Abstract
Paederia foetida is a plant with medicinal properties and is known for its diverse bioactive compounds such as beta-sitosterol and ursolic acid. It has been extensively utilized in both medicine and cooking by various communities in north India and Assam. The plant demonstrates numerous pharmacological characteristics, like anti diarrheal, Anti Ulcer including anti-inflammatory, antioxidant, ant diabetic, and anticancer effect. Even though the plant is traditionally used as a remedy for diarrhea and ulcers, we are examining the effects of the ethanol extract of Paederia foetida leaf on diarrhea induced by castor oil and ulcers induced by pyloric ligation in a Wister rat model. Investigations into the anti-diarrheal activity of these extracts at specified groups and concentrations involved performing castor-oil induced diarrhea and gastrointestinal motility tests. Additionally, and for the pylorus ligation induced ulcer model in Wister rats, Gastric protection was evaluated by assessing various parameters like gastric volume, total acidity, free acidity, and percentage inhibition of ulceration and also an acute-toxicity test was conducted using five different doses. The extract of plat leaf administrate orally to the Wister rat of the both ulcer and diarrheal model. For Diarrheal model we use loperamide as standard drug and for ulcer model we use ranitidine as standard drug. After evaluating and compare with stander drug for both models the extract gave significant result.
Keywords
Paederia foetid, castor oil induce diarrhea, Pyloric Ligation induce Ulcer, Antioxidant - DPPH assay.
Introduction
Diarrhea and ulcer is major health problem in developing countries now a days. The morbidity rate increases specially for infant and child. Diarrhea occurs due to an imbalance in electrolyte and fluid absorption and increases gastric mortality. The imbalance between water and sodium absorption is the major cause of diarrhea [1,2] . The peptic ulcer caused by an imbalance of gastric hydrochloric acid and also imbalance various enzyme imbalance and also cause oxidative stress [3]. In India various communities take various herbal plants for treatments of various diseases. Now a day WHO also approaches for herbal medication used for treatments of less adverse effects. Specially in north east of India Paederia foetida used for the treatment of diarrhea, ulcer rheumatoid arthritis, constipation, hepatic disorders, diabetes, itches, wounds, stomachache, coughs, asthma, pain, toothache, cancer, flatulency, body ache, typhoid, pneumonia, and bone fractures etc[4]. Paederia foetida belongs to the Rubiaceae family is a herbal traditional plant that plays a very important role in the treatment of various diseases. Paederia foetida is a fast-growing climbing plant, the length of the plant stem is 1.5 to 7 meters long[5]. The plant content pederin, paederone, and hentai content, iridoid glycoside, cetyl alcohol, volatile oil, palmitic acid, sitosterol, etc. The plant have foetida aroma due to the presence of methyl mercaptan[6,7]. The Paederia foetida plant leaf ethanolic extract have significant anti diarrheal and anti ulcer effect in Swiss–Wister strain mice by inhibiting G.I. motility and increasing water absorption from intestine and increasing gastric mucosal layer and decreasing acid secretion.[8,9].
MATERIALS AND METHODS:
Plant material:
The plant (Paederia foetida) was collected from Tezpur market, Assam, and authenticated by Department of Molecular Biology and Biotechnology, Tezpur University, Tezpur, Assam, India.
Preparation of extract:
Fresh leaves were washed thoroughly with distilled water and dried in the shade in a clean environment. The dried leaves were ground into powder by using an electrical grinder after which 500 gm of the coarse powder was suspended in 70% ethanol in a conical bottom flask and allowed to stand for 72 hours. The mixture was shaken at regular intervals. After 72 hours, the sample was filtered using Whatmann’s No 1 filter paper. The filtrates were then concentrated at 50o C using a rotary evaporator. Then the extract was refrigerated at 4o C until required for use [10,11].
