Abstract
Ocimum sanctum, Rubia cordifolia, Glycyrrhiza glabra, and Punica granatum are all used to treat various diseases and conditions in Ayurvedic medicine. In this study, we aimed to formulate and evaluate a polyherbal emulgel containing extracts from four different medicinal plants: O. sanctum, R. cordifolia, G. glabra, and P. granatum. Different gel formulations were created by combining a base component with extracts from O. sanctum, R. cordifolia, G. glabra, and P. granatum. pH, viscosity, rheology, appearance, and homogeneity were some of the criteria examined for each formulation. They were tested for evaporation and irritation of the epidermis with patches. The cup plate method was used to examine the antibacterial-antifungal activity of formulations against Escherichia coli, Pseudomonas aeruginosa, and Aspergillus niger. Results showed that the inhibition zones produced by all formulations were greater than those produced by the control. Antimicrobial characteristics can be found in polyherbal gels. Synergy between the plant components in the blend produced the desirable outcome.
Keywords
Antimicrobial activity, Patch test, Polyherbal gel, Evaluation test.
Introduction
Herbal remedies have always treated communicable infections. Traditional practitioners increasingly use folklore medicine for skin problems, and plant medications are crucial.1 This approach combined current biology and chemistry for discovery and treatment, making it popular in healthcare. Traditional medicine still contributes greatly to healthcare. Traditional polyherbal remedies are becoming more popular due to their perceived safety. Allopathic single-molecule medications have serious side effects.2
Skin protects the body from shocks, temperature, UV light, toxins, and other hazards. The epidermis, dermis, and subcutaneous fat tissues make up the skin from the outside in.3 The skin protects the body from germs. Human skin covers the most area. It can selectively distribute drugs and has a wide applicability area. It keeps medication concentrations high in surrounding tissues for a long time, lowering dose frequency.4 Skin has three main defensive functions: UV protection, antioxidant, and antibacterial. Skin health is important because it protects us. Compromised skin loses its barrier function.
Medical awareness has grown and faded with great civilizations, enriching their conventional experiences. After centuries, some believed folklore led to modern practice. Not entirely. Oriental old medicine never dies but evolves because disease and treatment ideas are deeply established in culture, spiritual dogmas, and ecological variables. Traditional folklore medicine also relates to human nature. The old ways of living are back in popularity. There has been a rise in interest in nontraditional healthcare, pesticide-free farming, organic foods, and environmentally friendly goods. Misuse of chemicals has negative consequences for both people and the environment. The use of herbal medicines and treatments is on the rise. Extensive drug release strategies use plant exudates, gums, mucilage, and sugars. Drugs can be delivered via ointments, suspensions, and emulsions. Scientific advancements have given Ayurveda and other traditional healing methods new life. Medicinal compounds found in these plants could be employed as stand-alone treatments or starting points for brand-new, unrefined medicines. Why? Because plants of different species create medicines based on distinct chemical principles. Modern scientists have studied traditional Indian medicine for decades. Since it’s a tried-and-true method, it’s better at treating numerous diseases. Ayurveda improves health across the board. Ayurveda cures arthritis, heart disease, diabetes, cancer, and immunological diseases. Folklore is gaining popularity. Traditional healthcare systems have relied on plant remedies to sustain human health for years.7 Ancient folklore systems are still used today. WHO began global CAM. CAM approaches have revolutionized herbal and contemporary medicine.8 Herbal medicine is booming. Herbal interest shows CAM’s popularity. Without scientific facts, caution is needed. Herbal medications include restrictions like, Traditional herbal drugs lack multiple herbs. Another limitation is non-drugstore drugs. Concerns include medicinal plant and contaminated medicine differences. Natural medication technology is a blessing and solution for all of us, where folklore knowledge plays a significant role and analytical techniques have been refreshed for quality control of plant-based drugs.9 New oriental medications, extensive pharmaceutical product research, decoctions, infusions, tinctures, and powders use herbs. Modern formulations, including topical ones administer drugs differently. Most ancient classics covered topical application. Charaka wrote a full chapter on topical formulation.10 Herbal medicines are popular in underdeveloped countries for primary health care because they are cheaper, more culturally acceptable, and less harmful. Some herbal remedies have been shown to be harmful in recent studies. Most herbal supplements sold today lack FDA approval as pharmaceuticals. Thousand-year-old practises can guide herbal formulation selection, preparation, and use. Therapeutic products must undergo rigorous scientific and clinical validation to be considered a viable alternative to mainstream medicine.11 Over the past 50 years, highthroughput screening and combinatorial chemistry have produced a variety of new medications, but natural products and their derivatives remain vital to pharmacopoeias. Natural products are used in numerous drug discovery and development initiatives because of their unmatched chemical diversity and distinctive mechanisms of action. Because of their wide range of biological target interactions, some of these naturally occurring chemicals have risen to prominence as some of the most vital pharmaceuticals in modern medicine.12
MATERIALS AND METHODS
Materials
Collection and identification of medicinal plants
Ocimum sanctum, Rubia cordifolia, Glycyrrhiza glabra, Pterocarpus marsupium, Nerium oleander and Punica granatum were procured from local market and were authenticated.13
Chemicals and drugs
All medications and chemicals were purchased from a local source and were of AR grade.
