Transdermal drug delivery has made an important contribution to medical practice. It is a medicated patch that delivers a specific amount of medication through the skin into the blood stream. An advantage of a transdermal drug delivery route over other types of medication delivery is that the patch provides a controlled release of the medication into the patient, usually through either a porous membrane covering a reservoir of medication or through body heat melting thin layers of medication embedded in the adhesive. The present investigation was aimed to formulate transdermal films of Spironolactone and Metformin HCL, which can be used for the treatment prevailing symptoms of PCOS using Solvent evaporation method and evaluated for physicochemical parameters like thickness, weight variation, moisture uptake, moisture content, folding endurance, and drug content values. Polycystic ovary syndrome (PCOS) is currently the leading cause of menstrual complications in women. It is characterized by clinical and/or biochemical hyperandrogenism, ovulation abnormalities and the presence of enlarged and/or polycystic ovaries in ultrasound images. It is often comorbid with hyperinsulinemia, dyslipidemia, overweight or obesity, and is a risk factor for the development of diabetes. Nine transdermal patches were prepared using different concentrations of Hydrooxypropyl methyl cellulose K4M (HPMC K4M) and Ethyl cellulose as polymers and Polyethylene glycol (PEG) as plasticizer. It was concluded that as the concentration of Plasticizer increases the folding endurance increases and as the concentration of Polymer increases thickness of patch and weight uniformity increases. Percentage moisture content and percentage moisture uptake decreases with increase in polymer concentration.
Transdermal, Patch, Polycystic, Ovary, Syndrome, PCOS
TRANSDERMAL DRUG DELIVERY SYSTEM (TDDM)
Transdermal drug delivery system has been in existence for a long time. In the past, the most commonly applied systems were topically applied creams and ointments for dermatological disorders. It is a widely accepted means of drug delivery system. The occurrence of systemic side-effects with some of these formulations is indicative of absorption through the skin. A number of drugs have been applied to the skin for systemic treatment. In a broad sense, the term transdermal delivery system includes all topically administered drug formulations intended to deliver the active ingredient into the general circulation. Transdermal therapeutic systems have been designed to provide controlled continuous delivery of drugs via the skin to the systemic circulation. [1] Most of the drug molecules penetrate through the skin through intercellular micro route. However, this route of delivery is challenged by the barrier nature of skin. The epidermis is the main barrier for penetration of the drug. [4] Therefore the role of permeation or penetration enhancers in TDDS is vital as they reversibly reduce the barrier resistance without damaging viable cells. [5] Several chemical enhancers such as sulphoxide, alcohols, fatty acids, polyols, urease and physical enhancers such as sonophorosis, electroporation, iontophorosis, and magnetophorosis have been used in TDDS. [4] Moreover, it overcomes various side effects like painful delivery of the drugs and the first pass metabolism of the drug occurred by other means of drug delivery systems. [1] They can even avoid gastrointestinal problems associated with drugs and low absorption. These therapeutic advantages reflect the higher marketing potential of TDDS. [5] So, this transdermal drug delivery system has been a great field of interest in the recent time. Many drugs which can be injected directly into the blood stream via skin have been formulated. [1] The appeal of using the skin as a portal of drug entry lies in case of access, its huge surface area, and systemic access through underlying circulatory and lymphatic networks and the noninvasive nature of drug delivery. Delivery of drugs through the skin for systemic effect, called transdermal delivery was first used in 1981. [9]The drug is mainly delivered to the skin with the help of a transdermal patch which adheres to the skin. [1] The advantages of delivering drugs through the skin include: [1] 1. Hepatic first pass metabolism, salivary metabolism and intestinal metabolism are avoided. 2. The ease of usage makes it possible for patients to self-administer. 3. Since the composition of skin structurally and biologically is the same in almost all the humans, there is minimal inter and intra patient variation. 4. Drugs showing gastrointestinal irritation and absorption can be suitably administered through the skin. 5. The risks, pain and inconvenience associated with parenteral therapy are evaded. 6. The release is more prolonged than oral sustained drug delivery systems. The following are some of the disadvantages of the transdermal delivery system: [1] 1. There is possibility of skin irritation due to the one or many of the formulation components. 2. Binding of drug to skin may result in dose dumping.
TRANSDERMAL PATCH
Nicotine patches were the first transdermal patch success raising the market value of TDDS in medicine to newer heights. [5] A transdermal patch, which may also be considered a Transdermal Drug Delivery System (TDDS), is defined as a flexible, multi-layered, pharmaceutical single dose preparation of varying size containing one or more active substances to be applied to the intact skin for systemic absorption. [9] The first commercially available prescription patch was approved by the U.S. Food and Drug Administration in December 1979 containing scopolamine for motion sickness. The highest selling transdermal patch in the United States was the nicotine patch which releases nicotine to help with cessation of tobacco smoking. The first commercially available vapour patch to reduce smoking was approved in Europe in 2007. In addition, various other patches are available in market including fentanyl, an analgesic for severe pain, nitroglycerin patches for angina, lidocaine patches, marketed as Lidoderm, relieve the peripheral pain of shingles (herpes zoster), Buprenorphine, marketed as BuTrans, as analgesia for moderate to severe chronic pain. It is also now commonly used offlabel, for pain from acute injuries and chronic pain. Flector (Diclofenac Epolamine) patch is an NSAID topical patch for the treatment of acute pain due to minor strains, sprains, and contusions. It is also being used in the treatment of pain and inflammation for chronic conditions benefiting from NSAIDs including fibromyalgia and arthritis. Recent developments expanded their use to the delivery of hormonal contraceptives, antidepressants and even pain killers and stimulants for Attention Deficit Hyperactivity Disorder (ADHD). [1] A transdermal patch has several components including Baking layer, drug containing layer, rate controlling membrane, adhesive and release liner which play a vital role in the release of the drug via skin. Though all layers may not be available in all types of TDP as there are several types of transdermal patches. [4] Transdermal patches usually contain an excess of active substance than that delivered to the patient during use. This excess is necessary to maintain a clinically effective rate of delivery over time and allow the minimum patch surface area. [9] Various types of patches along with various methods of applications have been discovered to deliver the drug from the transdermal patch. Because of its great advantages, it has become one of the highly research field among the various drug delivery system. [1] The biological properties of drug for preparing transdermal patch should be of short half-life, should not produce allergic response and the drug should be potent with a daily dose of the order of a few mg/day. [4]
The advantages of delivering drugs through the Transdermal Patch: [9]
Delivery via the transdermal route is an interesting option because transdermal route is convenient and safe. The positive features of delivery drugs across the skin to achieve systemic effects are:
- In case of an emergency, removing the patch at any point of time during therapy can instantly stop drug input.
- Avoidance of first pass metabolism.
- Avoidance of gastro intestinal incompatibility.
- Predictable and extended duration of activity.
- Minimizing undesirable side effects.
- Provides utilization of drugs with short biological half-lives, narrow therapeutic window.
- Improving physiological and pharmacological response.
- Avoiding the fluctuation in drug levels. 9. Inter and intra patient variations.
- Maintain plasma concentration of potent drugs.
- Termination of therapy is easy at any point of time.
- Greater patient compliance due to elimination of multiple dosing profile.
- Ability to deliver drug more selectively to a specific site.
- Provide suitability for self-administration.
- Enhance therapeutic efficacy.
The disadvantages of delivering drugs through the Transdermal Patch: [9]
- It can be used only for chronic conditions where drug therapy is desired for a long period of time including hypertension, angina and diabetes.
- Lag time is variable and can vary from several hours to days for different drug candidates.
- Cutaneous metabolism will affect therapeutic performance of the system.
- Not practical, when the drug is extensively metabolized in the skin and when molecular size is great enough to prevent the molecules from diffusing through the skin.
