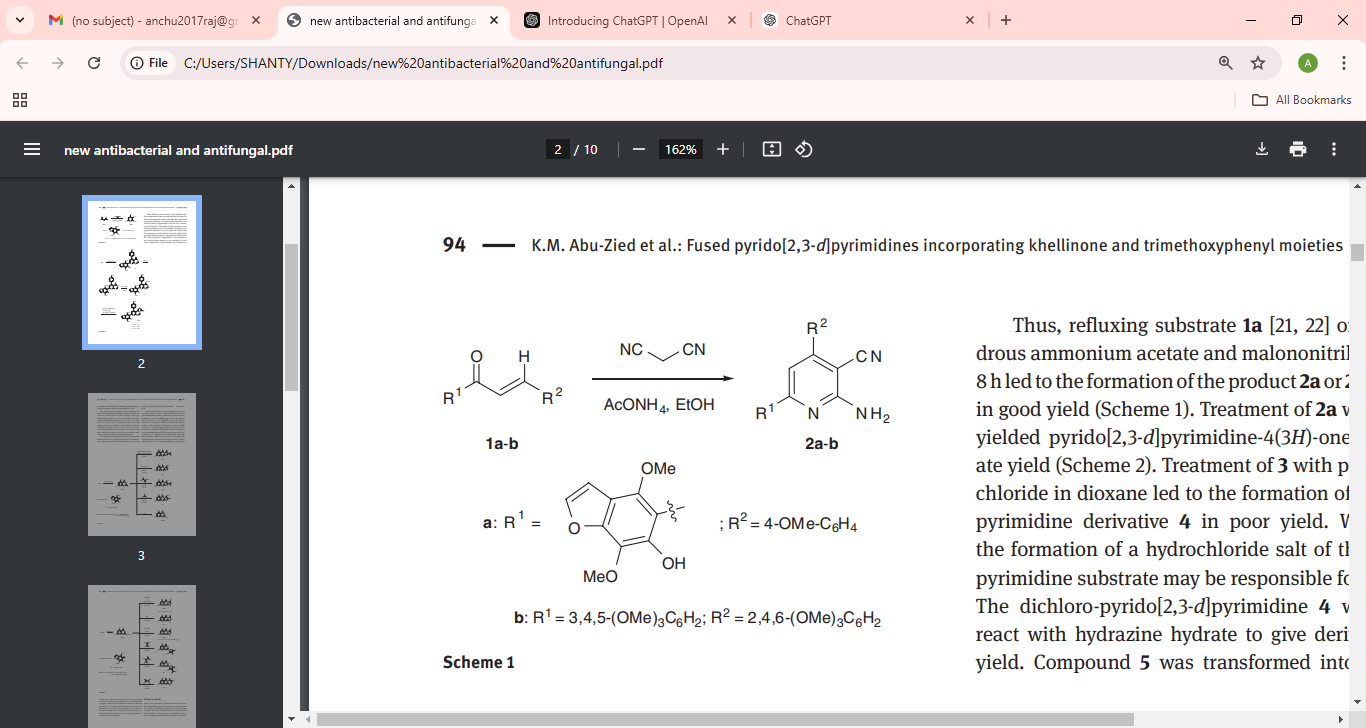
Scheme 1: Pyrido[2,3-d]pyrimidine intermediate formation and further derivatization steps.

Scheme 2: Pyridopyrimidine scaffolds (e.g., compound 3) are synthesized and further modified through chlorination and hydrazine treatment, introducing triazolo moieties (compounds 5, 6a–c) to explore new pharmacophore functionalities

Scheme 3: The pyrido[2,3-d]pyrimidine core (2a, 2b) is functionalized with dithione groups (7a, 7b) via carbon disulfide, enabling further cyclization reactions to yield thiazolo-fused pyrimidine derivatives (8–11).
