Novel drug delivery system (NDDS) is an expression mainly associated with the formulation of new pharmaceutical forms which have optimized characteristics such as smaller particle size.A suitable drug delivery system aims to provide an effective therapeutic dose at the targeted site at the right time and at suitable intervals until the desired cure is achieved.Novel drug delivery system (NDDS) is an expression mainly associated with the formulation of new pharmaceutical forms which have optimized characteristics such as smaller particle size. A suitable drug delivery system aims to provide an effective therapeutic dose at the targeted site at the right time and at suitable intervals until the desired cure is achieved.Traditional drug delivery methods, while effective in many cases, often face significant limitations. These include poor bioavailability, rapid clearance from the body, lack of site specific targeting, and undesirable side effects. These challenges underscore the need for more advanced drug delivery systems to overcome these limitations and provide more effective, safe, and patient friendly treatment Novel Drug Delivery System came into existence.Novel Drug Delivery System can provide controlled and sustained release of drugs, target specific tissues or cells, and reduce the frequency of dosing. For example, targeted drug delivery systems can direct therapeutic agents specifically to diseased cells, minimizing exposure to healthy tissues and thereby reducing side Carriers use in Novel Drug Delivery System are Phytosomes, Microneedle, Nanoparticles, Microspheres ,Liposomes for high permeability and high capacity of drugs to be loaded.This review article will provide an overview of types of carriers use in NDDS, Application and Mechanism of nanocarriers transport throughout the systemic circulation reaching the specific target.
Novel Drug Delivery System (NDDS), Microneedle (MN), Nanoparticles (NP), Enhanced Permeability and Retention (EPR).
Drug Delivery Systems (DDS) are technologies designed to transport therapeutic substances in the body to achieve a desired therapeutic effect. The primary goal of DDS is to deliver drugs in a controlled manner, ensuring that the active ingredients are released at the right place, at the right time, and in the right concentration. DDS plays a crucial role in modern medicine by enhancing the efficacy of treatments, reducing side effects, and improving patient compliance. The evolution of drug delivery technologies has been driven by the need to improve the therapeutic outcomes of medications. Initially, drug delivery was limited to simple formulations like tablets and injections, with minimal control over drug release and distribution [3]. Over the decades, advancements in material science, chemistry, and biotechnology have led to the development of more sophisticated DDS, such as controlled-release formulations, transdermal patches, and targeted delivery systems. The progression from conventional delivery methods to advanced technologies marks a significant shift in how drugs are administered and how treatment outcomes are optimised.
1.1Need for Advanced Drug Delivery Systems:
Traditional drug delivery methods, while effective in many cases, often face significant limitations. These include poor bioavailability, rapid clearance from the body, lack of sitespecific targeting, and undesirable side effects. These challenges underscore the need for more advanced drug delivery systems to overcome these limitations and provide more effective, safe, and patient friendly treatment options .Advanced Drug Delivery Systems (DDS) have been developed to address the shortcomings of traditional methods. These systems incorporate innovative technologies, such as nanoparticles, liposomes, and biodegradable polymers, to improve the delivery and efficacy of drugs. Advanced DDS can provide controlled and sustained release of drugs, target specific tissues or cells, and reduce the frequency of dosing. For example, targeted drug delivery systems can direct therapeutic agents specifically to diseased cells, minimizing exposure to healthy tissues and thereby reducing side effects. These advancements are critical in managing diseases that require precise and sustained drug administration, such as cancer, diabetes, and neurological disorders.
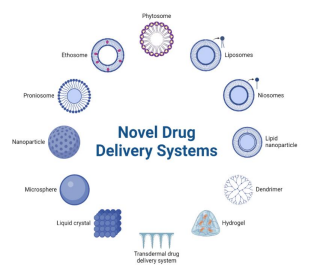
Figure no.1.Novel Drug Delivery System
2.Scope:
As the field of drug delivery continues to evolve, it is essential to understand the latest advancements and how they are being applied to improve therapeutic outcomes.
