Abstract
The recent study was to investigate the synergistic activity of Chitosan, Alum, and PVA cross-linked haemostatic film to stop bleeding during the war and other accidental causes. In India around 30- 40% of uncontrolled haemorrhage responsible for trauma-related death. Formulation of haemostatic patches was done by solvent casting method Aim: To evaluate the synergistic effect of high efficacy Chitosan and Alum for controlling blood loss. Methodsc: Prepare chitosan and alum hemostatic flim and evaluate the film by Swelling ratio and Percentage moisture content. In-vitro evaluation of the haemostatic film were done by Platelet count and Whole blood clotting test. In-vivo evaluation of the haemostatic film were done by Rat liver laceration model, Rat jugular vein model and Rat femoral artery model under these models we evaluate Haemostatic time measurement. After that we have go for histological evaluation of lasiration liver tissue. Results: The haemostatic film prepare properly and the all the evaluation were done properly according to mention protocol. We observed good result of chitosan alam haemostatic film compare to alam and chitosan film. Conclusion: A complex was produced by cross-linking with Chitosan, alum, and polyvinyl alcohol to form a haemostatic film through the solvent casting method. By using in vitro blood clotting models and in vivo animal bleeding models, it can be determined that the synergistic qualities of the haemostatic agents alum and chitosan produce a greater haemostatic effect. As a result, the haemostatic film can be applied to blood loss management. The mechanism of action of homeostasis will be improved by more assessment of the several molecular pathways linked to blood coagulation.
Keywords
Chitosan and Alum haemostatic film, Liver laceration model, Jugular vein model, Femoral artery model, Histopathology of liver.
Introduction
Haemorrhage is one of the major problems in the battlefield, disaster, and other accidental injuries around 30-40% of uncontrolled haemorrhages responsible for trauma-related deaths. Haemostasis is a procedure that keeps blood inside a broken blood vessel to avoid and stop bleeding. It is the first step of wound healing and blood clotting process. During haemostasis the physiological process to stop blood loss, three steps occur in a rapid sequence, a) Vasoconst-riction, b) Platelet plug formation, c) Clot formation. Such serious injury occurs with severe bleeding and leads to life-threatening. Haemostasis can be achieved by the chemical agent and mechanical agent and introduction of physical agent. In the last few decades, various novel effective haemostatic agents have been developed. [1,2] United States in 2003 approved the use of chitosan within the wound dressing to control excess blood loss and also used to decrease the growth of bacteria and fungi. Chitosan is produced by the deacetylation of chitin. Chitosan is a natural biodegradable polysaccharide extract from the marine source such as the structural element in the exoskeleton of crabs and shrimp and also obtained from the wall of fungi. Chitosan composed of D-glucosamine and N-acetyl D-Glucosamine by chitosan shells.[3] Haemostatic agents of chitosan are obtained from the chitosan salt by mixing with the organic acid. The haemostatic activity of chitosan is intracellular between the erythrocyte cell membrane which is negatively charged and the chitosan is positively charged compared leads to from thrombosis and the involvement of platelets at the site of wound chitosan have multiple application such as includes the formation of the biodegradable film, blends coating material and Nanocomposite.[4] Potassium alum is considered by FDA as a generally recognized safe substance. It is an inorganic salt with the formula Al K(SO4)2. Potassium aluminium sulphate is a metal sulphate composed of Potassium, Aluminium, and Sulphate ion in the ratio 1:1:2. Alum is freely soluble in water, insoluble in alcohol. Widely alum is used in dentistry as a haemostatic agent in the treatment of gum bleeding and chronic and acute gingivitis. The dose of Alum as an astringent is 0.5-5%. The main function of the alum is its astringent action. The astringent action is performed by the induction of the coagulation factor in the tissue layer until the crust is formed. The alum neutralizes the plasma protein and induces blood coagulation. Alum gives effective results in the inflammation of the nasal, gastrointestinal, and urinary passage and also reduces the swollen mucus membrane. In this following research work, the haemostatic activity of Chitosan Composite Alum haemostatic film will be checked. Physico-chemical properties of the haemostatic film will be assessed, as well as in-vitro evaluation of haemostatic effectiveness toxicity testing and In-vivo calibration of haemostatic effectiveness.[5,6,7]
MATERIALS AND METHODS:
Animals:
Male Wister rats (200-250 g body weight) bread in the animal house of Defence Research Laboratory, Defence Research & Development Organization, was used for the experiments. The animals were provided with standard pelleted feed and clean potable water ad-libitum and maintained at natural day/night cycle at an ambient temperature of 27±1ºC. All the animals were acclimatized for 10days in the laboratory environment prior to the study. For conducting all experiments with animals, we followed the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (Institute Regd. No.1127/GO/RBi/S/08/CPCSEA Dated: 27/03/20217 and experiment IAEC approval no 08 dated: 30/12/2020)
Preparation of Chitosan-Alum film:
The solvent casting method was used to create the formulation. Chitosan (1.5g) was dissolved in a solution of 1?etic acid. Using a magnetic stirrer, 8% PVA (polyvinyl alcohol) was applied to the solution to improve the permeability of the patch. Then, with constant stirring, add 5 g Alum until a film forms. The film was then poured onto a Petri dish and dried in a hot air oven at 40?C for 24 hrs.[8,9]
Physicochemical evaluation of Chitosan- Alum film:
Swelling ratio:
This experiment was carried out by cutting a 2x2 cm film, drying it at 40? C, and weighing it. Then it was put in a Petri dish and filled with deionised water and it was kept at room temperature for 1 hour. The final weight of the film was determined after 1 hour, and the swelling ratio was estimated.[10]
Swelling ratio = [final weight-initial weight/initial weight] ×100
Percentage moisture content:
The prepared film was weighed and kept in a desiccators containing calcium chloride at room temperature for 24 hrs. After 24 hrs the film was again weighed and the percentage moisture content was calculated.[11]
Percentage moisture content = [Initial weight- final weight/ final weight] ×100
In-vitro evaluation of the haemostatic film:
Platelet count:
Whole blood was collected from the rat into the EDTA tube. Then EDTA mixed blood was introduced with a prepared haemostatic film sample. Then previously prepared blood samples and Ammonium oxalate was mixed in a ratio of 1:20 in a micro-centrifuge tube. Then mixed blood was kept for 5 minutes for RBC lysis. Then haemocytometer was charged by the blood sample. Then charged haemocytometer was kept in the Petri dish for 5-10 min preventing dryness. Then haemocytometer was placed under the microscope at 40X to observe the platelet.[12]
Whole blood clotting test:
Rat whole blood was collected in a 0.109M (3.2%) tri-sodium citrate tube. The haemostatic film was cut into 1.0×1.0 cm and placed in the glass Petri dish. Then the haemostatic film was pre-warmed at 37?C for 5 min. About 200?l citrated whole blood was slowly be dispensed on the surface of the film. Then 20?L (0.2 M) CaCl2 solution was added. Then incubated at 37?C for 5 minutes. Then 25ml of distilled water was added carefully to the glass Petridis and were incubated for 5min at 37?C. Then the whole solution was taken in the falcon tube and was shaking at 30 rpm for 10 minutes after that absorbance of the sample was measured at 540 nm and distilled water was used as a reference. Then blood clotting index (BCI) of the haemostatic film was calculated.[13]
BCI = (Absorbance of sample/Absorbance of control) ×100
In-vivo evaluation of the haemostatic film:
Rat liver laceration model:
Twelve animals were weighed and randomly divided into 4 groups, 3 animals in each group. The group was as follows: Group 1 (Normal control), Group 2 (Treated with 1.5% Chitosan film), Group 3 (Treated with 5% Alum + Polyvinyl alcohol film), Group 4 (Treated with 1.5% Chitosan + 5% Alum + Polyvinyl alcohol film)
Surgical procedure:
The animals were given a normal diet and the furs were removed from the abdominal zone. After that, the anatomical position of the liver was determined. Rats were anesthetized by Ketamine i.p (100mg/kg). The betadine solution was applied to the skin and the abdominal cavity was opened and the incision was made by the scalpel (0.5 cm depth and 2 cm long).
Haemostatic time measurement:
After that, the haemostatic film was applied and then the haemostatic time was calculated. After controlling blood loss, the subcutaneous and the skin was closed by the suturing procedure and the analgesic was given by intraperitoneal injection. Histopathology of the liver was carried out.[14,15,16,17]
Rat jugular vein model:
Twelve animals were weighed and randomly divided into 4 groups, with 3 animals in each group. The group was as follows: Group 1 (Normal control), Group 2 (Treated with 1.5% Chitosan film), Group 3 (Treated with 5% Alum + Polyvinyl alcohol film), Group 4 (Treated with 1.5% Chitosan + 5% Alum + Polyvinyl alcohol film)
Surgical procedure:
The animals were given a normal diet and the fur was removed from the left ventrolateral neck region. After that, the anatomical position of the jugular vein was determined. Rats were anaesthetized by Ketamine i.p (100mg/kg). An incision was made centrically above the trachea and that exposes the left jugular vein. Then puncture was made on the jugular vein to achieve severe blood loss.