Microorganisms
We acquired the required micro-organisms from the D. Y. Patil Institute of Pharmaceutical Sciences & Research in Akurdi, Pune.
Animals
After receiving approval from the IAEC, we chose male and female wistar albino rats (180–220 gm) and Swiss albino mice (20–40 gm). All animals were housed in a conventional environment with a 12:12 light:dark cycle, 25 ± 2°C temperature, and humidity of 45 to 55%. Food and drink were not restricted for the duration of the trial for the animals. The hours of operation for all experiments were from 9:00 am to 6:00 pm.
Methods
Extraction of plant materials of selected plant
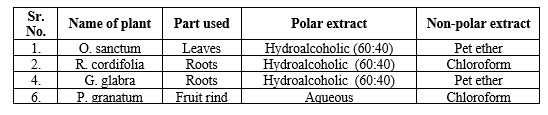
The raw material for the medicine was harvested from a single plant, dissected, dried, and powdered. Separate soxhlet extraction processes employing petroleum ether were applied to air-dried coarse powders of O. sanctum, R. cordifolia, G. glabra, and P. granatum to yield polar extracts. In addition, these extracts were extracted in succession using different polar solvents, such as water for P. granatum and a hydroalcoholic (60:40) solution for the others. The extracts were then concentrated till dry and stored in the fridge for future research.14 The powdered medicines were extracted with their respective non-polar solvents (Table 1) using the soxhlet extraction method to create non-polar extracts. The resulting extracts were filtered, evaporated to dryness to create a semisolid paste, and stored in the refrigerator until further analysis was performed.
Analysis of the extracts’ phytochemicals (Preliminary)
The following well-validated techniques were used for the preliminary phytochemical analysis of various extracts to document the presence of major chemical constituents.