- Transdermal delivery is neither practical nor affordable when required to deliver large doses of drugs through skin.
- Cannot administer drugs that require high blood levels
- Drug of drug formulation may cause irritation or sensitization.
- Not suitable for a drug, which doesn’t possess a favorable, o/w partition coefficient.
- The barrier functions of the skin of changes from one site to another on the same person, from person to person and with age.
The various steps involved in transport of drug from patch to systemic circulation are as follows:
- Diffusion of drug from drug reservoir to the rate controlling membrane.
- Diffusion of drug from rate limiting membrane to stratum corneum.
- Sorption by stratum corneum and permeation through viable epidermis.
- Uptake of drug by capillary network in the dermal papillary layer.
- Effect on target organ
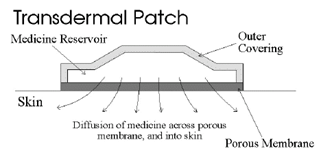
Figure 1: A typical transdermal patch showing diffusion of drug across porous membrane, and into skin.
COMPONENTS OF TRANSDERMAL PATCH: [1] [4] [5] [9]
The basic components of transdermal patch consists of polymer matrix / Drug reservoir, active ingredient (drug), permeation enhancers, pressure sensitive adhesive (PSA), backing laminates, release liner, and other excipients like plasticizers and solvents.
Polymer matrix:
Polymers are the backbone of a transdermal drug delivery system. Systems for transdermal delivery are fabricated as multilayered polymeric laminates in which a drug reservoir or a drug–polymer matrix is sandwiched between two polymeric layers: an outer impervious backing layer that prevents the loss of drug through the backing surface and an inner polymeric layer that functions as an adhesive and/or rate controlling membrane. Polymer selection and design must be considered when striving to meet the diverse criteria for the fabrication of effective transdermal delivery systems. The main challenge is in the design of a polymer matrix, followed by optimization of the drug loaded matrix not only in terms of release properties, but also with respect to its adhesion cohesion balance, physicochemical properties, compatibility and stability with other components of the system as well as with skin. [1]
The polymers utilized for TDDS can be classified as: [1]
- Natural polymers includes cellulose derivatives, zein, gelatin, shellac, waxes, gums, natural rubber and chitosan etc.
- Synthetic elastomers includes polybutadiene, hydrin rubber, polyisobutylene, silicon rubber, nitrile, acrylonitrile, neoprene, butyl rubber, etc.
- Synthetic polymers includes polyvinyl alcohol, polyvinylchloride, polyethylene, polypropylene, polyacrylate, polyamide, polyurea, polyvinylpyrrolidone, polymethylmethacrylate etc. The polymers like cross linked polyethylene glycol, eudragits, ethyl cellulose, polyvinylpyrrolidone and hydroxypropylmethylcellulose are used as matrix formers for TDDS. Other polymers like EVA, silicon rubber and polyurethane are used as rate controlling membrane.
The following criteria should be satisfied for a polymer to be used in a transdermal system: [9]
- Molecular weight, glass transition temperature, chemical functionality or polymer must allow diffusion and release of the specific drug.
- The polymer should permit the incorporation of a large amount of drug.
- The polymer should not react, physically or chemically with the drug.
- The polymer should be easily manufactured and fabricated into the desired product and in expensive.
- The polymer must be stable and must not decompose in the presence of drug and other excipients used in the formulation, at high humidity conditions, or at body temperature.
- Polymers and its degradation products must be non-toxic.
Drug:
The most important criteria for TDDS are that the drug should possess the right physicochemical and pharmacokinetic properties. Transdermal patches offer much to drugs which undergo extensive first pass metabolism, drugs with narrow therapeutic window, or drugs with short half-life which causes non-compliance due to frequent dosing. For example, drugs like rivastigmine for Alzheimer’s and Parkinson dementia, rotigotine for Parkinson, methylphenidate for attention deficit hyperactive disorder and selegiline for depression are recently approved as TDDS.
The selection of drug for transdermal drug delivery depends upon various factors: [9]
a. Physicochemical properties:
- The drug should have some degree of solubility in both oil and water (ideally greater than 1 mg/ml). 2. The substance should have melting point less than 200 °F. Concentration gradient across the membrane is directly proportional to the log solubility of drug in the lipid phase of membrane, which in turn is directly proportional to the reciprocal of melting point (in degree absolute of the drug). In order to obtain the best candidates for TDD, an attempt should be made to keep the melting point as low as possible.
- Substances having a molecular weight of less than 1000 units are suitable.
- A saturated aqueous solution of the drug should have a pH value between 5 and 9. Drugs highly acidic or alkaline in solution are not suitable for TDD; because they get ionized rapidly at physiological pH and ionized materials generally penetrate the skin poorly.
- Hydrogen bonding groups should be less than 2.
- Biological properties:
- Drug should be very potent, i.e., it should be effective in few mgs per day (ideally less than 25 mg/day).
- The drug should have short biological half-life.
- The drug should be non-irritant and non-allergic to human skin.
- The drug should be stable when in contact with the skin.
- The drug should not stimulate an immune reaction to the skin.
- Tolerance to drug must not develop under near zero order release profile of transdermal delivery. 7. The drug should not get irreversibly bound in the subcutaneous tissue.
- The should not get extensively metabolized in the skin
Permeation enhancers:
To increase permeability of stratum corneum so as to attain higher therapeutic levels of the drug permeation enhancers interact with structural components of stratum corneum i.e., proteins or lipids. The enhancement in absorption of oil soluble drugs is apparently due to the partial leaching of the epidermal lipids by the chemical enhancers, resulting in the improvement of the skin conditions for wetting and for trans-epidermal and trans-follicular permeation. The miscibility and solution properties of the enhancers used could be responsible for the enhanced transdermal permeation of water soluble drugs. [1] The penetration enhancer should be pharmacologically inert, non-toxic, non-allergenic, non-irritating and ability to act specifically, reversibly and for predictable duration. It should not cause loss of body fluids, electrolytes or other endogenous materials. [9] The permeation of drugs across the skin may also be enhanced by physical means including iontophorosis, electroporation, application of ultrasound (sonophoresis), and use of microscopic projection. Iontophorosis passes a few milliamperes of current to a few square centimeters of skin through the electrode placed in contact with the formulation, which facilitates drug delivery across the barrier. Electroporation is a method of application of short, high-voltage electrical pulses to the skin. After electroporation, the permeability of the skin for diffusion of drugs is increased by 4 orders of magnitude. The electrical pulses are believed to form transient aqueous pores in the stratum corneum, through which drug transport occurs. It is safe and the electrical pulses can be administered painlessly using closely spaced electrodes to constrain the electric field within the nerve-free stratum corneum. Application of ultrasound, particularly low frequency ultrasound, has been shown to enhance transdermal transport of various drugs including macromolecules. It is also known as sonophoresis. Transdermal patches with microscopic projections called microneedles were used to facilitate transdermal drug transport. Needles ranging from approximately 10-100 ?m in length are arranged in arrays. When pressed into the skin, the arrays make microscopic punctures that are large enough to deliver macromolecules, but small enough that the patient does not feel the permeation or pain. The drug is surface coated on the microneedles to aid in rapid absorption. They are used in development of cutaneous vaccines for tetanus and influenza. Various other methods are also used for the application of the transdermal patches like thermalporation, magnetophoresis, and photomechanical waves. However, these methods are in their early stage of development and require further detailed studies.
Pressure sensitive adhesive (PSA):
A PSA maintains an intimate contact between patch and the skin surface. It should adhere with not more than applied finger pressure, be aggressively and permanently techy, and exert a strong holding force. These include polyacrylate, polyisobutylene and silicon based adhesives. The selection of an adhesive is based on numerous factors, including the patch design and drug formulation. PSA should be physicochemical and biologically compatible and should not alter drug release. The PSA can be positioned on the face of the device (as in reservoir system) or in the back of the device and extending peripherally (as in case of matrix system).