3.Novel Drug Delivery System:
Novel drug delivery system (NDDS) is an expression mainly associated with the formulation of new pharmaceutical forms which have optimized characteristics such as smaller particle size, A suitable drug delivery system aims to provide an effective therapeutic dose at the targeted site at the right time and at suitable intervals until the desired cure is achieved . Before the novel drug delivery systems, conventional formulations were utilised, followed by immediate-release formulations, sustained release systems (often given through oral administration), and long-acting injectables. the performance of biotherapeutic agents when compared with their effect in the conventional dosage forms. A suitable drug delivery system aims to provide an effective therapeutic dose at the targeted site at the right time and at suitable intervals until the desired cure is achieved . Before the novel drug delivery systems, conventional formulations were utilised, followed by immediate-release formulations, sustained-release systems (often given through oral administration), and long-acting injectables. A suitable drug delivery system aims to provide an effective therapeutic dose at the targeted site at the right time and at suitable intervals until the desired cure is achieved . Before the novel drug delivery systems, conventional formulations were utilised, followed by immediate-release formulations, sustained-release systems (often given through oral administration), and long-acting injectables. Novel drug delivery systems comprise sophisticated technology merged into drug delivery systems. These systems often utilise carriers and their internal or external environment . Carriers that have been utilised in these DDSs include artificial drug carriers (nanoparticles, nanotubes, dendrimers, liposomes, ethosomes, aquasomes, polymersomes, niosomes, foams, hydrogels, cubosomes and quantum dots), natural drug carriers (nanoerythrosomes, exosomes) and macrophages.The minute size of nanoparticles and their high surface area to volume ratio make for high permeability and high capacity of drugs to be loaded. These nanoparticles can also cross physiological barriers due to their minute sizes. These systems are formed with materials to enhance permeation to target cells. The incorporation of novel technology into pharmaceutical formulations has led to the emergence of sophisticated drug delivery systems. These novel systems have been linked with higher efficacy, better patient compliance, better pharmacokinetics and improved targetability. Though these systems are more expensive than traditional drug delivery systems, they bear tremendous potential.
3.1. Types of Nanocarriers:
3.1.1. Organic Nanocarriers:
Organic nanocarriers, including liposomes, micelles, viral nano-carriers,dendrimers, solid lipid and polymeric nanoparticles, are often formed from natural and synthetic polymers, biodegradable and bio-compatible polymers. These carriers use covalent bonds and Vander Waal forces to formulate a smart drug delivery system. However, the choice of bonds depends on the type of solvent utilised, temperature, pH and other factors. Several advantages of organic nanocarriers include improved cell internalisation, excretion from the body in a biodegradable form, enhanced targeting of cells, tissues, and organs, and improved pharmacodynamics and pharmacokinetics. Different strategies for functionalising organic nanocarriers and improving their
efficacy include attaching ligands, polymer coatings, biocompatible materials and inorganic materials.
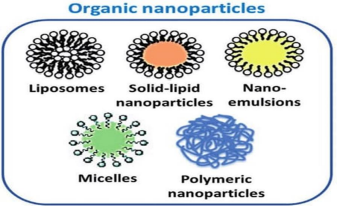
Figure no.2 Organic Nanoparticle
3.1.2. Inorganic Nanocarriers:
Inorganic nanoparticles have been explored as nanocarriers. They include carbon nanotubes, quantum dots, mesoporous silica, magnetic nanocarriers, gold nanocarriers, iron oxide nanoparticles, and calcium phosphate nanoparticles. Their advantages include non-toxicity, high stability, biocompatibility, hydrophilicity, high cellular uptake, no mi-crobial attack, and non-immunogenic response.