Pharmacognostic evaluation
The four extracts shortlisted after phytochemical analysis were individually subjected to various pharmacognostic tests.15
Emulgel’s preparation
To make the base gel, 50 mL of water was mixed with 0.1 gm of carbopol-934 polymer and stirred vigorously for 5 minutes to ensure uniform mixing. The ingredients were left alone for 24 hours at room temperature. To obtain the solution 15 mL of a 60 to 40% ethanol and water mixture was used to dissolve the hydroalcoholic extract. To make the emulgel, the solutions of hydroalcoholic extracts described above were added to the
Table 1: Details of selected plants and their extracts
polymer solution of carbopol-934 one at a time and thoroughly combined. Span 20 was diluted into light liquid paraffin to create the oil phase. To make the aqueous phase, tween 20 was dissolved in sterile water; then, methyl and propyl paraben were combined in propylene glycol; and, finally, the mixture was thoroughly stirred using a magnetic stirrer to ensure that the extracts and preservatives were evenly distributed throughout. After heating between 70 to 80? with constant stirring, the aqueous phase was cooled to room temperature. The pH of the emulgel was checked at regular intervals to ensure consistent dispersion and sodium hydroxide was added to bring it to a neutral pH.16
Acute Dermal Toxicity Study of Extracts
Pre-test procedure
The single acute dermal toxicity for the period of 14 days as per OECD guideline 402 was performed for each test formulation as follows,
Procedure
Five healthy rats (180–220 gm) were selected for each group. These separated rats were acclimatized for a period of 07 days. On 8th day, the fur from dorsal region skin was removed by shaving. (The area selected was approximately 10% of total body surface area). The respective pastes were uniformly applied on the shaven area. (A good contact for 24 hours was ensured using porous gauze dressing and non-irritating type). After 24 hours of application, residual pastes of test extracts was removed from the skin using wet cotton swab. These rats were macroscopically observed for various parameters (mentioned below) for next 14 days. On day 1, the observation was frequent i.e. for first 2 to 6 hours, while on subsequent days, it was once a day at a fixed time.17
Extracts with Antibacterial-Antifungal Properties
Procedures for obtaining test organisms and making stock Cultures
In order to conduct the antimicrobial testing, the following strains were collected. For bacteria E. coli and P. aeruginosa were used. For fungi A. niger was used. Mediums of nutrient broth and potato dextrose broth were made and sterilized in an autoclave at 121?. Following inoculation of bacteria and fungi into individual flasks and shaker incubation for 24 and 48 hours, fresh culture was prepared by visually adjusting the turbidity of the culture to meet the 0.5 McFarland turbidity standard.18
Cup plate method
In 100 µL of above fresh bacterial cell suspension/culture were poured in sterilized petri dishes (9 cm diameter) onto which 20 mL of sterile nutrient agar was poured and thoroughly mixed. It was allowed to solidify. In the plate of the nutrient agar medium, a cup cavity of 4 mm diameter were made with a sterilized cork-borer. These cups were filled with 50 µL of each dilution, i.e., emulgel formulation and standard [Ciprofloxacin 10 µL from stock solution of 0.1 mg/mL]. The petri dishes were incubated for 24 hours at 25 ± 2°C for bacteria and 48 hours 30 ± 2°C for fungi and the observations were recorded as the diameter of the inhibitory zone in mm. All experiments were repeated for three times.19
RESULTS
Pharmacogenetic Evaluation
Preliminary phytochemical evaluation of extracts
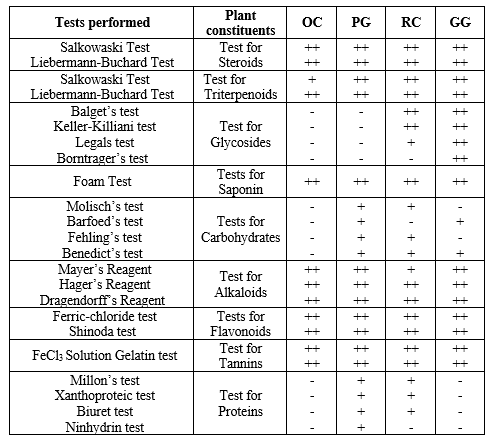
Extracts from P. granatum, O. sanctum, Glycirrhiza glabra, R. cordifolia were screened for their phytochemical content abbreviated in table as PG, OC, GG and RC, respectively. (Table 2).
Preliminary phytochemical investigation showed that the majority of the phytoconstituents were present in higher concentrations in extracts of O. sanctum, R. cordifolia, G. glabra, and P. granatum. Therefore, these four extracts were put to use in subsequent research.
Acute dermal toxicity study of formulation
The prepared formulation was evaluated for its acute dermal toxicity, where no erythema or edema was observed for the formulation (Tables 3 and 4). During the entire observation period, i.e. 14 days, There were no hazardous effects or noticeable changes in the coats/fur, eyes, or behavior of the treated animals. (Figure 1). All treated and controlled animals’ mean body weight was not altered significantly. The body’s total weight, organ weight, hemoglobin content, red blood cell count, white blood cell count, and platelet count all remained stable.