Haemostatic time measurement:
After that, the haemostatic film was applied and then the haemostatic time was calculated.[18,19,20,21]
Rat femoral artery model:
Twelve animals were weighed and randomly divided into 4 groups, with 3 animals in each group. The group was as follows: Group 1 (Normal control) Group 2 (Treated with 1.5% Chitosan film), Group 3 (Treated with 5% Alum + Polyvinyl alcohol film), Group 4 (Treated with 1.5% Chitosan + 5% Alum + Polyvinyl alcohol film)
Surgical procedure:
The animals were given a normal diet and the furs were removed from the left hind leg. Rats were anaesthetized by Ketamini.p (100mg/kg). After that 20-25 mm incisions were made to expose the femoral artery and vein. Then puncture was made on the femoral artery to achieve severe blood loss.
Haemostatic time measurement:
After that, the hemostatic film was applied and then the hemostatic time was calculated.[22,23,24,25]
Histology:
The animal was sacrificed by an overdose of diethyl ether, 3rd and 7th days after applying the hemostatic film at the site of liver laceration. The liver was excided out and wash with saline water. The liver tissues were fixed with 10% neutral buffer formalin for 24hr. The tissues were washed under running water for 12 hours. Dehydration procedures were performed by gradually increased methods (50%, 70%, 95%, and 100% alcohol). Then the tissue was put into the xylene and then paraffin. Blocks were made and sectioning was done at 5micron. Tissues were fixed with a slide by Meyer’s albumin and stain with hematoxylin and eosin. Then covered with coverslip by mounting liquid (D.P.X) and then microscopical evaluation was performed.[26,27,28]
RESULTS:
Preparation of Chitosan-Alum haemostatic film:
Table 1: Summarizes the quantity required for preparation of different type of film.
|
Formulation
|
Ingredients
|
| |
Chitosan (gm)
|
Potassium alum (gm)
|
Polyvinyl Alcohol (gm)
|
Acetic Acid (ml)
|
Deionized water
|
|
F1
|
1.5
|
--
|
--
|
1
|
Q.S
|
|
F2
|
--
|
5
|
8
|
--
|
Q.S
|
|
F3
|
1.5
|
5
|
8
|
1
|
Q.S
|
Figure 1: Different type of film after preparation. A) F1(Chitosan film), B) F2(Alum film), C) F3(Chitosan + Alum film)
For preparation of chitosan film we used chitosan which was dissolved in acetic acid similarly for preparation of alum film, alum was dissolved in polyvinyl alcohol and for the chitosan-alum film preparation chitosan was dissolved in acetic acid and alum dissolved in polyvinyl alcohol and mix the two solution (Table 1) and leave the solution for overnight for dry and appear properly (Figure 1).
Physicochemical evaluation of haemostatic film:
The observed result of the various physicochemical parameters of three haemostatic films (CH, alum, CH- alum) has shown. The values of swelling index and percentage moisture content of CH- alum film showed better result (Table 2).
Table 2: Evaluation parameter of the haemostatic film for three types of film.
|
Parameters
|
F1
|
F2
|
F3
|
|
%Swelling index
|
291.33
|
328.68
|
459.26
|
|
%Moisture content
|
3.04
|
4.484
|
6.731
|
In-vitro evaluation of haemostatic film:
Platelet count:
Chitosan is a potent inducer of platelet adhesion, and its mechanism of action might include increasing the expression of the GP IIb/ IIIa complex on platelet membranes. The purpose of this study was to conduct a platelet adhesion test in order to establish that chitosan has a clear effect on platelet count. The lowest level of platelet count is because of decrease of platelet count that leads of increased attraction of platelets to chitosan (Table-3) and the graphical result also shown significant (Figure-2).
Table 3: Summarizes the Platelet count of different sample haemostatic film.
|
SL.No.
|
Groups
|
Platelets count*109/L with SD
|
|
Sample 1(Mean+Sem)
|
Sample 2(Mean+Sem)
|
Sample 3(Mean+Sem)
|
-
|
Control
|
149.333 ± 21.221
|
143.66 ±10.016
|
143.33 ± 4.509
|
-
|
Chitosan
|
90.66 ± 3.78
|
91
|
92.33 ± 4.93
|
-
|
Alum
|
104 ± 3.05
|
104 ± 2.64
|
103.33 ± 3.05
|
-
|
Chitosan
+
Alum
|
105.33 ± 6.65
|
102.33 ± 5.77
|
100 ± 4.30
|
*[Values are expressed as Mean±Sem (n=3). * p<0>

Figure 2: Graphical representation of Platelet count of haemostatic film for three samples.
Whole blood clotting test:
The study on blood clotting mainly evaluates the performance of the haemostatic material to induce thrombosis in blood using an evaluation parameter called blood-clotting index (BCI). The smaller the BCI the stronger the haemostatic potential of the material. Thrombogenic activity among the three (CH, alum, and CH- alum) hemostatic films showed that the CH- alum haemostatic film had smaller BCI than the CH and alum haemostatic film The chitosan-alum film sown significant result (Table 4) in graphically also (Figure 3).