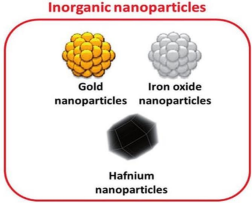
Figure no.3. Inorganic Nanoparticles
3.2.Types of carriers use in Novel Drug Delivery System: 3.2.1 Phytosome :
The flavonoid and terpenoid constituents of phyto extracts have better binding capacity to phosphatidylcholine. Phytosomes to be derived by the reaction between stoichiometric quantity of the phospholipid (phosphatidylcholine) and the extract
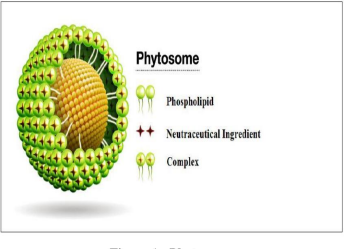
Figure no.4.Phytosomes
of standardized or polyphenolic derivatives (like flavonoids) in a non polar systemof solvent. The word "Phyto" indicates plant while others means cell-like. "Phyto" means plant. Phytosomes were the Method of vesicular supply of herbal extract phytoelectric ingredients and Lipid bound (one molecular phyto-constituent, bound to a phospholipid at least molecular). Phytosomes guard against degradation of important herbal extract componentsDigestive secretion and intestinal bacteria which have increased absorption Provides improved pharmacological and pharmacokinetic biological and improved availability Parameters of herbal extract traditional and the distinction between phytosomes and liposome.
Phytosome Benefits:
• Improved phospholipid complex bioavailability.
• Enhanced GIT absorption.
• Improved therapeutic results are attributed to increased bioavailability.
• High bioavailability requires less dosage.
• Greater stability. High lipophilicity causes high pentration and is thus used over liposomes in cosmetics.
• Phosphatidylcholine is not a carrier, but serves as a liver protection.
3.2.2.Microneedle
Microneedle technology is a mode of active transdermal drug delivery and is intended to be used a as a replacement to the traditional syringe injections. The MN array is used to penetrate the stratum corneum and deliver the drug with a minimally invasive action. These arrays are micro-sized needles with a height ranging from 25 to 2000 ?m. MNs have been used for different applications such as drug and vaccine delivery, cosmetic, and disease diagnostics. MN have various structural arrangements, shapes, forms, and materials along with different manufacturing methods which are further il lustrated in this review paper. Figure 3 show some current commercial MN devices. Donnelly et al. argued that 30% of the most recent scientific literature in “transdermal delivery technology” accounted for microneedle studies. The MN drug delivery route can be impacted by external environments such as skin physiology, physiochemical properties, and ambient conditions. These include the relative humidity and temperature in the vicinity of the application area. Too sparse (low humidity) will retard the release of drugs to the skin layers, however too high humidity (such as sweat) can interfere in the drug release kinetics due to excess water and presence of other salts thereby changing the osmotic gradient for transdermal drug de livery. Furthermore, an excess of perspiration can prevent the adhesion of the microneedle patch to the skin further retarding elution of drugs through the skin. Similarly, very low or very high pH ranges around the skin region can result in lower permeability of the drug into the stratus corneum and beyond excessive lipid films on the skin form a barrier layer can assist in trans dermal absorption. Raising the skin temperature can enhance permeation of drugs due to increase diffusivity and vasodilation of skin vessels. Microneedles (MNs) are tiny needle like structures used in drug delivery through layers of the skin. They are non-invasive and are associated with significantly less or no pain at the site of administration to the skin. MNs are excellent in delivering both small and large molecules to the subjects in need thereof. There exist several strategies for drug delivery using MNs, wherein each strategy has its pros and cons. Research in this domain lead to product development and commercialization for clinical use. Additionally, several MN-based products are undergoing clinical trials to evaluate its safety, efficacy, and tolerability. The present review begins by providing bird’s-eye view about the general characteristics of MNs followed by providing recent updates in the treatment of cancer using MNs. Particularly, we provide an overview of various aspects namely: anti cancerous MNs that work based on sensor technology, MNs for treatment of breast cancer, skin carcinoma, prostate cancer, and MNs fabricated by additive manufacturing or 3dimensional printing for treatment of cancer.
Advantages:
• A Microneedle is considered to be one of the best ways for transdermal drug delivery.
• Provide pain free experience.
• Avoid first pass metabolism.
• The MN array is long enough to penetrate the stratum corneum and short enough to prevent damage to the dermis or reach nerve endings thus painless.
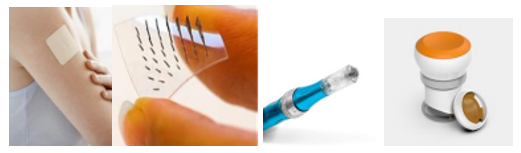
Figure no.5 Current microneedle devices (single needle with applicator, microneedles array patch, microneedles pen, microneedle pump patch, and microneedle roller).