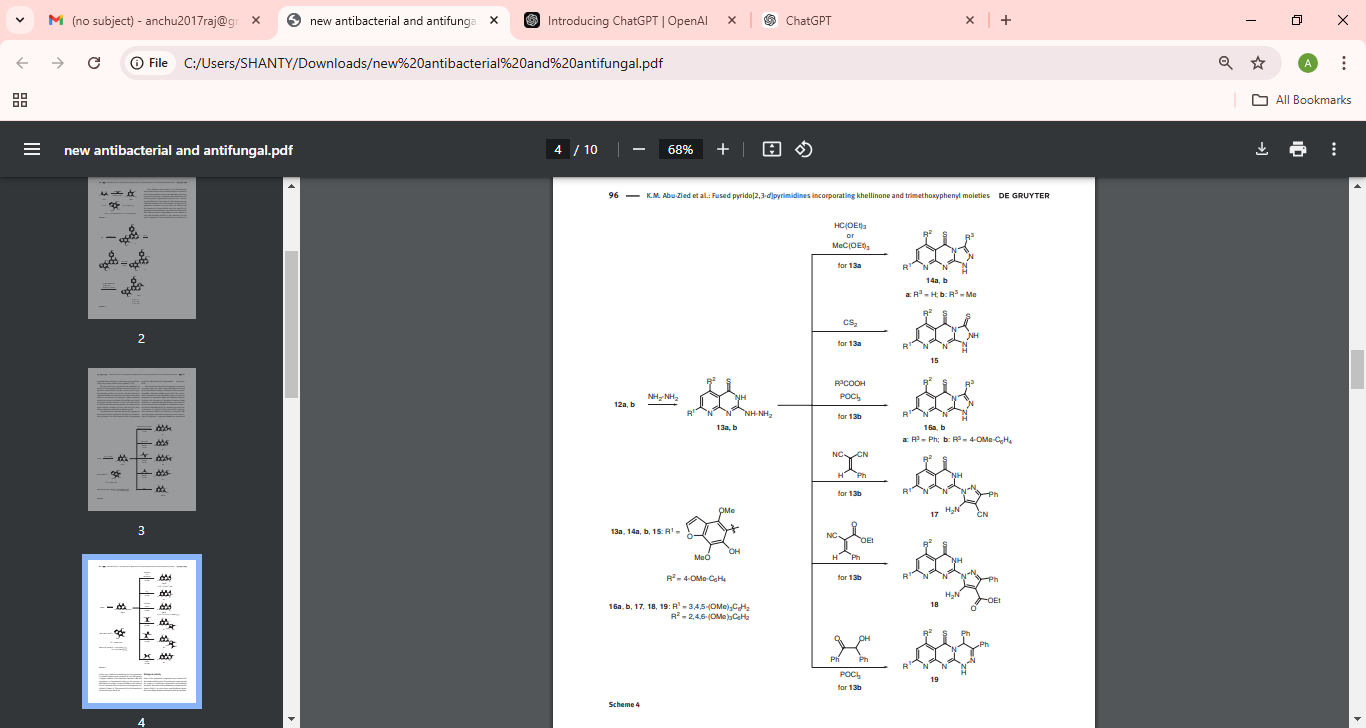
Scheme 4: Pyrido[2,3-d]pyrimidine dithione derivatives are elaborated into methylthio (12a, 12b) and hydrazino derivatives (13a, 13b), with subsequent cyclizations forming structurally diverse triazolo-pyrimidines (14a–16b), highlighting the versatility of this scaffold for creating biologically active leads.