Antioxidant activity:
Determination of Antioxidant Activity by DPPH scavenging assay:
This is known as a standard 2-2-Diphenyl-1-1picrylhydrazyl (DPPH) Assay. The DPPH assay is popular in natural product antioxidant studies. This assay is based on the theory that a hydrogen donor is antioxidant. The antioxidant effect is proportional to the disappearance of DPPH in test samples. Monitoring with a UV spectrometer has become the most commonly used method because of its simplicity and accuracy. DPPH shows a strong absorption maximum at 517 nm (purple). The color turns from purple to yellow followed by the formation of DPPH upon absorption of hydrogen from an antioxidant. This reaction is stoichiometric with respect to the number a hydrogen atoms absorbed. Radical scavenging activity of the ethanolic extract of Paederia foetida against stable DPPH was determined spectrophotometrically. Ascorbic acid used as a standard for this assay. 10mg of ascorbic acid was dissolved in 10ml of methanol to give concentrations of 20, 40, 60, 80, 100 µ/ml. Stock solutions of samples were prepared by dissolving 10mg of extract in 10 ml of ethanol to give concentration of 20,40, 60, 80, 100 µ/ml . 1ml of 0.3 Mm DPPH solutions was added to 2ml of each different concentrations of standard solution and incubated at dark for 30 mins at room temperature after it has been shaken vigorously. 1ml of 0.3 Mm DPPH solutions was added to 2ml of each different concentrations of standard solution and incubated at dark for 30 mins at room temperature after it has been shaken vigorously. 1ml of 0.3 Mm DPPH solutions was added to 2ml of methanol and this solution was taken as control and allowed to incubate at dark for 30 mins at room temperature. After 30 min, absorbance was measured at 517 nm taking methanol as blank using Multi Plate Reader (Spectramax plus 384). The percentage of inhibition was calculated by comparing the absorbance values of the control and test samples. All the tests were performed in triplicate. Ascorbic acid was used as a reference compound. The capability to scavenge the DPPH radical was calculated as the inhibition percentage of free radical by the sample/standard using the following formula: % Inhibition of DPPH scavenging activity = Ao-At/Ao *100, Where Ao is the absorbance of the control reaction and At is the absorbance of test/standard[12-16].
Animals:
Male and Female Wister rats (180–250 g body weight) bred in the Animal House Facility of the Defense Research Laboratory, Defense Research & Development Organization, Tezpur, Assam, were used for the experiments. The animals were provided with standard pelleted feed and clean potable water ad libitum and maintained at natural day/night cycle at an ambient temperature of 27±1ºC. All the animals were acclimatized for 10days in the laboratory environment prior to the study. For conducting all experiments with animals, we followed the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (Institute Regd. No.1127/GO/RBi/S/08/CPCSEA Dated: 27/03/20217 and experiment IAEC approval no 07 dated: 30/12/2020)
Acute toxicity test:
Thirty animals are taken and divided in to five groups for investigating acute toxicity and estimated LD50 values. The ethanolic extract was dissolved with CMC as vehicle. The extract administered orally to individual group according to body weight by following dose 100mg/kg, 200mg/kg, 400mg/kg, 800mg/kg and 1600mg/kg. LD50 was evaluated by recording mortality [17].
Ant diarrheal Activity:
Castor oil induced diarrhea:
We followed the method of Awouters et al to evaluate the ant diarrheal activity of 70% ethanolic extract of Paederia foetida leaf. Twenty-four animals were taken and randomly divided into four groups. The groups comprised of six animals each. The animals were fasted for 18 hours before the experiment but were provided with potable drinking water ad-libitum. The groups were as follows: Group I (Treated with CMC p.o. as per the volume given in treatment groups), Group II (Treated with Loperamidei.p.o. 5mg/kg), Group III ( Treated with PFEE p.o. 200mg/kg), Group IV ( Treated with PFE E p.o. 400mg/kg). After one hour the animals were given castor oil10ml/kg (0.965g/ml) for inducing diarrhea. Animals were placed back into individual cages and lined with white blotting paper. The paper was changed every one hour to observe each stool count and continue the process up to four hour. All wet and dry feces were counted and noted for individual animal. Then the total number of excreted feces was compared with control groups whereas total number of diarrheal feces of the control group was considered 100% [18-20].
Gastrointestinal motility test:
We carried out the experiment of gastrointestinal motility test according to the method described by Mascolo et al. Twenty-four animals were taken and randomly divided into four groups. The groups comprised of six animals each. The animals were fasted for 18 hours before the experiment but were provided with potable drinking water ad-libitum. The groups were as follows Group I (Treated with normal saline p.o. as per the volume given in treatment groups), Group II (Treated with Loperamidei.p.o. 5mg/kg), Group III (Treated with PFEEp.o. 200mg/kg), Group IV (Treated with PFEEp.o. 400mg/kg). After one hour the animals were given castor oil 10ml/kg(0.965g/ml) for inducing diarrhea.One hour after inducing diarrhea, Animals were given charcoal preparation (10% charcoal suspension in 5% gum acacia). ). One hour later animals were sacrificed by overdose of chloroform anesthesia and the distance traveled by the charcoal preparation from pylorus to caecum was measured [21-23].