Table 2: Preliminary phytochemical evaluation of extracts
Macroscopic Studies
Non-macroscopic studies
Table Organ Weight on 14th day:
None of the treated animals in the present study showed any significant change in the liver weight and kidney weight of the animals receiving the treatment of formulation in the present study (Table 5). Hematological studies were also performed (Table 6), and biochemical parameters were also studied and reported (Table 7). Gross necropsy At necropsy, no obvious pathological abnormalities were found in the control or formulation groups of rats that had received cutaneous dosages (Figures 2 and 3).
Histopathology:
Microscopic examination of the livers, kidneys, and skin of the experimental rats that underwent
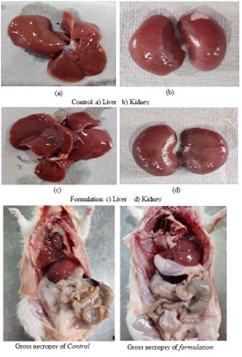
Figure 2: Gross pathology of control and formulation

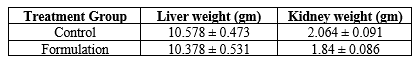
Table no 5 Effect of organ
Notes: Each value represents Mean ± SEM, n=5.
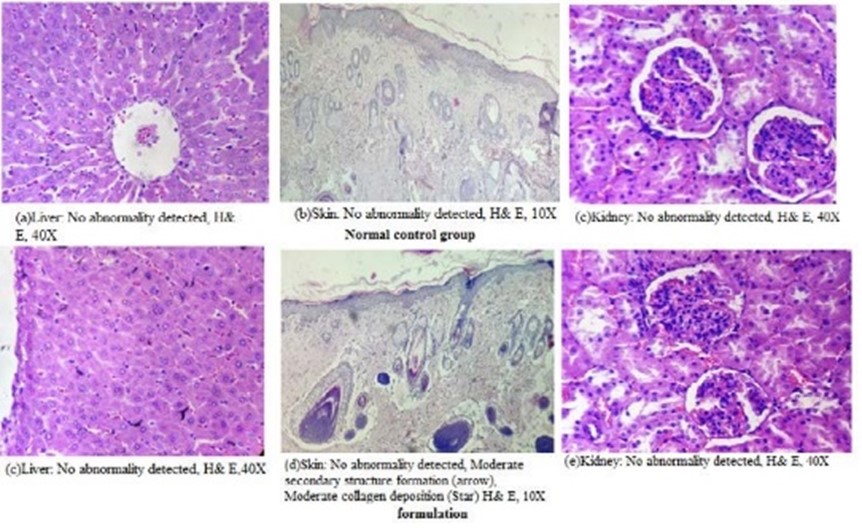
Figure 3: Histopathological findings of the normal control group and formulation rats exposed dermally
Table 3: Grading for Erythema
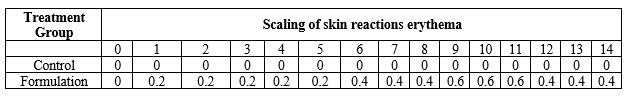
Note: each value represents mean value, n=5
Table 4 : Grading for edema formation
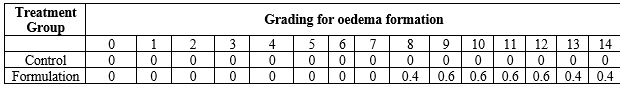
Note: each value represents mean value, n=5
Table 6: Haematology studies: Group Mean Haematology of animals in acute dermal toxicity studies

Notes: Each value represents Mean ± SEM, n=5.