Backing laminate:
The primary function of the backing laminate is to provide support. Backing layer should be chemical resistant and excipients compatible because the prolonged contact between the backing layer and the excipients may cause the additives to leach out or may lead to diffusion of excipients, drug or permeation enhancer through the layer. They should have a low moisture vapour transmission rate. They must have optimal elasticity, flexibility, and tensile strength. Examples of some backing materials are aluminium vapour coated layer, plastic film (polyethylene, polyvinyl chloride, polyester) and heat seal layer.
Release liner:
During storage release liner prevents the loss of the drug that has migrated into the adhesive layer and contamination. It is therefore regarded as a part of the primary packaging material rather than a part of dosage form for delivering the drug. The release liner is composed of a base layer which may be non-occlusive (paper fabric) or occlusive (polyethylene and polyvinylchloride) and a release coating layer made up of silicon or Teflon. Other materials used for TDDS release liner include polyester foil and metalized laminate.
Other excipients:
Various solvents such as chloroform, methanol, acetone, isopropanol and dichloromethane are used to prepare drug reservoir. In addition plasticizers such as dibutylpthalate, triethylcitrate, polyethylene glycol and propylene glycol are added to provide plasticity to the transdermal patch.
TYPES OF TRANSDERMAL PATCHES: [1]
Single layer drug in adhesive:
In this type the adhesive layer contains the drug. The adhesive layer not only serves to adhere the various layers together and also responsible for the releasing the drug to the skin. The adhesive layer is surrounded by a temporary liner and a backing.

Figure 2: Design of Single layer drug in adhesive transdermal patch.
Multi -layer drug in adhesive:
This type is also similar to the single layer but it contains an immediate drug-releaselayer and other layer will be a controlled release along with the adhesive layer. The adhesive layer is responsible for the releasing of the drug. This patch also has a temporary liner-layer and a permanent backing.

Figure 3: Design of Multi- layer drug in adhesive transdermal patch.
Vapour patch:
The patch containing the adhesive layer not only serves to adhere the various surfaces together but also serves as to release the vapour. The vapour patches are new to the market, commonly used for releasing the essential oils in decongestion. Various other types of vapour patches are also available in the market which are used to improve the quality of sleep and reduces the cigarette smoking conditions.
Reservoir system:
In this system the drug reservoir is embedded between an impervious backing layer and a rate controlling membrane. The drug releases only through the rate controlling membrane, which can be micro porous or non-porous. In the drug reservoir compartment, the drug can be in the form of a solution, suspension, gel or dispersed in a solid polymer matrix. Hypoallergenic adhesive polymer can be applied as outer surface polymeric membrane which is compatible with drug.

Figure 4: Design of Reservoir system transdermal patch.
Microreservoir system:
The system consists of microscopic spheres of drug reservoirs which releases drug at a zero order rate for maintaining constant drug levels. Microreservoir system is a combination of reservoir and matrixdispersion system. The aqueous solution of water soluble polymer is mixed with drug to form a reservoir. It is then followed by dispersing the solution homogeneously using high shear mechanical force in a lipophilic polymer to form thousands of microscopic drug reservoirs. Cross linking agents are added to stabilize the thermodynamically unstable dispersion by in-situ cross-linking the polymer.

Figure 5: Design of microreservoir transdermal patch.
VARIOUS METHODS FOR PREPARATION OF TDDS: [1] [9]
Circular Teflon mould method:
Solutions containing polymers in various ratios are used in an organic solvent. Calculated amount of drug is dissolved in half the quantity of same organic solvent. Enhancers in different concentrations are dissolved in the other half of the organic solvent and then added. Plasticizer (e.g., Di-N-butylphthalate) is added into the drug polymer solution. The total contents are to be stirred for 12 hrs and then poured into a circular Teflon mould. The moulds are to be placed on a levelled surface and covered with inverted funnel to control solvent vaporization in a laminar flow hood model with an air speed of 0.5 m/s. The solvent is allowed to evaporate for 24 h. The dried films are to be stored for another 24 h at 25±0.5°C in a desiccators containing silica gel before evaluation to eliminate aging effects. These types of films are to be evaluated within one week of their preparation.
Mercury substrate method:
The drug is dissolved in polymer solution along with plasticizer. It is followed by stirring for 10- 15 minutes to produce a homogenous dispersion and poured into a levelled mercury surface, covered with inverted funnel to control solvent evaporation.
“IPM membranes” method:
The drug is dispersed in a mixture of water and propylene glycol containing carbomer940 polymers and stirred for 12 h in magnetic stirrer. The dispersion is to be neutralized and made viscous by the addition of tri-ethanolamine. Buffer (pH 7.4) can be used in order to obtain solution gel, if the drug solubility in aqueous solution is very poor. The formed gel will be incorporated in the IPM (isopropyl myristate) membrane. 1.2.3.4. “EVAC membranes” method: In order to prepare the target transdermal therapeutic system, 1?rbopol reservoir gel, polyethelene (PE), ethylene vinyl acetate copolymer (EVAC) membranes can be used as rate control membranes. If the drug is not soluble in water, propylene glycol is used for the preparation of gel. Drug is dissolved in propylene glycol; carbopol resin will be added to the above solution and neutralized by using 5% w/w sodium hydroxide solution. The drug (in gel form) is placed on a sheet of backing layer covering the specified area. A rate controlling membrane will be placed over the gel and the edges will be sealed by heat to obtain a leak proof device.
Aluminium backed adhesive film method:
Transdermal drug delivery system may produce unstable matrices if the loading dose is greater than 10 mg. Aluminium backed adhesive film method is a suitable one. For preparation of same, chloroform is choice of solvent, because most of the drugs as well as adhesive are soluble in chloroform. The drug is dissolved in chloroform and adhesive material will be added to the drug solution and dissolved. A custom-made aluminium former is lined with aluminium foil and the ends blanked off with tightly fitting cork blocks.
Free film method:
Free film of cellulose acetate is prepared by casting on mercury surface. A polymer solution (e.g., 2% w/w) is prepared using organic solvent (e.g., chloroform). The optimized concentration of plasticizer is incorporated to the polymer solution (e.g., 40% w/w of polymer weight). Small volume (e.g., 5 ml) of polymer solution is poured in a glass ring which is placed over the mercury surface in a glass petri dish. The rate of evaporation of the solvent is controlled by placing an inverted funnel over the petri dish. The film formation is noted by observing the mercury surface after complete evaporation of the solvent. The dried film is separated out and stored between the sheets of wax paper in a desiccator until use. Free films of different thickness can be prepared by changing the volume of the polymer solution.
EVALUATION OF TRANSDERMAL PATCHES: [1] [4] [9]
Transdermal patches have been developed to improve clinical efficacy of the drug and to enhance patient compliance by delivering smaller amount of drug at a predetermined rate. This makes evaluation studies even more important in order to ensure their desired performance and reproducibility under the specified environmental conditions. These studies are predictive of transdermal dosage forms and can be classified into different types including physicochemical evaluation, in-vitro evaluation, and in-vivo evaluation. After the successful evaluation of physicochemical and in-vitro studies, in-vivo evaluations may be conducted.
Physicochemical Evaluation:
Thickness:
The thickness of transdermal film is determined by travelling microscope, dial gauge, screw gauge or micrometer at different points of the film.
Uniformity of weight:
Weight variation is studied by individually weighing 10 randomly selected patches and calculating the average weight. The individual weight should not deviate significantly from the average weight.
Drug content determination:
It can be determined by completely dissolving a small area (1 cm2 ) of polymeric film in suitable solvent of definite volume. The solvent is selected in which the drug is freely soluble. The selected area is weighed before dissolving in the solvent. The whole content is shaken continuously for 24 h in a shaker incubator followed by sonication and filtration. The drug in solution is assessed by appropriate analytical method.