Anti- ulcer studies in rat model:
Pylorus ligation(p.l.) induced ulcer:
Twenty-five animals were taken and randomly divided into five groups. The groups comprised of five animals each. The animals fasted for 24 hours before the experiment but provided with potable drinking water ad-libitum. The groups were as follows, Group I (Treated with CMC p.o. as per the volume given in treatment groups), Group II (Treated with CMC p.o. as per the volume given in treatment groups + P.L.), Group III (Treated with PFEE p.o. 100mg/kg + P.L.), Group IV (Treated with PFEEp.o. 200mg/kg +P.L.), Group V ( Treated with Ranitidine p.o. 10mg/kg + P.L). After 30 min of administration of drug pylorus ligation were done. After four-hour animals were sacrificed by over dose of ether[24,25].
Surgical procedure: After the treatment given to the rat the surgical procedure was done. The rats were anesthetized by diethyl ether. The abdomen of rat was opened by a midline incision. The stomach was taken out very carefully and legated the pylorus tightly by silk threat. After the ligation the stomach was put back to the position. The muscular and skin layer was stitched properly. Four-hours later the animals were sacrificed by overdose of diethyl ether and stomach was taken out, gastric juice was collected and the following processes were followed[25].
Gastric volume:
After 4hr of pyloric ligation the animals were sacrificed by over dose of diethyl ether. The stomach was excided out and washed with Luke worm water. The stomach content was taken and centrifuged at 4000rpm for 10min and the supernatant volume was measured as gastric volume [25].
Determination of total acidity and free acidity:
After 4hrs of pyloric ligation the animals were sacrificed by over dose of diethyl ether. The stomach was excided out and washed with Luke worm water. The stomach content was taken and centrifuged at 4000rpm for 10min the supernatant was collected. One ml of supernatant was taken in a conical flask and add 2-3 drop of topfer’s reagent. The gastric juice solution titrated with 0.1(N) NaOH, the red color turn into yellowish orange color. After that the volume was noted, it represented free acidity. Then in the titrated solution add 2 drop of phenolphthalein was added and again titrated with 0.1(N) NaOH solution and until red color was seen. The volume represents total acidity[25,26].
Estimation of total protein:
After 4hrs of pyloric ligation the animals were sacrificed by over dose of diethyl ether. The stomachs was excised out and washed with Luke worm water. The stomach content was taken and centrifuged at 4000rpm for 10min then1 ml of gastric juice was add with 9ml of 90% alcohol 0.1ml of alcoholic precipitate of gastric juice taken add 1ml of 0.1(n) NaOH 0.05ml solution take 4ml of NaOH added (kept for 10 min) then 0.4ml of phenol reagent was added (kept for 10 min) and absorbance was checked at with 610nm [25, 27].
Estimation of mucsin:
After 4hrs of pyloric ligation the animal was sacrificed by over dose of diethyl ether. The stomach was excised out and washed with Luke worm water. Stomach tissues were opened. The stomach tissues soaked for 1 hour in 0.1% alcian blue 8gx dissolved in 0.16(m) sucrose buffer with 0.05(m) sodium acetate and Ph should be 4.2 washed with 0.25(m) sucrose solution at 15, 45 mins tissue dip in 0.5(m) 10m MgCl2 for 1 hr add 10 ml diethyl ether absorbance in 605nm[25,28].
Histology:
After 4hr of pyloric ligation the animals were sacrificed by over dose of diethyl ether. The stomach was excised out and washed with Luke worm water. Stomach tissues were opened and cleaned properly. The stomach tissues were fixed with 10% neutral buffer formalin for 24hrs. After that the tissue was washed in running water for overnight. The tissues start dehydrating process by 50% alcohol and continue the process with 70%,95% and 100% alcohol. After that it was placed in Xylene and make paraffin blocks were made. Tissue cut in 5micron section. After that tissue fixed with slide by Meyers albumin and stained with hematoxylin and eosin. After that the tissues mount with cover slip by mounting liquid and go for microscopically evaluation [25, 29, 30]
RESULTS:
Antioxidant studies: DPPH scavenging activity:
The radical scavenging activity of 70% ethanolic extract of Paederia foetida is evident in the DPPH assay, where it was seen that the purple color changed to yellow colour after the hydrogen donated by the antioxidant was accepted by the DPPH radical thereby changing the colour that was read at 517 nm. The extract showed a strong hydrogen donating capacity and can powerfully scavenge DPPH radical. The maximum free radical scavenging activity and potency was interpolated from figure to give results as shown in table 1.