Table-4: Summarizes the blood clotting index of haemostatic film.
|
Sl. No.
|
Groups
|
Blood Clotting Index
|
|
1.
|
Control
|
100 ± 0
|
|
2.
|
Chitosan film
|
65.56 ± 6.03
|
|
3.
|
Alum film
|
80.41 ± 5.06
|
|
4.
|
Chitosan + Alum film
|
33.09 ± 0.67
|
*[Values are expressed as mean±SEM (n=3). * p<0>
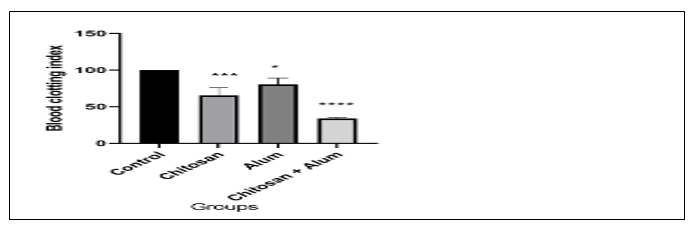
Figure 3: Graphical representation of blood clotting index of deferent haemostatic film.
In-vivo evaluation of haemostatic film:
Rat liver laceration model:
The surgical procedure were sown accordingly A) Fur were removed from the abdominal zone, B) Incision was made and liver was exposed, C) After liver laceration CH- alum haemostatic film was applied (Figure 4). In the rat liver laceration model (n=12), treatment of the surgical exposure and the transacted liver with three (CH, alum, and CH- alum) haemostatic film showed that the time for haemostasis was less in the CH- alum group with 23 ± 2.30 sec, (n=3). Where, Ch group showed 56 ± 1.73 sec, (n=3), and the alum group showed 112 ± 6.02 sec, (n=3). In addition, the blood loss in the CH- alum group showed 0.096 ± 0.032 ml, (n=3) was less than the CH group 0.805 ± 0.233 ml, (n=3) and alum group 0.620 ± 0.425 ml, (n=3). The chitosan-alum film gave significant result (Table 5) also in graphical (Figure 5).
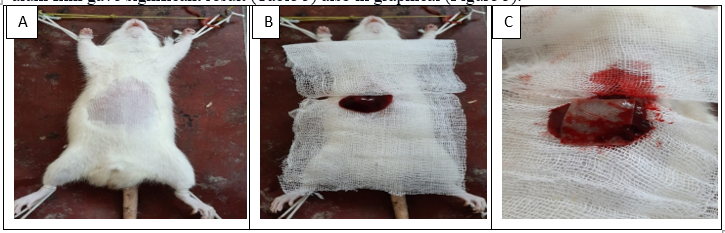
Figure 4: Steps for surgical procedure for liver laceration model accordingly A) Fur were removed from the abdominal zone, B) Incision was made and liver was exposed, C) After liver laceration CH- alum haemostatic film was applied.
Table-5: Haemostatic time(seconds) with SEM and Blood loss with SEM according groups
|
SL. NO.
|
Groups
|
Haemostatic time(seconds) with Mean+SEM
|
Blood loss(ml) with Mean+SEM
|
|
1.
|
Control
|
153 ± 9.07
|
3.175 ± 0.379
|
|
2.
|
Chitosan film
|
56 ± 1.73
|
0.805 ± 0.233
|
|
3.
|
Alum film
|
112 ± 6.02
|
0.620 ± 0.425
|
|
4.
|
Chitosan + Alum film
|
23 ± 2.30
|
0.096 ± 0.032
|
*[Values are expressed as Mean±SEM (n=3). * p<0>
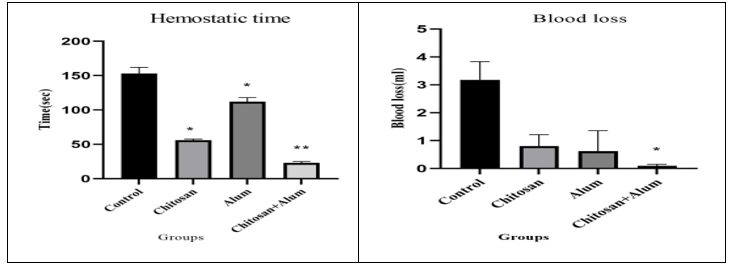
Figure 5: Graphical representation of Measurement of Haemostatic time (seconds) with Mean±SEM and Blood loss with Mean±SEM according groups
Rat jugular vein model:
Steps for surgical procedure for jugular vein model accordingly A) Jugular vein were isolated, B) Incision was made, C) After incision Chitosan-alum haemostatic film were applied(Figure 6). In this model (n=12) we used the same method as the liver laceration model, the jugular vein was exposed, and an incision was made then treatment with three (CH, alum, and CH- alum) haemostatic film to test the haemostatic time and blood loss in animals. The haemostatic time of the CH- alum group was 37.333 ± 1.452 sec, (n=3) which was lower than the CH group 61.333 ± 5.206 sec, (n=3) and alum group 114.333 ± 3.480 sec, (n=3). Moreover, CH- alum group 0.348 ± 0.044 ml, (n=3) showed lower blood loss than the CH group 0.433 ± 0.046 ml, (n=3) and the alum group 1.179 ± 0.234 ml, (n=3) and no re-bleeding was observed after removing test material. The chitosan-alum film gives significant result for haemostatic time measurement (Table 6) as well as graphically also (Figure 7).