Content uniformity test:
The test is applied as the gold standard to determine chemically the content of active constituent for each unit dose. The test is completed by performing assay to find out the content of drug material contained in polymeric film of the patch. According to USP the procedure consists of two stages. First stage consists of assaying the randomly selected ten units. It is followed by second stage to be performed on twenty more units when the first stage fails. Initially ten patches are selected and content is determined for individual patches. Test passes when all 10 unit doses have content ? 85 % and ? 115 % (RSD < 6>
Moisture content:
The prepared films are weighed individually and kept in a desiccators containing calcium chloride at room temperature for 24 h. The films are weighed again after a specified interval until they show a constant weight. The percent moisture content is calculated using following formula.
%Moisture Content = (???????????????????????????? ????????????????????? – ???????????????????? ?????????????????????) ???? 100 /???????????????????? ?????????????????????
Moisture Uptake:
Weighed films are kept in a desiccator at room temperature for 24 h. These are then taken out and exposed to 84% relative humidity using saturated solution of Potassium chloride in a desiccator until a constant weight is achieved. % moisture uptake is calculated as given below.
% Moisture Uptake = (???????????????????? ?????????????????????????????????????????????????? ?????????????????????)???? 100 /???????????????????????????? ?????????????????????
Folding Endurance:
Evaluation of folding endurance involves determining the folding capacity of the films subjected to frequent extreme conditions of folding. Folding endurance is determined by repeatedly folding the film at the same place until it break. The number of times the films could be folded at the same place without breaking gives the folding endurance value. [4]
Tensile Strength:
To determine tensile strength, polymeric films are sandwiched separately by corked linear iron plates. One end of the films is kept fixed with the help of an iron screen and other end is connected to a freely movable thread over a pulley. The weights are added gradually to the pan attached with the hanging end of the thread. A pointer on the thread is used to measure the elongation of the film. The weight just sufficient to break the film is noted. The tensile strength can be calculated using the following equation. [1]
Tensile Strength = ????/ ????.???? (1 + ???? /???? )
Where,
‘F’ is the force required to break.
‘a’ is width of film
‘b’ is thickness of film
‘L’ is length of film
‘l’ is elongation of film at break point
Water vapour transmission studies (WVT):
WVT is determined by taking one gram of calcium chloride in previously dried empty vials having equal diameters. The polymer films are pasted over the brim with the help of adhesive like silicon adhesive grease and then allowed to set for 5 minutes. The vials are accurately weighed and placed in humidity chamber maintained at 68 % RH. The vials are then weighed repeatedly up to seven consecutive days and an increase in weight was considered as a quantitative measure of moisture transmitted through the patch. [1]
Adhesive studies:
The therapeutic performance of TDDS can be affected by the quality of contact between the patch and the skin. The adhesion of a TDDS to the skin is obtained by using PSAs, which are defined as adhesives capable of bonding to surfaces with the application of light pressure. The adhesive properties of a TDDS can be characterized by considering the following factors. [5]
Peel Adhesion properties:
It is the force required to remove adhesive coating from test substrate. It is tested by measuring the force required to pull a single coated tape, applied to substrate at 180° angle. The test is passed if there is no residue on the substrate. [1]
Shear strength properties or creep resistance:
Shear strength is the measurement of the cohesive strength of an adhesive polymer i.e., device should not slip on application determined by measuring the time it takes to pull an adhesive coated tape off a stainless plate. [1]
In-vitro studies
In-vitro release studies
The amount of drug available for absorption to the systemic pool is greatly dependent on drug released from the polymeric transdermal films. Diffusion cells include Franz-diffusion cell and its modification Keshary-Chien Cell. In this method transdermal system is placed in between receptor and donor compartment of the diffusion cell. The transdermal system faces the receptor compartment in which receptor fluid (e.g., drug solution) is placed. The agitation speed and temperature are kept constant. The whole assembly is kept on magnetic stirrer and solution in the receiver compartment is constantly and continuously stirred throughout the experiment using magnetic beads. At predetermined time intervals, the receptor fluid is removed for analysis and is replaced with an equal volume of fresh receptor fluid. The concentration of drug is determined by suitable analytical method. The pH of the dissolution medium ideally should be adjusted to pH 5 to 6, reflecting physiological skin conditions. For the same reason, the test temperature is typically set at 32°C (even though the temperature may be higher when skin is covered). [9]
In-vitro permeation studies
After release from the polymeric films, drug reaches at skin surface is then passed to the dermal microcirculation by permeation through cells of epidermis and/or between the cells of epidermis through skin appendages. Usually permeation studies are performed by placing the fabricated transdermal patch with rat skin or synthetic membrane in between receptor and donor compartment in a vertical diffusion cell such as Franz diffusion cell or Keshary-Chien diffusion cell. The transdermal system is applied to the hydrophilic side of the membrane and then mounted in the diffusion cell with lipophillic side in contact with receptor fluid. The receiver compartment is maintained at specific temperature (usually 32±5°C for skin) and is continuously stirred at a constant rate. The samples are withdrawn at different time intervals and equal amount of buffer is replaced each time. The samples are diluted appropriately and estimated by suitable analytical method. The amount of drug permeated per square centimeter at each time interval is calculated. Many variables including design of system, patch size, surface area of skin, thickness of skin and temperature may affect the in-vitro properties of drug. Thus, the permeation studies involves preparation of skin, mounting of skin on permeation cell, setting of experimental conditions like temperature, stirring, sink conditions, withdrawing samples at different time intervals, sample analysis and calculation of flux (i.e., drug permeated per unit area per unit time). [9]
In-vivo Studies
In-vivo evaluations are the true depiction of the drug performance. The variables which cannot be taken into account during in-vitro studies can be fully explored during in-vivo studies. In-vivo evaluation of TDDS may be carried out using either animal models or human volunteers or both. [1]
Animal models
Considerable time and resources are required to carry out human studies, so animal studies are preferred at small scale. The most common animal species used for evaluating transdermal drug delivery systems are mouse, hairless rat, hairless dog, hairless rhesus monkey, rabbit, guinea pig etc. Based on the experiments conducted so far it is concluded that hairless animals are preferred over hairy animals in both in-vitro and in-vivo experiments. Rhesus monkey is one of the most reliable models for in-vivo evaluation of transdermal drug delivery. [1]
Human models:
The final stage of the development of a transdermal device involves collection of pharmacokinetic and pharmacodynamics data following application of the patch to human volunteers. Clinical trials are conducted to assess the transdermal systems including the efficacy, risk involved, side effects, and patient compliance. Phase-I clinical trials are conducted to determine mainly safety in volunteers and phase-II clinical trials determine short term safety and mainly effectiveness in patients. Phase-III trials indicate the safety and effectiveness in large number of patient population and phase-IV trials at post marketing surveillance are done for marketed patches to detect adverse drug reactions. Though human studies require considerable resources but they are the best to assess the performance of the drug. [1]
IDEAL PRODUCT REQUIREMENTS: [9]
- Shelf life up to 2 years.
- Small size patch (i.e., less than 40 cm2).
- Convenient dose frequency (i.e., once a day to once a week).
- Cosmetically acceptable (i.e., clear, white color).
- Simple packaging (i.e., minimum number of pouches and steps required to apply the system).
- Easy removal of the release liner (i.e., for children and elderly patients).
- Adequate skin adhesion (i.e., no fall off during the dosing interval and easy removal without skin trauma).
- No residue (i.e., “cold flow” around the edge of the patch in storage or after application to skin or beneath the patch after removal).
- No unacceptable dermal reactions (i.e., contact dermatitis, skin sensitization, photo toxicity, photosensitization, erythema, itching, stinging, burning, etc.).