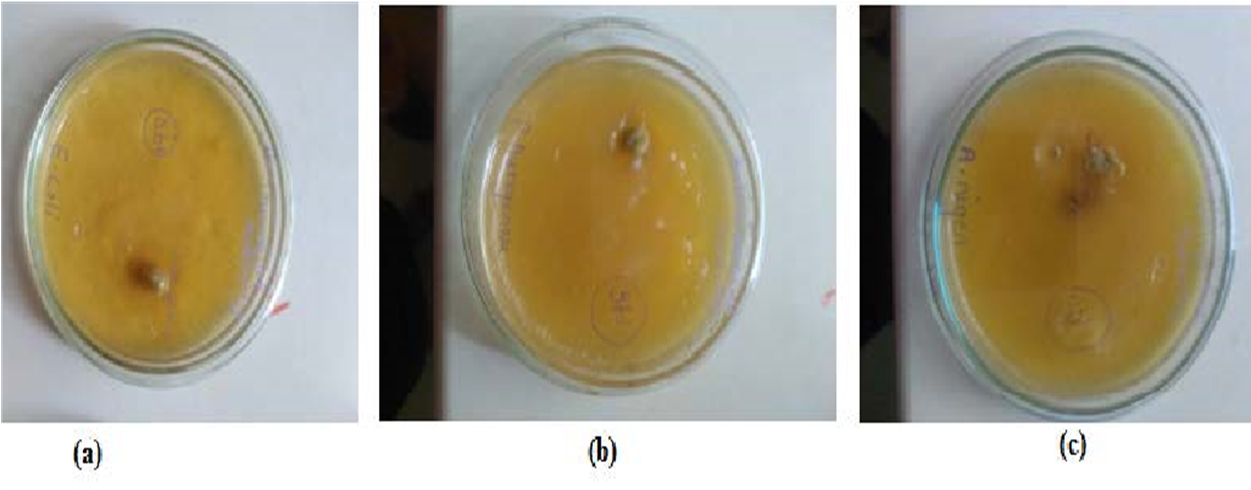
Figure 4: Antimicrobial activity of formulation on (a) E. coli, (b) P. aeruginosa and (c) A. niger
Table 7: Biochemical Parameters

Table 8: Antimicrobial activity of E. coli, P. aeruginosa bacteria and A. niger, C. albica fungi
dermal administration revealed no major changes or abnormalities.
Antimicrobial activity
Antimicrobial activity of emulgel formulation and standard ciprofloxacin 10 µL from stock solution of 0.1 mg/mL against E. coli (bacteria), P. aeruginosa (bacteria) and A. niger (fungi), was performed. Zone of inhibition of these test samples and standard are given in Table 8, Figure 4.
CONCLUSION
To obtain basic pharmaceuticals, plant components from certain plants were extracted. O. sanctum, R. cordifolia, G. glabra, and P. granatum each have their own polar extracts extracted using the soxhlet method and petroleum ether. Filtered, evaporated, and dried non-polar extracts were extracted using their corresponding non-polar solvents. The extracts were subjected to a preliminary phytochemical examination to document the existence of notable chemical components. Leaf samples from O. sanctum, R. cordifolia, G. glabra, and P. granatum were analyzed for their physical properties. Based on the preliminary phytochemical investigation, all four extracts appear to contain significant amounts of their respective phytoconstituents. There was no evidence of erythema or edema in an acute cutaneous toxicity trial of the formulation, and the average body weight of the treated and control animals did not differ significantly. Microscopically, the liver, kidneys, and skin showed no lesions. E. coli, P. aeruginosa, and A. niger were tested for susceptibility to the emulgel formulation and the standard.
REFERENCES
- Kale PS, Parekar PB, Shivpuje SS, Navghare VV, Savale MM, Surwase VB, Mane-Kolpe PS. Polyherbal Gel Development And Evaluation For Antifungal Activity. European Journal of Molecular & Clinical Medicine. 2022 May 22;9(3):5409-18.
- Goorani S, Zangeneh MM, Zangeneh A, Poorshamohammad C, Abiari M, Moradi R, Najafi F, Tahvilian R. Study of wound healing potential of Stevia rebaudiana ethanol extract in male rats. Res J Pharmacogn. 2018;5(1):23-30.
- Gauttam VK, Kalia AN. Development of polyherbal antidiabetic formulation encapsulated in the phospholipids vesicle system. Journal of advanced pharmaceutical technology & research. 2013 Apr;4(2):108.
- Singh S, Manvi FV, Nanjwade B, Nema RK. Antihyperlipidemic screening of polyherbal formulation of Annona squamosa and Nigella sativa. Inter. J. Toxicol. Pharmacol. Res. 2010;2(1):1-5.