3.3.3. Nanoparticles:
Nanoparticles (including nanospheres and nanocapsules of size 10-200 nm) are in the solid state and are either amorphous or crystalline. They are able to adsorb and/or encapsulate a drug, thus protecting it against chemical and enzymatic degradation. Nanocapsules are vesicular systems in which the drug is confined to a cavity surrounded by a unique polymer membrane, while nanospheres are matrix systems in which the drug is physically and uniformly dispersed. Nanoparticles as drug carriers can be formed from both biodegradable polymers and non-biodegradable polymers. In recent years, biodegradable polymeric nanoparticles have attracted considerable attention as potential drug delivery devices in view of their applications in the controlled release of drugs, in targeting particular organs/tissues, as carriers of DNA in gene therapy, and in their ability to deliver proteins, peptides and genes through the oral route. Nanoparticals the fundamental components of Nano technology. Nano particles size ranges from 1 to 100nm which are made up of metal, metal oxides, organic matter, carbon.Nanoparticles differ from various dimensions, to shapes and sizes apart from their material. Surface can be irregular with surface variations or a uniform. Among nanoparticles some are crystalline or amorphous with single or multi-crystal solids either agglomerated or loose. In the process of synthesizing new drugs, most drug candidates are insoluble or poorly soluble in water which causes a huge downfall for the pharmaceutical industry. One of the main reasons for a drugs insolubility is its complex and large molecular structure. It has been reported that over 65% of new active pharmaceutical ingredients (APIs) are either poorly soluble in water or insoluble. Due to their low aqueous solubility properties and high permeability, they are categorized as class II of the Biopharmaceutics Classification System (BCS), where the dissolution step is the rate limiting factor in drug absorption. The pharmaceutical industries are now facing a challenge to improve the dissolution characteristic of poorly water soluble drugs which is the key factor in enhancing drug bioavailability. For instance, they help to increase the stability of drugs/proteins and possess useful controlled release properties. This review predominately focused on synthesis of different types of nanoparticles using chemical, physical and biological methods. However, chemical and physical methods are expensive and harmful but biological method is simple, non-toxic, rapid and eco- friendly. It also explains about the characteristics of nanoparticles and concluded with various applications.
Figure no.6. Nanoparticles
Types of Nanoparticles:
1.Carbon-based NPs:
Fullerenes and carbon nanotubes (CNTs) are the two essential sub-categories of carbon based NPs. NPs of globular hollow cages, like allotropic forms of carbon, are found in fullerenes. Due to their electrical conductivity, high strength, structure, electron affinity, and adaptability, they have sparked significant economic interest. These materials have organized pentagonal and hexagonal carbon units, each of which is sp2 hybridized. While CNTs are elongated and form 1–2 nm diameter tubular structures. These fundamentally resemble graphite sheets rolling on top of one another. Accordingly, they are referred to as single-walled (SWNTs), double-walled (DWNTs), or multi-walled carbon nanotubes (MWNTs) depending on how many walls are present in the rolled sheets.
Figure no.7.Carbon Nanoparticles
2.Metal NPs:
Metal NPs are purely made of metals. These NPs have distinctive electrical properties due to well-known localized surface Plasmon resonance (LSPR) features. Cu, Ag, and Au nanoparticles exhibit a broad absorption band in the visible region of the solar electromagnetic spectrum. Metal NPs are used in several scientific fields because of their enhanced features like facet, size, and shape-controlled synthesis of metal NPs
Figure no.8 Metal Nanoparticles
3. Ceramics NPs:
Ceramic NPs are tiny particles made up of inorganic, non-metallic materials that are heat-treated and cooled in a specific way to give particular properties. They can come in various shapes, including amorphous, polycrystalline, dense, porous, and hollow, and they are known for heat resistance and durable properties. Ceramic NPs are used in various applications, including coating, catalysts, and batteries.