- Consistent biopharmaceutical performance (i.e., precision of the required pharmacokinetic and pharmacodynamics response between individuals and in the same individuals over time.
STRUCTURE OF SKIN
THE SKIN:
The skin of an average adult body covers a surface area of approximately 2 sq. m. and receives about one third of the blood circulating through the body and serves as a permeability barrier against the transdermal absorption of various chemical and biological agent. [9] The skin can be considered to have four distinct layers of tissues including non-viable epidermis (stratum corneum), viable epidermis, viable dermis and hypodermis (subcutaneous connective tissue). The epidermis is the relatively thin, tough, outer layer of the skin. The epidermis has keratinocytes. They originate from cells in the deepest layer of the epidermis called the basal layer. New keratinocytes slowly migrate up toward the surface of the epidermis. Stratum corneum (Non-viable epidermis) is the outermost portion of the epidermis, relatively waterproof and, when undamaged, prevents most bacteria, viruses, and other foreign substances from entering the body. The epidermis also protects the internal organs, muscles, nerves, and blood vessels against trauma. The outer keratin layer of the epidermis (stratum corneum) is much thicker. Viable Epidermis layer of the skin resides between the stratum corneum and the dermis and has a thickness ranging from 50-100 ?m. The structure of the cells in the viable epidermis is physiochemically similar to other living tissues. Cells are held together by tonofibrils. The water content is about 90%. The dermis, the skin's next layer, is a thick layer of fibrous and elastic tissue (made mostly of collagen, elastin and fibrillin) that gives the skin its flexibility and strength. The dermis contains nerve endings, sweat glands and oil glands, hair follicles, and blood vessels. The subcutaneous tissue also known as hypodermis is not actually accepted as a true part of the structured connective tissue. It is composed of loose textured, white, fibrous connective tissue containing blood and lymph vessels. Most investigators consider the drug permeating through the skin enter the circulatory system before reaching the hypodermis where the fatty tissue serve as a depot of the drug.
PATHWAYS OF SKIN PERMEATION:
An important function of the skin is to protect the body from the external environment, and it is normally a very effective barrier to the permeation of active substances. However, for certain active substances, depending on their physicochemical properties, passive diffusion is possible to achieve a therapeutic effect. [6] Drug molecules permeate through skin surface by the different potential pathways including through the sweat ducts, through the hair follicles and sebaceous glands or directly across the stratum corneum. Since the last few years there is a point of debate among scientists for the relative importance of the shunt or appendageal route of transport across the stratum corneum and is further complicated by the lack of a suitable experimental model to permit separation of the these pathways. [1] Most of the drug molecules penetrate through the skin through intercellular micro route and therefore the role of permeation or penetration enhancers in TDDS is vital as they reversibly reduce the barrier resistance of the stratum corneum without damaging viable cells. [5]
BASIC PRINCIPLES OF TRANSDERMAL PERMEATION:
Earlier, skin was considered as an impermeable protective barrier, but later investigations were carried out which proved the utility of skin as a route for systemic administration. [1] Transdermal permeation is based on passive diffusion. Before a topically applied drug can act either locally or systemically, it must penetrate the stratum corneum – the skin permeation barrier. In the initial transient diffusion stage drug molecules may penetrate the skin along the hair follicles or sweat ducts and then absorbed through the follicular epithelium through the intact stratum corneum becomes the primary pathway for transdermal permeation. The release of a therapeutic agent from a formulation applied to the skin surface and its transport to the systemic circulation is a multistep process, which involves: [9]
-
- Dissolution with skin and release from the formulation.
- Partitioning into the skin’s outermost layer, the stratum corneum.
- Diffusion through the SC, principally via a lipidic intercellular pathway.
- Partitioning from the SC into the aqueous viable epidermis, diffusion through the viable epidermis and into the upper dermis, and uptake into the papillary dermis and into the microcirculation.

Blood tests.
Your blood may be analyzed to measure hormone levels. This testing can exclude possible causes of menstrual abnormalities or androgen excess that mimics PCOS. You might have additional blood testing to measure glucose tolerance and fasting cholesterol and triglyceride levels. [7]
An ultrasound.
Your doctor checks the appearance of your ovaries and the thickness of the lining of your uterus. A wandlike device (transducer) is placed in your vagina (transvaginal ultrasound). The transducer emits sound waves that are translated into images on a computer screen. During a transvaginal ultrasound, your doctor or a medical technician inserts a wandlike device (transducer) into your vagina while you lie on your back on an exam table. The transducer emits sound waves that generate images of your pelvic organs, including your ovaries. On an ultrasound image (inset), a polycystic ovary shows many follicles. Each dark circle on the ultrasound image represents a fluid-filled follicle in the ovary. Your doctor may suspect PCOS if you have 20 or more follicles in each ovary. [7]

Figure 7: An Ultrasound Diagnosis
MANAGEMENT OF PCOS: [2] [3] [7] [11]
PCOS treatment focuses on managing your individual concerns, such as infertility, hirsutism, acne or obesity. Specific treatment might involve lifestyle changes or medication.
Lifestyle changes:
Your doctor may recommend weight loss through a low-calorie diet combined with moderate exercise activities. Even a modest reduction in your weight- for example, losing 5 percent of your body weight- might improve your condition. Losing weight may also increase the effectiveness of medications your doctor recommends for PCOS, and can help with infertility.
Medications:
To regulate your menstrual cycle, your doctor might recommend:
1. Combination birth control pills.
Pills that contain estrogen and progestin decrease androgen production and regulate estrogen. Regulating your hormones can lower your risk of endometrial cancer and correct abnormal bleeding, excess hair growth and acne. Instead of pills, you might use a skin patch or vaginal ring that contains a combination of estrogen and progestin.
2. Progestin therapy.
Taking progestin for 10 to 14 days every one to two months can regulate your periods and protect against endometrial cancer. Progestin therapy doesn't improve androgen levels and won't prevent pregnancy. The progestin-only minipill or progestin-containing intrauterine device is a better choice if you also wish to avoid pregnancy.
To help you your doctor might recommend:
1. Clomiphene (Clomid). This oral anti-estrogen medication is taken during the first part of your menstrual cycle.
2. Letrozole (Femara). This breast cancer treatment can work to stimulate the ovaries.
3. Metformin (Glucophage, Fortamet, others). This oral medication for type 2 diabetes improves insulin resistance and lowers insulin levels. If you don't become pregnant using clomiphene, your doctor might recommend adding metformin. If you have prediabetes, metformin can also slow the progression to type 2 diabetes and help with weight loss.
4. Gonadotropins. These hormone medications are given by injection.
5. Birth control pills. These pills decrease androgen production that can cause excessive hair growth.
6. Spironolactone (Aldactone). This medication blocks the effects of androgen on the skin. Spironolactone can cause birth defect, so effective contraception is required while taking this medication. It isn't recommended if you're pregnant or planning to become pregnant.
7. Eflornithine (Vaniqa). This cream can slow facial hair growth in women.
8. Electrolysis. A tiny needle is inserted into each hair follicle. The needle emits a pulse of electric current to damage and eventually destroy the follicle. You might need multiple treatments.
Lifestyle and home remedies:
To help decrease the effects of PCOS, try to:
1. Maintain a healthy weight. Weight loss can reduce insulin and androgen levels and may restore ovulation. Ask your doctor about a weight-control program, and meet regularly with a dietitian for help in reaching weight-loss goals.
2. Limit carbohydrates. Low-fat, high-carbohydrate diets might increase insulin levels. Ask your doctor about a low-carbohydrate diet if you have PCOS. Choose complex carbohydrates, which raise your blood sugar levels more slowly.
3. Be active. Exercise helps lower blood sugar levels. If you have PCOS, increasing your daily activity and participating in a regular exercise program may treat or even prevent insulin resistance and help you keep your weight under control and avoid developing diabetes.