Table 1: DPPH radical scavenging activity of ascorbic acid and 70% Paederia foetida extracts.
|
Concentration(µg/ml)
|
Ascorbic Acid
|
Extract
|
|
20
|
55.1297
|
58.9466
|
|
40
|
71.299
|
79.3364
|
|
60
|
82.3965
|
82.3965
|
|
80
|
94.4274
|
86.8416
|
|
100
|
98.3894
|
95.3164
|
Acute toxicity test:
P. foetida leaf 70% ethanolic extract was given up to 1600 mg/kg body weight for the purpose of determining the LD50 dose. Throughout the course of the 14-day observation period, we saw no overt indications of acute toxicity or mortality.
Ant diarrheal Activity:
Castor oil induced diarrhea:
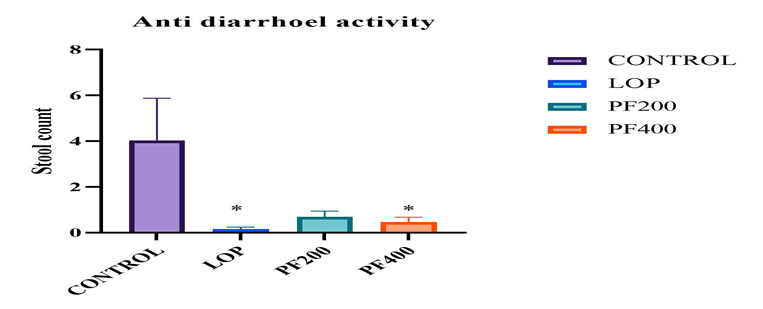
In the castor oil induced diarrhea experiment the Paedera foetida leaves extract produce significant anti-diarrheal effect on Wister rat. The ethanolic extract dose of 200mg/kg and 400mg/kg display significant (p<0>± 0.24(PFEE 200mg/kg), 0.266 ± 0.179 (PFEE 400mg/kg) and 0.166 ± 0.117 (LP 5mg/kg)
Table - 2: Effect of PFEE on castor oil induce diarrhea.
|
Anti-Diarrheal Model
|
|
Group
|
Treatment
|
Feces count (Mean ±Sem)
|
|
Control
|
Castor oil 10ml/kg
|
4.03 ± 0.71
|
|
Positive
|
Loperamide 5mg/kg
|
0.166 ± 0.117
|
|
Extract low dose
|
PFEE 200mg/kg
|
0.6 ± 0.24
|
|
Extract high dose
|
PFEE 400mg/kg
|
0.266 ± 0.179
|
*[Values are expressed as mean±SEM (n=6). *p<0>
Gastrointestinal motility test
Figure - 1: Number of diarrheal Feces (Effect of PFEE on castor oil induce diarrhea)
The extract of P. foetida have significantly (p<0>± 5.42 cm, positive group (loperamide) 34.41 ± 3.71 cm, the low dose of extract group 64.21 ± 4.91 cm and the high dose of extract group 39.33 ± 3.61 cm.
Table - 3: Effect of PFEE to decrease the gastric motility.
|
Gastric Motility Test
|
|
Group
|
Treatment
|
Distance travel (Mean ±Sem)
|
|
Control
|
Castor oil 10ml/kg
|
92.5± 5.42
|
|
Positive
|
Loperamide 5mg/kg
|
34.41 ± 3.71
|
|
Extract low dose
|
PFEE 200mg/kg
|
64.21 ± 4.91
|
|
Extract high dose
|
PFEE 400mg/kg
|
39.33 ± 3.61
|
*[Values are expressed as mean±SEM (n=6). * p<0>

Figure - 2: Distance travel of charcoal in pylorus to cecum
Anti- Ulcer Studies In Rat Model
Gastric volume:
The Peaderia foetida leaves extract gave significant (p<0>± 0.89, for control group (CMC+P.L.) 6.66 ± 0.52, for standard (RANITIDINE+P.L.) group 2.7 ± 0.11, for extract low dose (PFEE 100mg/kg) group 4.28 ± 0.29 and for extract high dose (PFEE 200mg/kg) group 3.1 ± 0.12.