Table 1 : Anti microbial activity of selected compounds
|
Tested compounds and standards(µg/mL)
|
E.coli(G-)
|
S.aureus(G+)
|
B.subtilis(G+)
|
|
1
|
++
|
++
|
+++
|
|
2a
|
+++
|
++
|
+++
|
|
3
|
+++
|
+++
|
+++
|
|
4
|
+
|
+
|
+
|
|
5
|
++
|
++
|
++
|
|
6a
|
+++
|
++
|
+++
|
|
6b
|
++
|
++
|
++
|
|
6c
|
+++
|
++
|
+++
|
|
7a
|
+++
|
++
|
+++
|
|
12a
|
+++
|
++
|
+++
|
|
13a
|
++
|
++
|
++
|
|
14a
|
+++
|
++
|
+++
|
|
14b
|
+++
|
++
|
+++
|
|
14c
|
++
|
++
|
++
|
|
levofloxacin
|
+++
|
+++
|
+++
|
|
nystatin
|
-
|
-
|
-
|
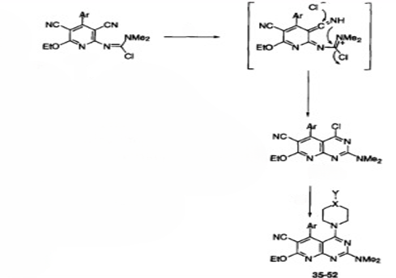
2.4 Anti- Histaminic Activity (12)
Jose M. Quintela et al prepared a number of pyrido[2,3-d]pyrimidine via nucleophilic substitution and cyclization on chloroamidines. Compounds 35, 37, 49, 50, 51 and 52 52 showed moderate (>25% inhibition) in pre incubation immunologically triggered secretion assays as the drug is added with the antigen in colon cancer therapy those showed activity only it though there when the drug was added Simultaneously such. In the chemically stimulated studies the inhibition was still stronger in 42, 43 and 49 in which only 30 – 50 % histamine liberation was achieved in the control’s group. Compound 49 appeared as a good inhibitor during immunological mediated stimulation while non immunological inhibition was also seen but many of the tested compounds inhibited under only one of the conditions or the other not both (42 and 43).
2.5Analgesic Activity (13,14,15)
A number of pyrido[2,3-d]pyrimidine derivatives have been designed, synthesized by Ichiro Takasaki et al and subsequently tested for antagonist potency on PAC1 receptor. In this study they synthesized twenty one PA-8 structure based derivatives. Among them, the compounds (2Amino-5-(3-trifluoromethoxy-phenyl)-5,8-dihydro-3H,6H-pyrido[2,3-d]pyrimidine-4,7-dione) exhibited more potent antagonistic activities than PA-8.The pyrido[2,3-d]pyrimidine skeletion was constructed using three component reaction which is indicated in scheme 5.
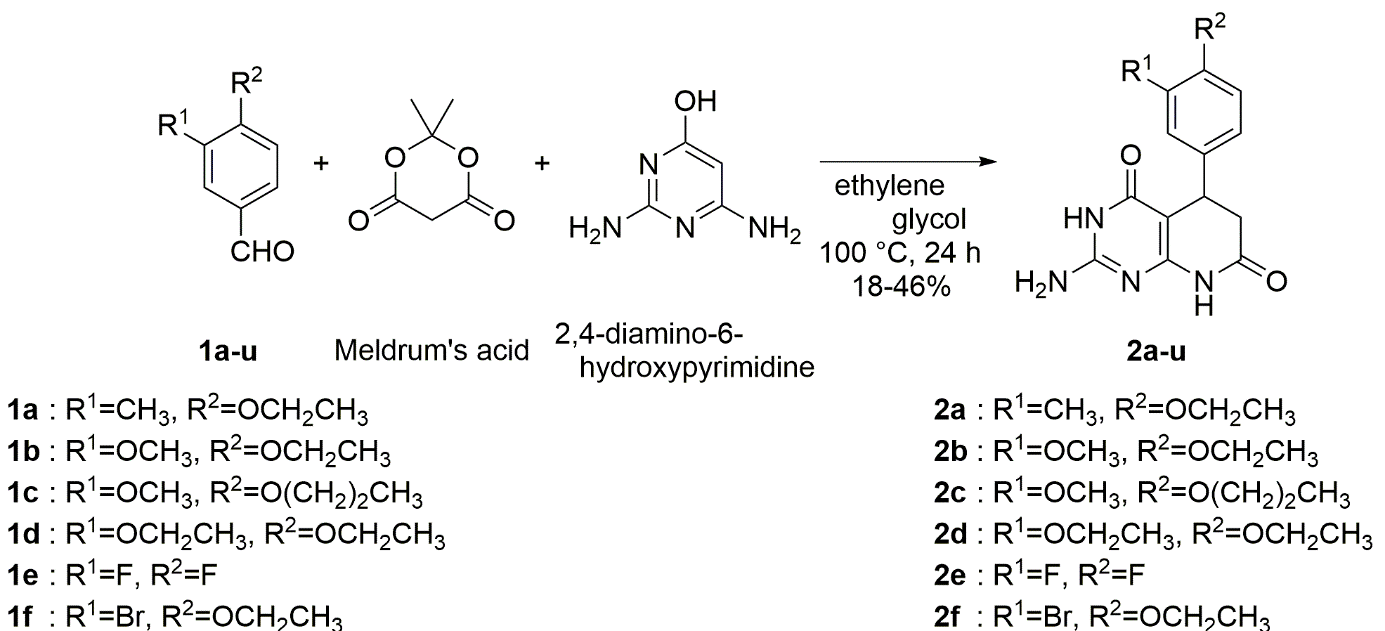
Scheme 5: Construction of pyridopyrimidine skeleton using 3 component reaction.