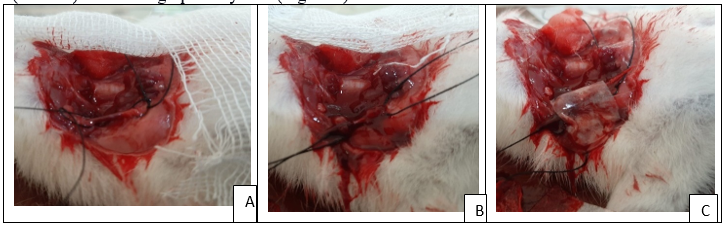
Figure 6: Steps for surgical procedure for jugular vein model accordingly A) Jugular vein were isolated, B) Incision was made, C) After incision Chitosan-alum haemostatic film were applied.
Table 6: Haemostatic time (seconds) with Mean±SEM and Blood loss (ml) with Mean±SEM according to all groups.
|
SL. NO.
|
Groups
|
Hemostatictime(seconds) with Mean±SEM
|
Blood loss(ml) with Mean±SEM
|
|
1.
|
Control
|
148.333 ± 2.728
|
2.322 ± 0.064
|
|
2.
|
Chitosan film
|
61.333 ± 5.206
|
0.433 ± 0.046
|
|
3.
|
Alum film
|
114.333 ± 3.480
|
1.179 ± 0.234
|
|
4.
|
Chitosan + Alum film
|
37.333 ± 1.452
|
0.348 ± 0.044
|
*[Values are expressed as Mean±SEM (n=3). * p<0>
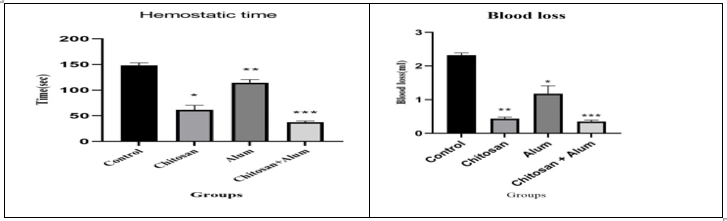
Figure 7: Graphical representation of Measurement of Haemostatic time (seconds) with Mean±SEM and Blood loss (ml) with Mean±SEM according groups.
Rat femoral artery model:
The steps involve in femoral artery model accordingly A) Femoral artery was isolated, B) Incision was made, C) Haemostatic film was applied (Figure 8). In the rat femoral artery model (n=12), we used the same method as the previous to test the haemostatics time and blood loss. The haemostatics time of the CH- alum group was 40.333 ± 0.882 sec, (n=3) which was lower than the CH group 78.000 ± 4.359 sec, (n=3) and alum group 103.00 ± 2.646 sec, (n=3). Moreover, CH- alum group 0.410 ± 0.0217 ml, (n=3) showed lower blood loss than the CH group 1.559 ± 0.055 ml, (n=3) and the alum group 1.763 ± 0.276 ml, (n=3) and no re-bleeding was observed after removing test material. The chitosan-alum film gave significant result (Table 7) also in graphical (Figure 9).
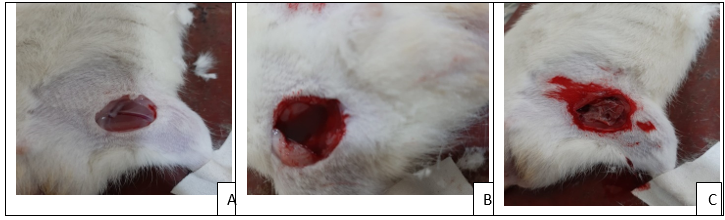
Fig 8: The steps involve in femoral artery model accordingly A) Femoral artery was isolated, B) Incision was made, C) Haemostatic film was applied.