- Damanhouri ZA, Ahmad A. A review on therapeutic potential of Piper nigrum L. Black Pepper): The King of Spices. Med. Aromat. Plants. 2014;3(3):161.
- Aiyalu R, Govindarjan A, Ramasamy A. Formulation and evaluation of topical herbal gel for the treatment of arthritis in animal model. Brazilian Journal of Pharmaceutical Sciences. 2016 Jul;52:493-507.
- Mahmoud DA, Hassanein NM, Youssef KA, Abou Zeid MA. Antifungal activity of different neem leaf extracts and the nimonol against some important human pathogens. Brazilian Journal of Microbiology. 2011;42:1007-16.
- Velraj M, Soumya D, Sndhukavi D. Antibacterial and antifungal activity of herbal gel from the ethanolic extract of stem bark of Bauhinia variegate Linn. Int J Pharm Sci Rev Res. 2016;41:53-6.
- Chellathurai BJ, Anburose R, Alyami MH, Sellappan M, Bayan MF, Chandrasekaran B, Chidambaram K, Rahamathulla M. Development of a Polyherbal Topical Gel for the Treatment of Acne. Gels. 2023 Feb 17;9(2):163.
- Dubey S, Dixit AK. Preclinical evidence of polyherbal formulations on wound healing: A systematic review on research trends and perspectives. Journal of Ayurveda and Integrative Medicine. 2023 Mar 1;14(2):100688.
- Gupta A. Fundamentals of skin wound healing and repair: A brief review on cellular and molecular pathophysiologic basis of wound healing. Natural Polymers in Wound Healing and Repair. 2022 Jan 1:1-8.
- Kavitha KS, Baker S, Rakshith D, Kavitha HU, Yashwantha Rao HC, Harini BP, Satish S. Plants as green source towards synthesis of nanoparticles. Int Res J Biol Sci. 2013 Jun;2(6):66-76.
- Khan AD, Rastogi V, Lavhale PM, Jain J. Novel approaches for herbal drug delivery in wound healing: A review. Indian Journal of Pharmaceutical Sciences. 2022 Apr 14;84(2):247-60.
- Rajad S, Karodi R, Dhanake K, Kohakde S, Bendre S. Formulation And Evaluation of Polyherbal Mouth Ulcer Gel Containing Bombax ceiba Thorn Extract and Psidium guajava Leaf Extract. Journal of Coastal Life Medicine. 2023 Mar 2;11:845-57.
- Rathi V, Pal R, Sandhu KS. Formulation and evaluation of serum containing polyherbal extracts of Cinnamomum cassia & aloe vera for the treatment of wound infection. Journal of Advanced Medical and Dental Sciences Research. 2022 Aug 1;10(8):104-9.
- Rieger S, Zhao H, Martin P, Abe K, Lisse TS. The role of nuclear hormone receptors in cutaneous wound repair. Cell biochemistry and function. 2015 Jan;33(1):1-3.
- Rogers C, Gobbi A. The Optimization of Natural Healing. Bioorthopaedics: A New Approach. 2017:3-24.
- Sohail T, Khan RA, Imran H, Fareed G, Yasmeen S. Development and Evaluation of Antimicrobial Poly-herbal Gel Formulation for the Treatment of Various Skin Infections. RADS Journal of Pharmacy and Pharmaceutical Sciences. 2022 Oct 31;10(3):92-9.
- Umadevi A, Kumari C, Kumar PA, Am HS, Divya K, Hisana PV. Development and evaluation of polyherbal gel for antifungal activity. International Journal of Current Pharmaceutical Research. 2018 Sep 15;10(5):40-3.


 Rasika. A.Waykar *
Rasika. A.Waykar *
 S.R.Ghodake
S.R.Ghodake
 H. V. Kamble
H. V. Kamble




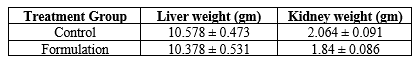







 10.5281/zenodo.11222261
10.5281/zenodo.11222261