Table - 4: Effect of PFEE on gastric volume.
|
Gastric volume
|
|
Group
|
Treatment
|
Mean ± Sem
|
|
Negative control
|
CMC
|
1.80 ± 0.89
|
|
Control
|
CMC+PL
|
6.66 ± 0.52
|
|
Standard
|
RANITIDINE+PL
|
2.7 ± 0.11
|
|
PFEE low dose
|
PF-100+PL
|
4.28 ± 0.29
|
|
PFEE high dose
|
PF-200+PL
|
3.1 ± 0.12
|
All the values are mean±SEM n=5. ***P<0>
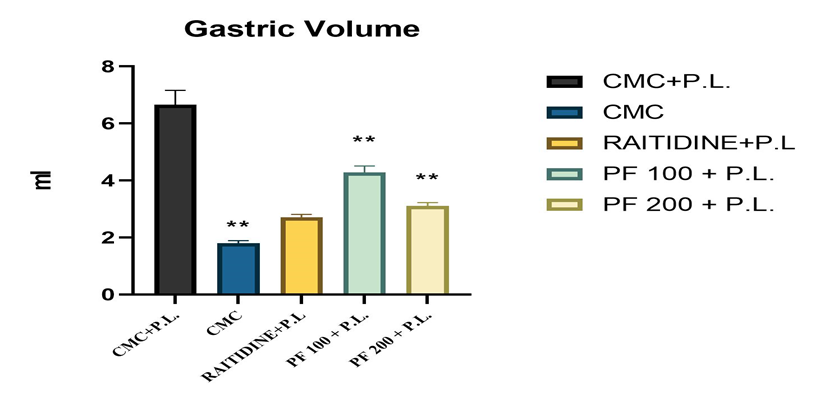
Figure - 3: Effect of PFEE on gastric volume.
Determination of Total Acidity and Free Acidity:
The Paederia foetida leaf extract gave significant (p<0>± 2.55, for the positive control (CMC 10ml/kg +P.L) group 87.2 ± 2.72, for the standard treatment (Ranitidine 5mg/kg+P.L.) group 30 ± 2.5, for the low dose of PFEE extract (PFEE 100mg/kg + P.L.) group 46.8 ± 2.65 and for the high dose of PFEE extract (PFEE 200mg/kg + P.L.) group36.60 ± 1.43. The value for the total acidity for the negative control (CMC 10mg/kg) group76.8 ±2.87, for the positive control (CMC 10ml/kg +P.L) group 128.6 ± 3.72, for the standard treatment (Ranitidine 5mg/kg+P.L.) group 37.80 ± 2.35, for the low dose of PFEE extract (PFEE 100mg/kg + P.L.) group 52 ± 1.89and for the high dose of PFEE extract (PFEE 200mg/kg + P.L.) group 41.9 ± 2.41.
Table - 5: Effect of PFEE on free acidity and total acidity
|
Acid Output
|
Free Acidity (Mean ±Sem)
|
Total Acidity (Mean ±Sem)
|
|
CMC
|
65.2 ± 2.55
|
76.8 ±2.87
|
|
CMC+PL
|
87.2 ± 2.72
|
128.6 ± 3.72
|
|
RANITIDINE+PL
|
30 ± 2.5
|
37.80 ± 2.35
|
|
PF-100+PL
|
46.8 ± 2.65
|
52 ± 1.89
|
|
PF-200+PL
|
36.60 ± 1.43
|
41.9 ± 2.41
|
All the values are mean±SEM n=5. ***P<0>
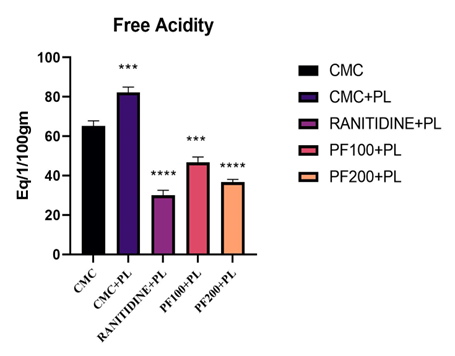
Figure - 4: Effect of PFEE on Free Acidity.

Figure - 5: Effect of PFEE on Total Acidity.
Estimation of total protein:
The Paederia foetida leaf extract reduce the protein secretion on stomach compare to control and standard with the PFEE groups. The value of the protein in stomach for negative control group (CMC) 87.80 ± 4.01, for control group (CMC+P.L.) 93.20 ± 4.7, for standard (RANITIDINE+P.L.) group 86 ± 3.4, for extract low dose (PFEE 100mg/kg) group 80 ± 2.8 and for extract high dose (PFEE 200mg/kg) group 87.60 ± 2.64.