Table 7: Measurement of Haemostatic time (seconds) with Mean±Sem and Blood loss (ml) with Mean±Sem according groups.
|
SL. NO.
|
Groups
|
Haemostatic time (seconds) with Mean±Sem
|
Blood loss (ml) with Mean±Sem
|
|
1.
|
Control
|
151.667 ± 1.453
|
1.697± 0.051
|
|
2.
|
Chitosan film
|
78.000 ± 4.359
|
1.559 ± 0.055
|
|
3.
|
Alum film
|
103.00 ± 2.646
|
1.763 ± 0.276
|
|
4.
|
Chitosan + Alum film
|
40.333 ± 0.882
|
0.410 ± 0.0217
|
*[Values are expressed as Mean±Sem (n=3). * p<0>
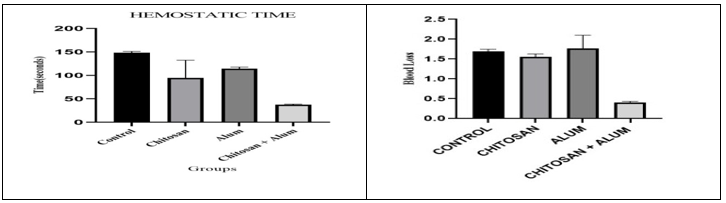
Figure 9: Haemostatic time (seconds) with SEM and Blood loss (ml) with SEM according groups.
Histological and Microscopic evaluation:
In the histological examination of the first application, the haemostatics barrier was observed on the cut surface of the liver that consists of film and clot mixture. 3 days later, the film form was nearly absorbed, and the macrophage layer was observed between the liver parenchyma and the haemostatics barrier. After 7 days no necrosis was observed in the liver parenchyma under the macrophage barrier. The haemostatics barrier was seen to be started organizing. When hematoxylin and eosin-stained tissue specimens assessed under light microscopy, mild inflammation was observed. Serious inflammation was not noted. The film was absorbed completely (Figure 10).
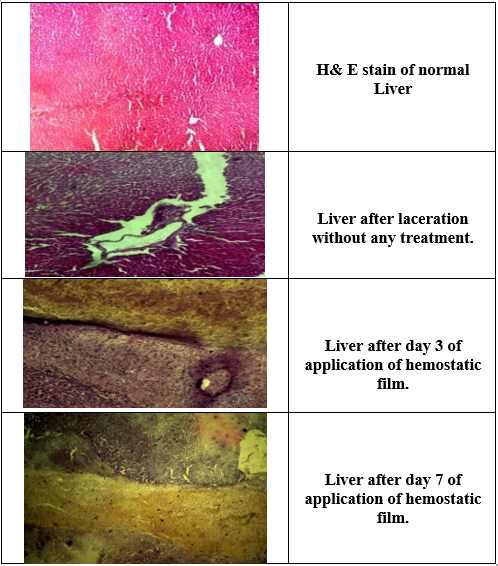
Figure 10: Microscopically Evaluation of liver laceration treated by chitosan-alum film.
DISCUSSION:
Both chitosan and alum are proven haemostatic materials that act at the primary stage of haemostasis. In this study, chitosan with alum was mixed together and cross-linked using polyvinyl alcohol to obtain a haemostatic film (CH-alum). Chitosan is an abundant biodegradable polymer and alone has bio adhesive properties. Protonated Chitosan haemostatic agent works by an interaction between the cell membrane of platelets which are negatively charged and rapid formation of thrombus. On the other hand, alum was traditionally used as a haemostatic agent. Al3+ ions of alum neutralized the negatively charged colloidal particles of blood proteins, forms coagulation. Positive ions of alum cause precipitation of negatively charged plasma protein and form a barrier at the site of injury. Polyvinyl alcohol was hydrophilic that increased film permeability and drug diffusion. Synergistic activity of chitosan composite alum haemostatic film was evaluated by using in vivo animal models such as (rat liver laceration model, rat jugular vein model, and rat femoral artery model). In-vitro evaluation of haemostatic film also carried by using two methods platelet count method and whole blood clotting assay. Studies of In-vivo haemostatic model have shown that chitosan alum haemostatic film produces a significant effect on haemostatic time. It also reduces blood loss. This is an improved and better result than the other chetosan and alum haemostatic film. Thus, taking into account that chitosan composite alum haemostatic film is an important pre-requisite natural polymeric material that can be used in the management of haemorrhage. Further, in-vitro evaluation gives a proper conclusion about the mechanism of the haemostasis of Chitosan which incorporates Alum haemostatic film. According to data of platelet count, Chitosan film had shown a decrease amount of platelet count. That gave which gives the clear result that protonated Chitosan attracted the negatively charged platelet at the site of injury and also showed effective results of Chitosan composite Alum film(CH- alum). Moreover, the data of the whole blood clotting assay suggests that the smaller the Blood Clotting Index (BCI) the stronger the haemostatic activity. CH- alum film gives a significant effect in comparison to Chitosan and alum alone haemostatic film. Hence from the data of in-vivo and in-vitro evaluation of haemostatic film we found CH- alum film showed rapid coagulation compare to chitosan and alum alone film.