Of the compounds synthesized, 2o showed more potent antagonistic action than PA-8 in an in vitro pharmacological assay. With a yield of 78%, the new antagonist 2o has the benefit of being formed by a single step process. Also, compound 2o was found to have stronger analgesic activities against PACAP- and SNL-induced mechanical allodynia. According to the results obtained, 2o demonstrated advantageous ADME and PK properties. In summary, these results indicate that 2o has potential to become a useful analgesic for neuropathic pain.
3. CONCLUSION
Pyridopyrimidines are versatile class of heterocyclic compounds with a significant potential and biological activities including anticancer, antimicrobial, antiviral, analgesic and antihistaminic. Their ability to target key enzymes and signalling pathways has made them promising candidates. Thus we can conclude that pyridopyrimidine’s unique structural framework and ongoing advancement in structural modification enhances their potential as scaffold for innovative drug development.
4. Future Perspectives
The review highlights that pyridopyrimidine scaffolds exhibit a wide range of beneficial biological activities, including anti-cancer, anti-viral , anti-bacterial, anti-histaminic and analgesic effects. This indicates a promising avenue for future research. The development of pyridopyrimidine derivatives with these properties suggests they could provide effective and selective therapeutic options with fewer unwanted side effects.
5. ACKNOWLEDGEMENT
The authors are thankful to MAR DIOSCORUS COLLEGE OF PHARAMCY management, Thiruvanathapuram , for providing all facilities for the present investigation.
REFERENCES
- Shweta Agarwal and Ranjana Mehrotra, An overview of Molecular Docking, JSM Chemistry, 4(2), 1024
- Wadood A, Ahmed N, Shah L, Ahmad A, Hassan H, Shams S. In-silico drug design: An approach which revolutionarised the drug discovery process. OA Drug Design & Delivery 2013 Sep 01;1(1):3.
- Monisha Sivanandhan, Amutha Parasuraman, In silico Molecular Docking and ADMET predictions of Pyrido[2,3-d]pyrimidine-2,4(1H,3H)-Dione Analogues as promising Antimicrobial, Antioxidant and Anticancer agents, Polycyclic Aromatic Compounds.2024, 44,(2),1273-1290.
- Pratiba Yadav and Kamal Shah; Pyridopyrimidines as anticancer agents; ECS Trancation, 2022, 107(1), 11577.
- Mohamed M. Hammouda, Khaled M. Elattar Ayman Y. El Khateeb · Sahar E. Hamed Amany M. A. Osman, Developments of pyridodipyrimidine heterocycles and their biological activities, 2023, 28, 927–964 , https://doi.org/10.1007/s11030-023-10623-9
- Salwa F. Mohamed, Dina H. Elnaggar, Heba S. Abd-Elghaffar, Mohamed A. Elsayed, Abd El-Galil E. Amr, Eman S. Abou-Amra, Dina N. Abd-elshafy, Synthesis, antiviral screening, and in silico investigations of new pyrimidine and pyridopyrimidine derivatives: Finding new therapeutic prospects to combat the herpes simplex virus (HSV-1), Journal of Molecular Structure, February 2025, 1322, 1(15) 140246.