Table - 6: Effect of PFEE on Total Protein
|
Total Protein
|
|
Group
|
Treatment
|
Protein(MEN±SEM)
|
|
Negative control
|
CMC
|
87.80 ± 4.01
|
|
Positive control
|
CMC+PL
|
93.20 ± 4.7
|
|
Standard
|
RANITIDINE+PL
|
86 ± 3.4
|
|
PFEE low dose
|
PF-100+PL
|
80 ± 2.8
|
|
PFEE high dose
|
PF-200+PL
|
87.60 ± 2.64
|
All the values are mean±SEM n=5. ***P<0>

Figure - 6: Protein estimation of stomach tissue
Estimation of mucsin:
The Peaderia foetida leaves extract gave significant (p<0>± 8.21, for control group (CMC+P.L.) 222.4 ± 6.08, for standard (RANITIDINE+P.L.) group 293.6 ± 7.48, for extract low dose (PFEE 100mg/kg) group 238.8 ± 4.79 and for extract high dose (PFEE 200mg/kg) group 288.6 ± 6.60.
Table - 7: Effect of PFEE on Total Mucsin
|
Total Mucsin
|
|
Group
|
Treatment
|
Mucsin(MEN±SEM)
|
|
Negative control
|
CMC
|
171.2 ± 8.21
|
|
Positive control
|
CMC+PL
|
222.4 ± 6.08
|
|
Standard
|
RANITIDINE+PL
|
293.6 ± 7.48
|
|
PFEE low dose
|
PF-100+PL
|
238.8 ± 4.79
|
|
PFEE high dose
|
PF-200+PL
|
288.6 ± 6.60
|
All the values are mean±SEM n=5. ***P<0>

Figure - 7: Musin estimation of stomach tissue
Histopathological Studies:
In the histological studies as shown in the Figure 8, severe inflammatory cell infiltration in the mucosa and submucosa, as well as diffuse mucosal ulcers, are observed in the control group. Mostly necrosis and acute inflammatory cells were seen in a diffuse mucosal ulceration, and necrosis was also seen in some of the glands. Lamina propria, muscularis mucosa, and intact epithelium are present in the stomach mucosa of the group that received a modest dose of PFEE (100 mg/kg). Moderate mononuclear inflammatory infiltration is observed in the epithelial cells and the submucosa exhibits oedema with few congested vascular spaces. Lamina propria, muscularis mucosa, and intact epithelium are all present in the stomach mucosa of the group that received a high dose of PFEE (200 mg/kg) in the region under study. There is minimal mononuclear inflammatory infiltration and sparse vascular gaps when the epithelial cells intervene. Lamina propria, muscularis mucosa, and intact epithelium are all present in the stomach mucosa of the standard group that received ranitidine (10 mg/kg) in the region under study. Mononuclear inflammatory infiltration and clogged vascular spaces are observed interfering with the epithelial cells. Vascular areas are dilated and crowded in the connective tissues stroma that makes up the submucosa. It looks like a normal muscularis propria.
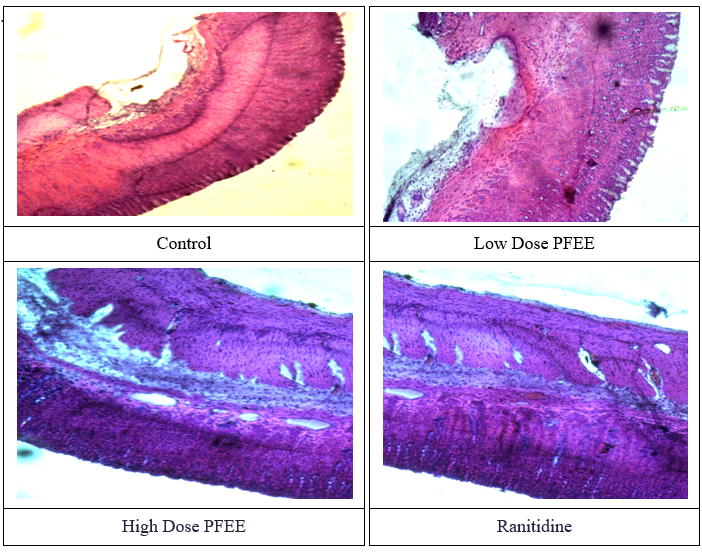
Figure – 8: Microscopically Evaluation of Wister Rat stomach Tissue.