CONCLUSION:
A complex was produced by cross-linking with Chitosan, alum, and polyvinyl alcohol to form a haemostatic film through the solvent casting method. From the result and discussion, it can be concluded that the synergistic properties of haemostatic agents Chitosan and Alum show better haemostatic effect by performing in vivo animal bleeding models and in vitro blood clotting models. Hence the haemostatic film can be used as in the management of blood loss. Further estimation of different molecular pathways associated with blood coagulation will improve the mechanism of action of haemostasis.
Declarations:
ACKNOWLEDGEMENT:
We are grateful to the Defence Research Laboratory (DRL), Defence Research and Development Organization and P.G. Institute of Medical Science for providing the necessary resources and facility.
Author’s Contribution:
Malay Besra Design this study, performs this study, conducted the data analysis and prepares manuscript.
Mir Irfan Soyel performs this study, conducted the data analysis and prepares manuscript.
Biplab Kumar Chakra have done Proof reading
Nilanjan Adhikari have supply resource compilations.
Sumit Maji have supply resource compilations
All authors read and approved the fnal manuscript.
Funding:
This work was supported by the Defence Research Laboratory (DRL), Defence Research and Development Organization and P.G. Institute of Medical Science.
Data availability:
The data that support the fndings of this study are available from the corresponding author, Malay Besra, upon reasonable request.
Ethics approval and consent to participate:
We followed the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (Institute Regd. No.1127/GO/RBi/S/08/CPCSEA Dated: 27/03/20217 and experiment IAEC approval no 08 dated: 30/12/2020) for the animal excrement supply by
Defence Research Laboratory (DRL), Defence Research and Development Organization.
Consent for publication:
Not applicable.
Competing interests:
The authors declare that they have no competing interests.
REFERENCES
- Hu Z, Zhang DY, Lu ST, Li PW, Li SD. Chitosan-based composite materials for prospective hemostatic applications. Marine drugs. 2018 Aug;16(8):273.
- Dong F, Wang C, Duan J, Zhang W, Xiang D, Li M. Puerarin attenuates ovalbumin-induced lung inflammation and hemostatic unbalance in rat asthma model. Evidence-Based Complementary and Alternative Medicine. 2014 Jan 1;2014.
- Feng C, Li J, Wu GS, Mu YZ, Kong M, Jiang CQ, Cheng XJ, Liu Y, Chen XG. Chitosan-coated diatom silica as hemostatic agent for hemorrhage control. ACS applied materials & interfaces. 2016 Dec 21;8(50):34234-43.
- Janvikul W, Uppanan P, Thavornyutikarn B, Krewraing J, Prateepasen R. In vitro comparative hemostatic studies of chitin, chitosan, and their derivatives. Journal of applied polymer science. 2006 Oct 5;102(1):445-51.
- Janvikul W, Uppanan P, Thavornyutikarn B, Krewraing J, Prateepasen R. In vitro comparative hemostatic studies of chitin, chitosan, and their derivatives. Journal of applied polymer science. 2006 Oct 5;102(1):445-51.
- Hu Z, Lu S, Cheng Y, Kong S, Li S, Li C, Yang L. Investigation of the effects of molecular parameters on the hemostatic properties of chitosan. Molecules. 2018 Nov 30;23(12):3147.
- Hu Z, Lu S, Cheng Y, Kong S, Li S, Li C, Yang L. Investigation of the effects of molecular parameters on the hemostatic properties of chitosan. Molecules. 2018 Nov 30;23(12):3147.
- Moura JM, Farias BS, Rodrigues DA, Moura CM, Dotto GL, Pinto LA. Preparation of chitosan with different characteristics and its application for biofilms production. Journal of Polymers and the Environment. 2015 Dec;23:470-7.
- Han D, Yan L, Chen W, Li W. Preparation of chitosan/graphene oxide composite film with enhanced mechanical strength in the wet state. Carbohydrate Polymers. 2011 Jan 10;83(2):653-8.
- Mathew S, Brahmakumar M, Abraham TE. Microstructural imaging and characterization of the mechanical, chemical, thermal, and swelling properties of starch–chitosan blend films. Biopolymers: Original Research on Biomolecules. 2006 Jun 5;82(2):176-87.
- Aguirre-Loredo RY, Rodríguez-Hernández AI, Morales-Sánchez E, Gómez-Aldapa CA, Velazquez G. Effect of equilibrium moisture content on barrier, mechanical and thermal properties of chitosan films. Food chemistry. 2016 Apr 1;196:560-6.