DRUG PROFILE:
Metformin:


Spironolactone:


REVIEW OF LITERATURE
-
- Alam I., et al (2013): Now a day about 74% of drugs are taken orally and are found not to be as effective as desired. To improve such characters transdermal drug delivery system was emerged. Drug delivery through the skin to achieve a systemic effect of a drug is commonly known as transdermal drug delivery and differs from traditional topical drug delivery. Transdermal drug delivery systems (TDDS) are dosage forms involves drug transport to viable epidermal and or dermal tissues of the skin for local therapeutic effect while a very major fraction of drug is transported into the systemic blood circulation. The adhesive of the transdermal drug delivery system is critical to the safety, efficacy and quality of the product. Topical administration of therapeutic agents offers many advantages over conventional oral and invasive methods of drug delivery. Several important advantages of transdermal drug delivery are limitation of hepatic first pass metabolism, enhancement of therapeutic efficiency and maintenance of steady plasma level of the drug. This review article provides an overview of TDDS, its advantages over conventional dosage forms, drug delivery routes across human skin, permeation enhancers, and various components of transdermal patches, types of transdermal patches, methods of preparation and its methods of evaluation.[1]
- Badawy A., et al (2011): Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women. The clinical manifestation of PCOS varies from a mild menstrual disorder to severe disturbance of reproductive and metabolic functions. Management of women with PCOS depends on the symptoms. These could be ovulatory dysfunction-related infertility, menstrual disorders, or androgen-related symptoms. Weight loss improves the endocrine profile and increases the likelihood of ovulation and pregnancy. Normalization of menstrual cycles and ovulation could occur with modest weight loss as little as 5% of the initial weight. The treatment of obesity includes modifications in lifestyle (diet and exercise) and medical and surgical treatment. In PCOS, anovulation relates to low follicle-stimulating hormone concentrations and the arrest of antral follicle growth in the final stages of maturation. This can be treated with medications such as clomiphene citrate, tamoxifen, aromatase inhibitors, metformin, glucocorticoids, or gonadotropins or surgically by laparoscopic ovarian drilling. In vitro fertilization will remain the last option to achieve pregnancy when others fail. Chronic anovulation over a long period of time is also associated with an increased risk of endometrial hyperplasia and carcinoma, which should be seriously investigated and treated. There are androgenic symptoms that will vary from patient to patient, such as hirsutism, acne, and/or alopecia. These are troublesome presentations to the patients and require adequate treatment. Alternative medicine has been emerging as one of the commonly practiced medicines for different health problems, including PCOS. This review underlines the contribution to the treatment of different symptoms. [2
- Bednarska S., et al (2017): Polycystic ovary syndrome (PCOS) is currently the leading cause of menstrual complications in women. It is characterized by clinical and/or biochemical hyperandrogenism, ovulation abnormalities and the presence of enlarged and/or polycystic ovaries in ultrasound images (12 or more small bubbles located circumferentially and/or ovarian volume > 10 mL). It is often comorbid with hyperinsulinemia, dyslipidemia, overweight or obesity, and is a risk factor for the development of diabetes and cardiovascular diseases (CVDs). The treatment of patients with PCOS depends on the prevailing symptoms. The aim of this paper is to present the etiopathogenesis, clinical and biochemical implications, and non-pharmacological and pharmacological treatment options – those approved by worldwide scientific organizations as well as new therapies whose initial results are encouraging enough to prompt researchers to explore them further. [3]
- Budhathoki U., et al (2016): This study was carried out to develop matrix based transdermal patches containing Atenolol. A 2 factors HPMC (hydroxyl propyl methyl cellulose) K4M and PVP (Polyvinyl Pyrolidone) 3 level (23) factorial design was done using Design Expert® which gave 13 experiments. The patches were prepared by Solvent casting method. Propylene glycol (3%) and Tween 80 (6%) were used as plasticizer and permeation enhancer respectively. Physicochemical characteristics and In-Vitro permeation study of formulated transdermal patches were carried out. Contour plot suggested 770mg of PVP and 265mg of HPMC K4M in Optimized formulation. [4]
- Committee for Medicinal Products for Human Use (CHMP), (2014): This guideline addresses new marketing authorization applications (including generic or abridged applications) and subsequent variation submissions for transdermal patches for systemic delivery. Guidance is provided on the quality requirements for the description, development, manufacture, characterization of excipients, control of drug product, packaging and stability of transdermal patches. In particular, in vitro performance testing with respect to drug release, adhesion and skin permeation is discussed, together with its relation to clinical and in vivo performance. It should be read in conjunction with the Guideline on the Pharmacokinetic and clinical evaluation of modified-release dosage forms. Transdermal patches are designed to provide a controlled delivery of the active substance(s) through the skin, principally by diffusion, resulting in a defined rate and extent of systemic delivery of active substance. [5]
- Costello M., (2005): Polycystic ovary syndrome (PCOS) is the most common endocrinopathy in women and is associated with both reproductive and metabolic disorders. This article discusses the health risks, clinical assessment and investigations consistent with the new internationally agreed definition of PCOS, available treatments, and the long term monitoring of women with PCOS. Women with PCOS have an increased risk of endometrial carcinoma, type 2 diabetes mellitus, and possibly cardiovascular disease. Polycystic ovary syndrome is a diagnosis of exclusion of other causes of hyperandrogenism. Screening for diabetes is important. Treatment is directed at the presenting symptom as the primary cause is unknown. Long term medical treatment may include the oral contraceptive pill or metformin. Long term surveillance is recommended for the early detection and treatment of potential metabolic complications. [6]
- Graham A., et al (2016): Polycystic ovarian syndrome (PCOS) is a common and complex gynaecological endocrine disorder. The spectrum of clinical features includes ovulatory dysfunction (oligomenorrhoea/ amenorrhoea) and hyperandrogenism leading to hirsutism, alopecia and acne. These symptoms can cause considerable patient distress and be difficult to manage. In addition, PCOS is associated with metabolic syndrome, reproductive difficulties, long-term cardiovascular issues and endometrial cancer. This review provides an overview of PCOS, with a focus on therapeutics in primary care and when to refer for specialist treatment.[7]
- Jalwal P., et al (2010): The administration of drugs by transdermal route offers the advantage of being relatively painless. The appeal of using the skin as a portal of drug entry lies in case of access, its huge surface area, and systemic access through underlying circulatory and lymphatic networks and the noninvasive nature of drug delivery. Delivery of drugs through the skin for systemic effect, called transdermal delivery was first used in 1981, when Ciba-Geigy marketed Transderm V (present day marketed as Transderm Scop) to prevent the nausea and vomiting associated with motion sickness. Transdermal drug delivery offers controlled release of the drug into the patient, it enables a steady blood level profile, resulting in reduced systemic side effects and, sometimes, improved efficacy over other dosage forms. The main objective of transdermal drug delivery system is to deliver drugs into systemic circulation through skin at predetermined rate with minimal inter and intrapatient variation. [8] Kriplani P., et al (2018): Transdermal drug delivery has made an important contribution to medical practice. It is a medicated patch that delivers a specific amount of medication through the skin into the blood stream. An advantage of a transdermal drug delivery route over other types of medication delivery is that the patch provides a controlled release of the medication into the patient, usually through either a porous membrane covering a reservoir of medication or through body heat melting thin layers of medication embedded in the adhesive. The present investigation was aimed to formulate transdermal films of nonsteroidal antiinflammatory drug, Diclofenac sodium using mercury substrate method and evaluated for physicochemical parameters like thickness, weight variation, moisture uptake, moisture content, folding endurance, and drug content values. Three transdermal patches were prepared using different concentrations of ethyl cellulose. It was concluded that as the concentration of polymer increases the thickness of patch, weight uniformity and folding endurance increases. Percentage moisture content and percentage moisture uptake decreases with increase in polymer concentration. [9]