DISCUSATION:
Paederia foetida leaves use as a herbal traditional medicine for long time in various tribal community in India. Not only the plant leaves but also the bark of the plant is also used for the treatment of the patients. The ethanolic extract of plant leaves have significant (p<0>
Ulcer is the most common disease now a days. The most common cause of ulcer is the increase gastric acid secretion and damage of the mucosal layer associated with helicobacter pylori interaction. The plant leaves extract emerges as new treatment of the ulcer management. As we know castor oil increases the secretion and decreases absorption so the stomach acid secretion will increase. In the pyloric ligation model acid content varies which induces acute ulcer in rat stomach. The standard group ranitidine works as a proton pump inhibitor which reduces the gastric acid secretion. The plant leaves extract inhibits the hyper secretary activity which reduces the gastric acid secretion. Also act as mucosal protective agent that’s why it helps to reduces the ulcerogenic activity. In the above experiment we see that as the plant leaves inhibit the hyper secretary activity. It reduces the gastric acid secretion as well as it reduces the gastric volume and it was established by this experiment. The plant leaf extract hasw significantly anti-diarrheal and anti-ulcer effect in Wister rat models from above the experiment.
CONCLUSION:
The 70% ethanolic extract of Paederia foetida leaves have significant activity as anti-diarrheal and anti-ulcer effect from overall experiment. The anti-diarrheal activity is due to decrease in the gastric motility and the anti-ulcer activity due to inhibition of the gastric acid secretion and protect the mucosal layer of stomach. The advance research needs to identify the specific compounds of plant which act as anti- diarrheal and anti-ulcer agents in specific pathway.
Conflict of interest
There is no conflict of interest.
Author’s Contribution
Concept and execution Mir Irfan Soyel and Malay Besra Resource compilation Nilanjan Adhikari, Amartya Banerjee
Proof reading and editing Dr. Biplab Kumar Chakra
ACKNOWLEDGEMENT
We are grateful to the Defense Research Laboratory (DRL), Defense Research and Development Organization and P.G. Institute of Medical Sciences for providing the necessary resources and facility.
REFERENCES
- Coker MF, Berky S, Pandou C. New development in acute diarrhea current problem. Paediatrics. 1998;24:15?17.
- Besra, S.E., Gomes, A., Chaudhury, L., Vedasiromoni, J.R., Ganguly, D.K., 2002. Antidiarrhoeal activity of seed extract of Albizzia lebbek Benth. Phytotherapy Research 16, 529–533.
- Silpi Chanda, Lokesh Deb, Raj Kumar Tiwari , Kuldeep Singh and Sayeed Ahmad Gastroprotective mechanism of Paederia foetida Linn. (Rubiaceae) – a popular edible plant used by the tribal community of North-East India, BMC Complementary and Alternative Medicine, 2015:304:2-9
- Liang Wang, Yiping Jiang, Ting Han, Chengjian Zheng and Luping Qin A Phytochemical, Pharmacological and Clinical Profile of Paederia foetida and P. scandens Natural Product Communications2014: Vol. 9 (6):880
- Capasso, F., Mascolo, N., Izzo, A.A., Gaginella, T.S., 1994. Dissociation of castor oil-induced diarrhoea and intestinal mucosal injury in rat: effect of NG-nitro-l-arginine methyl ester. British Journal of Pharmacology 113,1127–1130.
- Prodip Roy, Hira Das, Mir Samim Ali, Pradipta Sarkar and Soumi Chattopadhyay a review on paederia foetida as a medicinal plant and it's pharmacological activities, World Journal of Pharmacy and Pharmaceutical Science, 2021: Vol 10:637
- Basu KR, Basu BD. Indian medicinal plants. In: Bishen Singh, Mahendra P Singh, editors. 1987;4(2):1796?1799.
- Khushbu, C., Anar, P., Mayuree, P., Carol, M., Roshni, S. and Subodh, Paederia foetida Linn. As a potential medicinal plant, A Review. Journal of Pharmacy Research 2010:3(12), pp.3135-3137.
- Coker MF, Berky S, Pandou C. New development in acute diarrhea current problem. Paediatrics. 1998; 24:16?18.
- Mohanasundari C, Natarajan D, Srinivasan K, Umamaheshwari S, Ramachandran A. Antibacterial properties of Passifl ora foetida a common exotic medicinal plant. Afr J Biotechnol 2007; 6:2650-3.
- Khandelwal KR. Practical Pharmacognosy. 11th ed. Pune: Nirali Prakashan; 2004. p. 149-56.