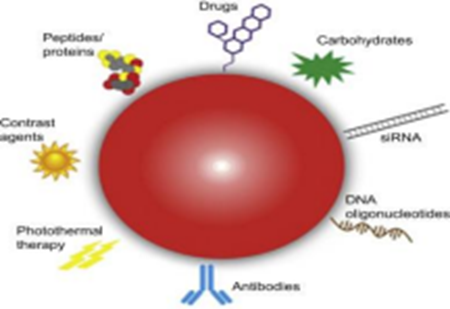
Figure no.9 Ceramic Nanoparticles
4. Lipid-based NPs:
These NPs are helpful in several biological applications because they include lipid moieties. Lipid NPs typically have a diameter of 10–1,000 nm and are spherical. Lipid NPs, i.e., polymeric NPs, have a solid lipid core and a matrix consisting of soluble lipophilic molecules
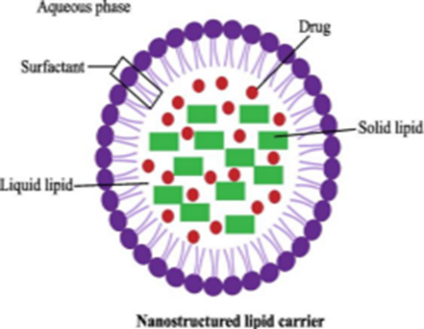
Figure no.9 Lipid base nanoparticles
5. Semiconductor NPs:
Semiconductor NPs have qualities similar to metals and non-metals. That is why Semiconductor NPs have unique physical and chemical properties that make them useful for various applications. For example, semiconductor NPs can absorb and emit light and can be used to make more efficient solar cells or brighter light-emitting diodes (LEDs). They can make smaller and faster electronic devices, such as transistors, and can be used in bio imaging and cancer therapy.
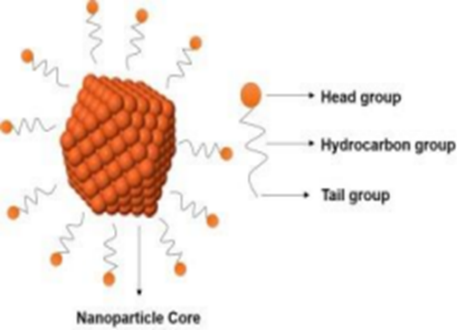
Figure no.10 Semiconductor Nanoparticles
6. Polymeric NPs:
Polymeric NPs with a size between 1 and 1,000 nm can have active substances surface adsorbed onto the polymeric core or entrapped inside the polymeric body. These NPs are often organic, and the term polymer nanoparticle (PNP) is commonly used in the literature to refer to them. They resemble Nano spheres or Nano capsules for the most part.
Figure no.11 Polymeric Nanoparticles
Applications of Nanoparticles:
• More specific drug targeting and delivery,
•Reduction in toxicity while maintaining therapeutic effects,
• Greater safety and biocompatibility, and.
• Faster development of new safe medicines.
3.3.4. Microspheres:
Microspheres are one of the most explored drug carriers for site-specific delivery of cargoes in a controlled and sustained manner. They are biocompatible, biodegradable, and are capable of surface modifications. A variety of polymers have been explored which can encapsulate drugs with different physicochemical properties. All these properties, together with the different strategies makes them a deserving candidate for targeting to various sites of the body, including lungs, liver, bone, brain, colon, eye, and other malignant tissues in different parts of the body. They are also capable of targeting the macrophages and to cellular organelles like mitochondria. The objective of this review is to highlight the applications of microspheres in the field of targeted drug delivery system. Microspheres can be targeted to the desired location by active or passive targeting strategies. Passive targeting is based upon the size and general surface properties of the microspheres such as degree of hydrophobicity, surface charge, and non-specific adhesion, which directs them towards the particular organ. Additionally, the approach confers special properties to the carriers which aid them to cross physiological barriers like the specialized epithelia and Blood Brain Barrier (BBB).On the other hand, active targeting is mostly associated with ligand-receptor recognition, referred to as secondary active targeting. Apart from this, targeting to cell organelles i.e. tertiary targeting, and to different organs of the body i.e. primary targeting is also accomplished by microspheres. Indeed, microspheres can be tailored to target a specific site by a combination of more than one targeting approaches too.The choice of the targeting approach depends upon the intended area of human body, since each human body part has a different physiology. Size based targeting is mainly a cornerstone approach for lung targeting and liver targeting, as the drug cargo has to reach and get retained in the smallest capillary network of the lungs and in the arteries surrounding the tumours in the liver .Surface modifications either by linking the microspheres with targeting moieties or by coating them with materials with special properties like acid resistant or mucoadhesive polymers can be used for targeting almost all the organs including lungs, brain, colon, eyes, tumours, etc. Apart from the normally explored targeting strategies, very specific approach lies in bone targeting, where inorganic materials are used to deliver the drugs to the bony tissues.