- Winchester JF, MacKay JM, Forbes CD, Courtney JM, Gilchrist T, Prentice CR. Hemostatic changes induced in vitro by hemoperfusion over activated charcoal. Artificial Organs. 1978 Aug;2(3):293-300.
- Buriuli M, Kumari WG, Verma D. Evaluation of hemostatic effect of polyelectrolyte complex-based dressings. Journal of Biomaterials Applications. 2017 Nov;32(5):638-47.
- Midi A, Kumandas A, Ekici H, Bayraktar F, Karapirli K, Karahan S, Turk M, Ozyurek HE. Investigation of the efficacy of algan hemostatic agent in liver laceration model in rats. EJMO. 2019;3(1):37-42.
- Morgan CE, Prakash VS, Vercammen JM, Pritts T, Kibbe MR. Development and validation of 4 different rat models of uncontrolled hemorrhage. JAMA surgery. 2015 Apr 1;150(4):316-24.
- Binneto?lu K, Kumandas A, Ekici H, Özbayku? AC. Comparison of the algan hemostatic agent with floseal in rat liver laceration bleeding model. The Eurasian Journal of Medicine. 2022 Feb;54(1):36.
- Trooskin SZ, Pierce RA, Dear SB, Flancbaum L, Mackenzie JW, Greco RS, Boyd CD. The effect of viable omentum on early bile leakage and healing of liver lacerations. Journal of Trauma and Acute Care Surgery. 1989 Jan 1;29(1):47-50.
- Feng J, Fitz Y, Li Y, Fernandez M, Puch IC, Wang D, Pazniokas S, Bucher B, Cui X, Solomon SB. Catheterization of the carotid artery and jugular vein to perform hemodynamic measures, infusions and blood sampling in a conscious rat model. Journal of visualized experiments: JoVE. 2015 Jan 30(95):51881.
- Smith DW, Bailes JE, Fisher JA, Robles J, Turner RC, Mills JD. Internal jugular vein compression mitigates traumatic axonal injury in a rat model by reducing the intracranial slosh effect. Neurosurgery. 2012 Mar 1;70(3):740-6.
- Nasir S, Aydin MA, Karahan N, Demiryürek D, Sargon M. New microvenous anastomosis model for microsurgical training: external jugular vein. Journal of reconstructive microsurgery. 2006 Nov;22(08):625-30.
- Raake W, Elling H. Rat jugular vein hemostasis-a new model for testing antithrombotic agents. Thrombosis research. 1989 Jan 1;53(1):73-7.
- Okada T, Harada T, Bark DH, Mayberg MR. A rat femoral artery model for vasospasm. Neurosurgery. 1990 Sep 1;27(3):349-56.
- Matsuno H, Uematsu T, Nagashima S, Nakashima M. Photochemically induced thrombosis model in rat femoral artery and evaluation of effects of heparin and tissue-type plasminogen activator with use of this model. Journal of pharmacological methods. 1991 Jul 1;25(4):303-17.
- Clatterbuck RE, Oshiro EM, Hoffman PA, Dietsch GN, Pardoll DM, Tamargo RJ. Inhibition of vasospasm with lymphocyte function-associated antigen—1 monoclonal antibody in a femoral artery model in rats. Journal of neurosurgery. 2002 Sep 1;97(3):676-82.
- Bowman G, Dixit S, Bonneau RH, Chinchilli VM, Cockroft KM. Neutralizing antibody against interleukin-6 attenuates posthemorrhagic vasospasm in the rat femoral artery model. Neurosurgery. 2004 Mar 1;54(3):719-26.
- Kohlmeier RE, Dimaio VJ, Sharkey F, Rouse EA, Reeves KE. The timing of histologic changes in liver lacerations. The American Journal of Forensic Medicine and Pathology. 2008 Sep 1;29(3):206-7.
- Saviano A, Ojetti V, Zanza C, Franceschi F, Longhitano Y, Martuscelli E, Maiese A, Volonnino G, Bertozzi G, Ferrara M, La Russa R. Liver trauma: management in the emergency setting and medico-legal implications. Diagnostics. 2022 Jun 13;12(6):1456.
- Bilal HM, Riaz F, Munir K, Saqib A, Sarwar MR. Histological changes in the liver of diabetic rats: A review of pathogenesis of nonalcoholic fatty liver disease in type 1 diabetes mellitus. Cogent Medicine. 2016 Dec 31;3(1):1275415.


 Malay Besra*
Malay Besra*
 Mir Irfan Soyel
Mir Irfan Soyel
 Biplab Kumar Chakra
Biplab Kumar Chakra
 Nilanjan Adhikari
Nilanjan Adhikari
 Sumit Maji
Sumit Maji










 10.5281/zenodo.14851453
10.5281/zenodo.14851453