- S. Afroz a, M. Alamgir a,b, M.T.H. Khanc,d, S. Jabbar c,N. Nahar a, M.S.K. Choudhuri, Antidiarrhoeal activity of the ethanol extract of Paederia foetida Linn. (Rubiaceae), Journal of Ethnopharmacology 2006:105:125-130.
- Lu, Y.; Shipton, F. N.; Khoo, T. J.; Wiart, C. Antioxidant activity determination of Citronellal and crude extracts of Cymbopogon citratus by 3 different methods. Pharmacol. Pharm. 2014; 5: 395–400.
- Khalil H, Ismail H, Taye A, Kamel M. Gastroprotective effect of Lippa nodifl ora L. extracts in ethanol induced gastric lesions. Pharmacog Mag 2007; 3:973-1296.
- Sedlak J, Lindsay RH. Estimation of total protein- bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 1968; 25:1192-205.
- Kind PR, King EJ. Determination of serum alkaline phosphatase. Clin Path 1954; 7:322.
- Litchfield JT, Wilcoxon F. A simplified method of evaluating dose?effect experiments. J Pharmacol Exp Ther. 1949; 96:99?113.
- Awouters F, Niemegeers CJE, Lenaerts FM, et al. Delay of castor oil diarrhea in rats; a new way to evaluate inhibitors of prostaglandin biosynthesis. J Pharmacol Pharma. 1998;30(1):41?45.
- Besra, S.E., Gomes, A., Chaudhury, L., Vedasiromoni, J.R., Ganguly, D.K., 2002. Antidiarrhoeal activity of seed extract of Albizzia lebbek Benth. Phytotherapy Research 16, 529–533.
- Capasso, F., Mascolo, N., Izzo, A.A., Gaginella, T.S., 1994. Dissociation of castor oil-induced diarrhoea and intestinal mucosal injury in rat: effect of NG-nitro-l-arginine methyl ester. British Journal of Pharmacology 113, 1127–1130.
- Donowitz, M., Welsh, M.J., 1987. Regulation of mammalian small intestine, electrolyte secretion. In: Johnson, L.R. (Ed.), Physiology of the Gastrointestinal, Tract. Raven Press, New York, pp. 1351–1388.
- Gaginella, T.S., Phillips, S.F., Dozois, R.R., Go, V.L.W., 1978. Stimulation of adenylate cyclase in homogenates of isolated intestinal epithelial cells from hamsters. Effects of gastrointestinal hormones, prostaglandins, and deoxycholine and recinoleic acid. Gastroenterology 74, 11–15.
- Mascolo N, Izzo AA, Autore G, et al. Nitric oxide and castor oil induced diarrhea. J Pharmacol Exp Ther. 1994; 268(1):291?295.
- R. Sathish, Alok Sahu, K. Natarajan Antiulcer and antioxidant activity of ethanolic extract of Passiflora foetida L. Indian Journal of Pharmacology, June 2011, Vol 43 336-337
- Silpi Chanda, Lokesh Deb, Raj Kumar Tiwari, Kuldeep Singh and Sayeed Ahmad, Gastroprotective mechanism of Paederia foetida Linn. (Rubiaceae) – a popular edible plant used by the tribal community of North-East India, Chanda et al. BMC Complementary and Alternative Medicine (2015) 15:304, 3-4
- Hawk PB, Oser BL, Summerson HW. Practical Physiological Chemistry. 12th Edn. London: Churchill; 1947; 347p.
- Lowry CH, Rose borough NI, Farr AL, et al. Protein Measurement with Folin Phenol Reagent. J Biol Chem. 1951; 193: 265–75p.
- Goel RK, Bhattacharya SK. Gastroduodenal Mucosal Defense and Mucosal Protective Agents. Indian J Exp Biol. 1991; 29: 701–14p.
- Baron TH, Langman MT, Wastell C. Stomach and Duodenum. In: Bouchier IA, (Ed.). Recent Advances in Gastroenterology. London: Churchill Livingston; 1980; 23p.
- Menguy R, Masters YF. Effects of Aspirin on Gastric Mucus Secretion. Surg Gynecol Obstet. 1965; 120: 92–98p


 Irfan Soyel*
Irfan Soyel*
 Malay Besra
Malay Besra
 Sumit Maji
Sumit Maji
 Biplab Kumar Chakra
Biplab Kumar Chakra
 Nilanjan Adhikari
Nilanjan Adhikari








 10.5281/zenodo.14325063
10.5281/zenodo.14325063