Figure no.12. Microspheres
Advantages of Microspheres:
•Decreased size of microsphere contributes increased surface area thereby increases the potency of the poorly soluble material
•Dose frequency and adverse effects can be reduced.
• Increased patient compliance.
• Drug packaged with polymer prevent drug from enzymatic cleavage therefore the drug can be protected from various enzymes.
• Enhances bioavailability.
• Gastric irritation can be reduced.
? Types of materials use in Microspheres:
Materials used in the formulation of microsphere Microspheres are usually made of polymers, they are classified as follows.
• Synthetic Polymers
• Natural polymers
A. Synthetic polymers are of two types:
a) Non-biodegradable polymers Eg- Acrolein, Polymethylmethacrylate (PMMA), Epoxy polymers, Glycidyl methacrylate
b) Biodegradable polymers Eg- Glycolides and their co polymers, Poly alkyl cyano acrylates, Poly anhydrides and lactides.
B. Natural polymers – These are obtained from different sources such as proteins, carbohydrates and chemically modified carbohydrates. Proteins: Albumin, Collagen and gelatin Carbohydrates: Agarose, Carrageenan, Starch, chitosan, Chemically modified carbohydrates: Poly dextran, Poly starch.
Types of Microspheres:
Microspheres are classified into different types. They are of following 1. Bioadhesive microspheres
2. Magnetic microspheres
3. Floating microspheres
4. Radioactive microspheres
5. Polymeric microspheres
• Bioadhesive microspheres:
Sticking of drug to the membrane by using the watersoluble property of the water-soluble polymers is called adhesion. Sticking or adhesion of drug delivery system to the mucosal membrane such as buccal, nasal, ocular, rectal etc can be termed as bioadhesion. This type of microsphere provides prolonged residence time at the target site and provide better therapeutic action.
• Magnetic microspheres:
This type of delivery system localizes drug to the target site. In this type of delivery system, a drug or therapeutic radioisotope bound to a magnetic component is injected in the systemic circulation, which is then stopped with powerful magnetic field in the disease/target site. Magnetic microspheres are molecular particles which are small enough to move across capillaries without creating an esophagealocclusionn(<4>
• Floating microspheres:
In floating type, the bulk density is less than the gastric fluid and so remains buoyant in stomach without affecting gastric emptying rate. The drug is released slowly at the desired rate, and the system is found to be floating on gastric contents and decrease gastric residence and increases fluctuation in plasma concentration. Moreover, it also reduces chances of dose dumping. It produces prolonged effect and so reduces dosing frequencies.
• Radioactive microspheres:
Radio embolization therapy microspheres sized 10-30nm are of larger than the diameter of the capillary bed when they come across. They are injected in the arteries that lead them to tumour of interest so all these conditions radioactive microspheres deliver high radiation dose to the targeted areas without damaging the normal surrounding tissues. Here radioactivity is not released from microsphere but acts within a radioisotope in typical distance. The different types of radioactive microspheres are ? emitters, ? emitters and ? emitters. • Polymeric microspheres
Polymeric microspheres can be classified as biodegradable polymeric microsphere and synthetic polymeric microspheres
i. Biodegradable polymeric microspheres Natural polymers such as starch are used as they are biodegradable, biocompatible and also bioadhesive in nature. Biodegradable polymers prolong the residence time when contact with mucous membrane due to its high degree of swelling property with aqueous medium resulting in gel formation. The rate and extent of drug release are controlled by concentration of polymer and the release pattern a sustained manner. This type of microspheres shows prolonged residence time at the site of application.
ii. Synthetic polymeric microspheres Synthetic polymeric microspheres are widely used in clinical application, moreover they are also used as bulking agent, fillers, embolic particles, drug delivery vehicles etc. and proved to be safe and biocompatible but the main disadvantage of these kind of microspheres, are they tend to migrate away from the injection site and lead to potential risk, embolism and further organ damage.