- Lucidi R. (2019): Women with polycystic ovarian syndrome (PCOS) have abnormalities in the metabolism of androgens and estrogen and in the control of androgen production. PCOS can result from abnormal function of the hypothalamic-pituitary-ovarian (HPO) axis. A woman is diagnosed with polycystic ovaries (as opposed to PCOS) if she has 20 or more follicles in at least 1 ovary. The major features of PCOS include menstrual dysfunction, anovulation, and signs of hyperandrogenism. Exclude all other disorders that can result in menstrual irregularity and hyperandrogenism, including adrenal or ovarian tumors, thyroid dysfunction, congenital adrenal hyperplasia, hyperprolactinemia, acromegaly, and Cushing syndrome. An ovarian biopsy may be performed for histologic confirmation of PCOS; however, ultrasonographic diagnosis of PCOS has generally superseded histopathologic diagnosis. An endometrial biopsy may be obtained to evaluate for endometrial disease, such as malignancy. Lifestyle modifications are considered first-line treatment for women with PCOS. Pharmacologic treatments are reserved for so-called metabolic derangements, such as anovulation, hirsutism, and menstrual irregularities. [10]
- Radosh L, (2009): Polycystic ovary syndrome is a condition present in approximately 5 to 10 percent of women of childbearing age. Diagnosisncan be difficult because the signs and symptoms can be subtle and varied. These may include hirsutism, infertility, menstrual irregularities, and biochemical abnormalities, most notably insulin resistance. Treatment should target specific manifestations and individualized patient goals. When choosing a treatment regimen, physicians must take into account comorbidities and the patient’s desire for pregnancy. Lifestyle modifications should be used in addition to medical treatments for optimal results. Few agents have been approved by the U.S. Food and Drug Administration specifically for use in polycystic ovary syndrome, and several agents are contraindicated in pregnancy. Insulin-sensitizing agents are indicated for most women with polycystic ovary syndrome because they have positive effects on insulin resistance, menstrual irregularities, anovulation, hirsutism, and obesity. Metformin has the most data supporting its effectiveness. Rosiglitazone and pioglitazone are also effective for ameliorating hirsutism and insulin resistance. Metformin and clomiphene, alone or in combination, are first-line agents for ovulation induction. Insulinsensitizing agents, oral contraceptives, spironolactone, and topical eflornithine can be used in patients with hirsutism.
- Patel K, et al, (2012): The purpose of this research was to develop a matrix-type transdermal therapeutic system containing drug Diclofenac acid, Pressure Sensitive Adhesive (PSA) like acrylic adhesive by the solvent evaporation technique. Different concentrations of Labrasol, oleic acid and triacetin were used to enhance the transdermal permeation of diclofenac acid. Polyethylene monolayer film as a backing membrane and Silicone coated polyester film as a release liner preferred in preparation of transdermal patches. Formulated transdermal patches were physically evaluated with regard to percentage moisture absorption, thickness, weight variation, drug content, tensile strength, % elongation, folding endurance. All prepared formulations indicated good physical stability. In vitro skin permeation studies of formulations were performed by using Franz diffusion cells. Formulation containing 5% drug, 85?hesive solution and 10% triacetin as permeation enhancer showed best in vitro skin permeation through human cadaver skin as compared to all other formulations. The results rate was found to follow zero order kinetics. These results indicate that the formulation F3 has shown optimum release in concentration independent manner. Stability study indicates that drug remains stable for six months and primary irritation study indicated that the transdermal patches are non-irritant. [11]
- Patel D., et al, (2012): A transdermal patch is a medicated adhesive patch that is placed on the skin to deliver a specific dose of medication through the skin and into the bloodstream. Often, this promotes healing to an injured area of the body. An advantage of a transdermal drug delivery route over other types of medication delivery such as oral, topical, intravenous, intramuscular, etc. is that the patch provides a controlled release of the medication into the patient, usually through either a porous membrane covering a reservoir of medication or through body heat melting thin layers of medication embedded in the adhesive. The main disadvantage to transdermal delivery systems stems from the fact that the skin is a very effective barrier; as a result, only medications whose molecules are small enough to penetrate the skin can be delivered in this method. A wide variety of pharmaceuticals are now available in transdermal patch form. [12]
- Patel R., et al, (2009): The purpose of this research was to develop a matrix-type transdermal therapeutic system containing drug Aceclofenac with different ratios of hydrophilic (hydroxyl propyl cellulose) and hydrophobic (ethyl cellulose) polymeric systems by the solvent evaporation technique by using 15 % w/w of dibutyl phthalate to the polymer weight, incorporated as plasticizer. Different concentrations of oleic acid and isopropyl myristate were used to enhance the transdermal permeation of Aceclofenac. The physicochemical compatibility of the drug and the polymers studied by differential scanning calorimetry and infrared spectroscopy suggested absence of any incompatibility. Formulated transdermal films were physically evaluated with regard to thickness, weight variation, drug content, flatness, tensile strength, folding endurance, percentage of moisture content and water vapour transmission rate. All prepared formulations indicated good physical stability. In-vitro permeation studies of formulations were performed by using Franz diffusion cells. Formulation prepared with hydrophilic polymer containing permeation enhancer showed best in-vitro skin permeation through rat skin (Wistar albino rat) as compared to all other formulations. The results followed the release profile of Aceclofenac followed mixed zero-order and first-order kinetics in different formulation. However, the release profile of the optimized formulation F9 (r2 = 0.9935 for Higuchi) indicated that the permeation of the drug from the patches was governed by a diffusion mechanism. Formulation F9 showed highest flux among all the formulations and 1.369 fold enhancements in drug permeation. These results indicate that the formulation containing 15 % of oleic acid with 10 % Isopropyl myristate give better penetration of Aceclofenac through rat skin. [13] Prausnitz M., et al, (2008): Transdermal drug delivery has made an important contrib ution to medical practice, but has yet to fully achieve its potential as an alternative to oral delivery and hypodermic injections. Firstgeneration transdermal delivery systems have continued their steady increase in clinical use for delivery of small, lipophilic, low-dose drugs. Secondgeneration delivery systems using chemical enhancers, non cavitational ultrasound and iontophoresis have also resulted in clinical products; the ability of iontophoresis to control delivery rates in real time provides added functionality. Thirdgeneration delivery systems target their effects to skin’s barrier layer of stratum corneum using microneedles, thermal ablation, microdermabrasion, electroporation and cavitational ultrasound. Microneedles and thermal ablation are currently progressing through clinical trials for delivery of macromolecules and vaccines, such as insulin, parathyroid hormone and influenza vaccine. Using these novel second- and third-generation enhancement strategies, transdermal delivery is poised to significantly increase impact on medicine. [14]
- Sheth N., et al, (2011): Since oral bioavailability of Propranolol Hydrochloride is poor due to high first pass metabolism different matrix- type transdermal patches incorporating Propranolol Hydrochloride were formulated with an objective to study the effect of polymers on transdermal release of the drugs. The polymers selected for sustaining the release of drug were polyvinylpyrrolidone, Hydroxypropylmethycellulose (HPMC) and Ethyl cellulose (EC). The patches were formulated using combination of polymers and propylene glycol as plasticizer. The physicochemical evaluation of the polymer matrices was performed for suitability. In vitro permeation studies were performed using rat abdominal skin as the permeating membrane in Franz diffusion cell. The result indicated that maximum release was obtained at 2% solution of EC. Optimized batch was evaluated for permeation enhancement through rat skin using natural permeation enhancer Eugenol and it was concluded that permeation enhancement through Eugenol was comparable to the commercially available permeation enhancer Dimethyl sulfoxide 1% (DMSO). All the films were found to be stable at 37ºC and 45ºC with respect to their physical parameters and drug content. [17]