- F. Buron, J.Y. Merour , M. Akssira , G. Guillaumet , S. Routier , Recent advances in the chemistry and biology of pyridopyrimidines, European Journal of Medicinal Chemistry,2024, 95,76-95
- Adarsh Kumar, Kuber Kumar Bhagat, Ankit Kumar Singh, Harshwardhan Singh, Tanuja Angre, Amita Verma, Habibullah Khalilullah, Mariusz Jaremko, Abdul-Hamid Emwase and Pradeep Kumar, Medicinal chemistry perspective of pyrido[2,3-d] pyrimidines as anticancer agents, RSC Adv., 2023, 13, 6872–6908.
- M. M. Gineinah, M. N. Nasr, S. M. Badr and W. M. ElHusseiny, Synthesis and antitumor activity of new pyrido [2,3-d]pyrimidine derivatives, Med. Chem. Res., 2013, 22(8), 3943–3952.
- K. M. Abu-Zied, T. K. Mohamed, O. K. Al-Duiaj and M. E. Zaki, A simple approach to fused pyrido[2,3-d] pyrimidines incorporating khellinone and trimethoxyphenyl moieties as new scaffolds for antibacterial and antifungal agents, Heterocycl. Commun., 2014, 20(2), 93–102.
- Nuran Kahrimana, K?vanç Pekera, Vildan Serdaro?lua, Ali Ayd?nb , Asu Ustac, Seda Fandakl?a, Nurettin Yayl?, Novel 2-amino-4-aryl-6-pyridopyrimidines and N-alkyl derivatives: Synthesis, characterization and investigation of anticancer, antibacterial activities and DNA/BSA binding affinities, Bioorganic chemistry, 2020, 99, 10385.
- José M. Quintela, a Carlos Peinador, a Luis Botana, b Manuel Estévez b and Ricardo Riguera, Synthesis and Antihistaminic Activity of 2-Guanadino-3- cyanopyridines and Pyrido [2,3-d] -pyrimidines, Bioorganic & Medicinal Chemistry, 1997, Vol. 5, No. 8, pp 1543-1553.
- Ichiro Takasaki , Ai Watanabe, Takuya Okada, Daisuke Kanayama, Ryota Nagashima , Miyu Shudo, Ayaka Shimodaira, Kazuto Nunomura, Bangzhong Lin, Yurie Watanabe, Hiroaki Gouda, Atsuro Miyata, Takashi Kurihara, Naoki Toyooka, Design and synthesis of pyrido[2,3-d]pyrimidine derivatives for a novel PAC1 receptor antagonist, European Journal of Medicinal Chemistry, 2022, 231, 114160.
- Tu S, Wang Q, Xu J, Zhu X, Zhang J, Jiang B, Jia R, Zhang Y, Zhang J, An efficient onepot synthesis of 5-aryl substituted 2-amino-5,8-dihydropyrido[2,3-D] pyrimidin-4,7- diones under microwave irradiation without catalyst. J. Heterocyclic Chem, 2006, 43, 855–858. https://doi.org/10.1002/jhet.5570430407.
- Tu S, Zhang J, Zhu X, Xu J, Zhang Y, Wang Q, Jia R, Jiang B, Zhang J, New potential inhibitors of cyclin-dependent kinase 4: Design and synthesis of pyrido[2,3- d]pyrimidine derivatives under microwave irradiation. Bioorg. Med. Chem. Lett., 2006, 16, 3578–3581. https://doi.org/10.1016/j.bmcl.2006.03.084.


 Dr. S. Sreeja*
Dr. S. Sreeja*
 Abin Alex Binoy
Abin Alex Binoy
 S. R. Anchu
S. R. Anchu
 V. S. Athira
V. S. Athira
 L. Venkitachalam
L. Venkitachalam
 K. Nandhini
K. Nandhini













 10.5281/zenodo.14722775
10.5281/zenodo.14722775