3.3.5.Liposomes:
The Greek term "liposome" is made up of two words: "Lipos" means fat and "Soma" means the flesh. Liposomes were first discovered in 1964 by Dr. Alec D. Bangham. A number of materials, including cholesterol, non-toxic chemicals, sphingolipids, glycolipids, long-chain fatty acids, and a layer of proteins, can be used to formulate liposomes, which are small, rounded spheres. In the recent past, a lot of research has been concentrated on the delivery of genes. The phospholipid membrane of liposomes is 4-5 nm thick, and the liposome size varies i.e. 30 nm to micrometer scale. Monolayer and bilayer forms are referred to as micelles and liposomes, respectively. Because of their unique properties, liposomes are used in the distribution of drugs. A number of papers focus on biological applications, and new developments in liposomal methodology.
? Components of Liposomes:
1.Phospholipid
2.Cholesterol
Phospholipids: Phospholipids are the main auxiliary components of natural films. The most prevalent phospholipid used in liposomal composition is phosphatidylcholine (PC). An amphophilic molecule called phosphatidylcholine contain. A hydrophilic polar head, phosphocholine . A glycerol bridge .A match of hydrophobic acyl hydrocarbon chain.
Cholesterol: Almost all commercially available products can use cholesterol (Chol), another essential component of the liposome membrane. An increase in cholesterol can affect a variety of processes, including the compression of lipid chains and bilayer organization, modification of membrane fluidity/rigidity, progression of the effect on medication release, and solidity of liposomes. The structure of these membranes is significantly altered when eight sterols are joined in a liposome bilayer. Cholesterol itself does not form a bilayer structure. Cholesterol acts as a buffer. The membrane becomes more stable over the stage transition, while the film becomes less prevalent and slightly more porous beneath the stage shift. Exceptionally high concentrations of it can be integrated into phospholipid membranes, up to 1:1 or even 2:1 molar proportions of cholesterol to phospholipids.
Figure no.13.Liposomes
4. Mechanism of Nano carrier transport throughout the systemic circulation reaching the specific target:
4.1.Active Targetting:
Active targeting is an advanced strategy used to ensure selectivity and specificity of smart Nano system to the targeted site/organ/cells. Commonly, targeting of antitumor drugs to the cancerous tissue has become the main strategy to reduce the effects the drugs might have on healthy tissues. The technique of active targeting should be considered during preparation of the smart noncolloidal system via functionalization of the surface of the carrier with ligands, which specially bind with its corresponding receptor on the surface of the targeted cell. Ligand—receptor attachment can guarantee that the Nano system will be optimally delivered to the dis eased cells rather than the surrounding healthy tissues. The affinity of binding between the ligand and the receptor which is overexpressed on the surface of the targeted cell is the most important factor affecting the delivery of the drugs.
Figure no.11.Active Targeting
4.2. Responsive to stimulate targeting:
This is a newer targeting strategy, is still under investigation as several limitations have emerged after its clinical application. The main concept of this approach is that smart nanosystems start to release their encapsulated drug content after exposure to an external trigger. These triggers might be pH, temperature, light, ultrasound, or magnetic /electric field. To optimally apply this technique, one or more of the nanosystem components should be sensitive to these triggers. The advantages of responsive to stimuli targeting include:
? enhancing of nanosystem internalization and binding to the targeted cells
? controlling of the drug release
? reducing of unwanted side effects
? efficient drug distribution throughout the tumor mass and
? improving the bioavailability of some insoluble class II/IV chemotherapeutic agents.
Figure no.12. Responsive to Stimuli Targeting
4.3.Passive Targeting:Passive targeting is the primary pathway for a colloidal
Nano system via the enhanced permeability and retention (EPR) effect. The EPR effect has been extensively investigated in previous studies. These studies illustrated that the EPR effect highly depends on the degree of vascularity and efficiency of lymphatic drainage at the site of targeting. Increased leakage of blood vessels and inefficient lymphatic drainage might enhance the EPR effect and achieve better accumulation of Nano carriersin targeted tissues [4]. Fortunately, a crucial gain from the EPR effect is that it can be used to maximize the delivery of noncolloidal systems to tumour’s or cancer ous tissues due to their enhanced vascular permeability when compared with healthy tissues.