- Singh A., et al, (2016): Duloxetine hydrochloride is an antidepressant drug also approved for diabetic neuropathy, anxiety disorders, and fibromyalgia requiring repeated administration on chronic basis. The objective of this study was to develop a transdermal drug delivery system for duloxetine hydrochloride as a once daily dosage form. Transdermal patches were prepared by solvent evaporation method employing controlled release grades of HPMC in presence or absence of plasticizer PEG-400. FTIR and Differential scanning calorimetry ruled out drug polymer interactions. Standard procedures were used to analyze the prepared films for various physicochemical parameters, drug release (Franz diffusion cell) and skin irritation test. The formulations were uniform in their physical characteristics with low water vapor absorption, moisture loss and water vapor transmission implying excellent quality and uniformity in patch characteristics. The patches were devoid of hypersensitivity reactions on rat skin. The in-vitro and ex-vivo drug release studies for all the formulations showed that the first dose of the drug was released in 2.0-3.0 h and nearly complete release (94%) was achieved in 24 h. Transdermal patches were successfully prepared for duloxetine hydrochloride and their evaluation suggested excellent quality and uniformity in patch characteristics. This can have potential applications in therapeutic arena offering advantages in terms of reduced dosing frequency, improved patient compliance and bioavailability. [18]
- Sirisha V., et al, (2012): The purpose of this research was to develop a matrix-type transdermal therapeutic system containing drug propranolol hydrochloride with different ratios of hydrophobic (eudragit’s) polymeric systems by the solvent evaporation technique by using 30 % w/w of di-butyl phthalate to the polymer weight, incorporated as plasticizer. The physicochemical compatibility of the drug and the polymers studied by infrared spectroscopy suggested absence of any incompatibility. Formulated transdermal films were physically evaluated with regard to thickness, weight variation, drug content, flatness, folding endurance moisture. All prepared formulations indicated good physical stability. In-vitro permeation studies of formulations were performed by using Franz diffusion cells.It shown that drug release follows zero order and the mechanism of release is diffusion from the polymer. [19]
- Tracy L., et al, (2014): Polycystic ovary syndrome is now a well-recognized condition affecting 6%-25% of reproductive-aged women, depending on the definition. Over the past 3 decades, research has launched it from relative medical obscurity to a condition increasingly recognized as common in internal medicine practices. It affects multiple systems, and requires a comprehensive perspective on health care for effective treatment. Metabolic derangements and associated complications include insulin resistance and diabetes, hyperlipidemia, hypertension, fatty liver, metabolic syndrome, and sleep apnea. Reproductive complications include oligo- /amenorrhea, sub-fertility, endometrial hyperplasia, and cancer. Associated psychosocial concerns include depression and disordered eating. Additionally, cosmetic issues include hirsutism, androgenic alopecia, and acne. This review organizes this multi-system approach around the mnemonic “MY PCOS” and discusses evaluation and treatment options for the reproductive, cosmetic, and metabolic complications of this condition. [20]
- Williams T, et al, (2016): Polycystic ovary syndrome is the most common endocrinopathy among reproductive-aged women in the United States, affecting approximately 7% of female patients. Although the pathophysiology of the syndrome is complex and there is no single defect from which it is known to result, it is hypothesized that insulin resistance is a key factor. Metabolic syndrome is twice as common in patients with polycystic ovary syndrome compared with the general population, and patients with polycystic ovary syndrome are four times more likely than the general population to develop type 2 diabetes mellitus. Patient presentation is variable, ranging from asymptomatic to having multiple gynecologic, dermatologic, or metabolic manifestations. Guidelines from the Endocrine Society recommend using the Rotterdam criteria for diagnosis, which mandate the presence of two of the following three findings hyperandrogenism, ovulatory dysfunction, and polycystic ovaries—plus the exclusion of other diagnoses that could result in hyperandrogenism or ovulatory dysfunction. It is reasonable to delay evaluation for polycystic ovary syndrome in adolescent patients until two years after menarche. For this age group, it is also recommended that all three Rotterdam criteria be met before the diagnosis is made. Patients who have marked virilization or rapid onset of symptoms require immediate evaluation for a potential androgen-secreting tumor. Treatment of polycystic ovary syndrome is individualized based on the patient's presentation and desire for pregnancy. For patients who are overweight, weight loss is recommended. Clomiphene and letrozole are firstline medications for infertility. Metformin is the first-line medication for metabolic manifestations, such as hyperglycemia. Hormonal contraceptives are first-line therapy for irregular menses and dermatologic manifestations. [21
MATERIALS AND METHODS:
Materials:
All the chemicals used in this research were of standard pharmaceutical grade. Spironolactone and Metformin HCL was procured as a gift sample from Cipla Pharmaceuticals. Ethyl Cellulose, Polyethylene glycol 400(PEG 400), Methanol, Hydrooxypropyl methyl cellulose K4M (HPMC K4M), and Mercury were bought from Powder Pack PVT. LTD. Borivali, Mumbai. Square shape Bandage was bought from local market. Other materials used in the study such as chloroform, di-n-butyl-phthalate (DBP), etc.) Were of analytical grade.
Methods:
Ethyl Cellulose (EC) and Hydrooxypropyl methyl cellulose K4M (HPMC K4M) was used as polymer for the formulation of Transdermal Patch. Polyethylene glycol 400 (PEG 400) was used as a plasticizer, di-n-butyl-phthalate (DBP) is used as penetration enhancer. The polymer was dissolved in chloroform: methanol (1:1) solvent. The drug was dispersed uniformly in the viscous solution with continuous stirring. The DBP was also added on the same time. The resulting mass was poured into leveled mercury surface in a Petri dish covered with inverted funnel. The Petri dish was left undisturbed at room temperature for one day. The patch was obtained intact by slowly lifting from the Petri dish. Then the prepared patch was attached in the bandage to get the final product. The final patch was stored in the polyethelene bag for further evaluation.room temperature for one day. The patch was obtained intact by slowly lifting from the Petri dish. Then the prepared patch was attached in the bandage to get the final product. The final patch was stored in the polyethelene bag for further evaluation.
Table 1. Formulation design.
General procedure for the synthesis of chalcones by Claisen-Schmidt condensation
- Conventional Method of Synthesis
Equimolar quantities (0.001mol) of 2-acetylthiophene and respective aldehydes (0.001mol), were mixed and dissolved in minimum amount (3ml) of alcohol, to this aqueous potassium hydroxide solution (30%) was added slowly and mixed occasionally for 24 hrs, at room temperature. Completion of the reaction was identified by observing on precoated TLC plates of Merck. After completion of the reaction, the reaction mixture was poured into crushed ice, if necessary acidified with dil HCl. The solid separated was filtered and dried. It was purified by recrystallization or by column chromatography performed on silica gel (100-200 Mesh, Merck), using ethyl acetate and hexane mixture as mobile phase.5
- Microwave Assisted Synthesis
Equimolar quantities (0.001mol) of 2-acetylthiophene and respective aldehydes (0.001mol) were mixed and dissolved in minimum amount (3ml) of alcohol; to this aqueous potassium hydroxide solution (30%) was added slowly and mixed. The entire reaction mixture was microwave irradiated for about 2-6 minutes at 180 watts, then kept aside for 1-3 hrs. Completion of the reaction was identified by observing on precoated TLC plates of Merck. After completion of the reaction, the reaction mixture was poured into crushed ice, if necessary acidified with dil HCl. The solid separated was filtered and dried. It was purified by recrystallization or by column chromatography performed on silica gel (100-200 mesh, Merck), using ethylacetate and hexane mixture as mobile phase. 6

Table 1. Formulation design.


 Preeti gupta *
Preeti gupta *

















 10.5281/zenodo.11045487
10.5281/zenodo.11045487