Figure no.13. Passive Targeting
5. Aim and Objectives:
5.1. Aim: To design develop and characterized a Novel Drug Delivery System.
5.2. Objective:
• Increase bioavailability
• Improve control delivery of drug.
• Improve patient compliance.
• Easy to administer
• Safe and reliable
• To improve patient compliance and treatment outcome
CONCLUSION:
In Conclusion NDDS have shown a great potential in enhancement bioavailability, solubility and permeability of the drugs. This conclusion provides a comprehensive overview of NDDS highlighting their advantages, challenges and impact on healthcare. NDDS increase accessibility to healthcare services and in the advancement in pharmaceutical research and development.
REFERENCES
- Faisal Khaled Aldawood, Abhay Andar and Salil Desai; A Comprehensive Review on Microneedles : Types, Materials,Process, Characterization and Application; Polymers;2021;13162815.
- Athira K,Vineetha,Krishnanda Kamath K,A R Shabaraya;Microspheres As A Novel Drug Delivery System-A Review;International Journal of Pharmaceutical Science Review and Research;2022;160-166.
- M.Mohan Varma, K.T.Sunil Kumar and I.Durga Srivalli;A Review On Nanoparticles:Synthesis,Characterization And Application;World Journal Of Pharmaceutical And Medical Research;2021;169-179.
- Snehal Ashok Gavhane ,Aditi Tukaram Gade;The Novel Drug Delivery System; International Journal of Creative Research Thought(IJCRT);2021;2320-2882;9.
- Munibah Qureshi, Cláudia Viegas, Sofia O.D. Duarte, Michael Girardi, Adeeb Shehzad, Pedro Fonte; Camptothecin-loaded mesoporous silica nanoparticles functionalized with CpG oligodeoxynucleotide as a new approach for skin cancer treatment; International Journal of Pharmaceutics;2024;124340;660.
- Sanket J Soni;Review on advanced drug delivery systems: innovations in formulation and delivery technologiesThe Journal of Multidisciplinary Research;2024;6-13;4(2).
- Ugochi Ewii,Adaeze Linda Onugwu,Victor C. Nwokpor,Ikanke-Abasi Akpaso;Novel Drug Delivery Systems: Insight into Self-Powered and Nano-Enabled Drug Delivery Systems; Nano Transmed;2024;100042.
- Umashankar M S Professor,Yashi Srivastava,Abdul Rahman Mohammed Al Ansari;A comprehensive review on various Novel Drug Delivery Systems and their challenges in designing of Herbal drug;Eur chem bull;2023;1309-1337;6.
- Saurabh raj Pandey,Hariom Rajput;A Review Article on Novel Drug Delivery System;Journal of research in Pharmaceutical science;2024;21-31;10.
- Ugochi E. Ewii, Adaeze L. Onugwu, Victor C. Nwokpor, Ikanke-abasi Akpaso, Toochukwu E. Ogbulie, Bibiana Aharanwa, Chinonye Chijioke Ngozi Verla, Callistus Iheme, Cosmas Ujowundu, Chioma Anyiam, Anthony A. Attama;Novel drug delivery systems: Insight into self-powered and nano-enabled drug delivery systems;Nano Transmed;2024;100042;3.
- Xingdi Wu, Mengyuan Hu, Yilu Cai, Fan Jia, Yang Ye, Naiji Yu, Min Chen, Kaijun Wang;Nano-based drug delivery systems for the treatment of non-infectious uveitis;Advances in Ophthalmology Practice and Research;2024.


 Chetan Ghulaxe *
Chetan Ghulaxe *
 Arti Nimkar
Arti Nimkar
 Asmita Choukade
Asmita Choukade
 Dhanshri Ulhe
Dhanshri Ulhe











 10.5281/zenodo.14581381
10.5281/zenodo.14581381