Abstract
Osmotic drug delivery systems (ODDS) are a type of controlled release technology that utilizes the principles of osmosis to deliver drugs in a precise and sustained manner. These systems typically consist of a core containing the drug and an osmotic agent, surrounded by a semipermeable membrane. Water from the surrounding environment draws in through the membrane due to the osmotic pressure generated by the core, pushing the drug solution out through a predefined delivery orifice in a controlled manner. Osmotic drug delivery devices, such as the Rose and Nelson pump, Higuchi Leeper pump, Higuchi Theeuwes pump, Elementary osmotic pump, etc., are covered by a number of patents. There are several methods for creating osmotically controlled drug delivery systems (OCDDS), such as the sandwiched osmotic tablet system, telescopic capsule for delayed release, controlled porosity osmotic pump, osmotic bursting osmotic pump, and push-pull osmotic pump. Drug solubility, systemic osmotic pressure, drug delivery orifice size, and type and thickness of the rate-controlling membrane are some of the variables that affect how quickly the drug is released from the formulation. The current review primarily focuses on the fundamental elements of an OCDDS, different parameters controlling drug release from these systems and OCDDS types, commercially available preparations, patents, and in vivo evaluation. ODDS offer a promising approach for achieving precise and prolonged drug delivery, enhancing therapeutic efficacy and patient convenience and holding great potential for reducing the side effects of the drug and optimising medication delivery.
Keywords
Osmotic drug delivery system, osmosis, controlled drug delivery, delivery orifice, semipermeable membrane
Introduction
An OCDDS stands out as most promising advancements drug delivery technologies that employs osmotic pressure as a main force for delivering of active agents in controlled manner. Since Rose and Nelson developed an implantable pump in 1955 the osmotic drug delivery system has made a significant progress. In this system, osmotic pressure and osmogens were used for controlled delivery of drugs (for up to 10 – 16 hrs). Both in human pharmaceuticals and in veterinary medicine Osmotic systems for controlled drug-delivery applications are well established.1 A high degree of In vitro/In vivo correlation is achieved due to drug release from OCDDS is independent of pH and hydrodynamic conditions of the body because of the nature of the rate-controlling membrane and the design of the deliver orifice used in osmotic systems. Osmotic pump-controlled release preparation is a unique drug delivery system with controlled osmotic pressure difference between the internal and external of the semipermeable membrane as drug delivery power. In modern times, osmotic tablets have been developed where the delivery orifice is created by incorporating a leachable component in the coating. The water-soluble component dissolves, and an osmotic pumping system result when the tablet comes in contact with solution. Later, water permeates into the core through the microporous membrane, establishing an osmotic gradient and consequently regulating the release of drug2.
OSMOSIS:
The movement of a solvent from a solution of lower solute concentration to a higher solute concentration through an ideal semipermeable membrane is called osmosis. The pressure applied to the higher-concentration side to inhibit solvent flow is called the osmotic pressure. Osmosis is the process that can control the drug delivery system. Osmotic pressure created due to the imbibition of fluid from the external environment into the dosage form regulates the delivery of drugs from the osmotic device2. The rate of drug delivery from the osmotic pump is directly proportional to the osmotic pressure developed due to the imbibition of fluids by osmogen. Osmotic pressure represented as a colligative property of a solution, where the osmotic pressure's magnitude remains unaffected by the quantity of individual solute entities within the solution. Therefore, the rate at which drugs are released from osmotic devices relies on the solubility, molecular weight, and coefficient of the solute (osmogen)1.
EVOLUTION OF OSMOTIC DRUG DELIVERY SYSTEM:
Rose and Nelson, were initiators of osmotic drug delivery after 75 years of discovery of osmosis principle. In 1955 the first osmotic implantable pump and in 1975 the elementary osmotic pump for oral delivery of drugs was introduced. In 1970 implantable osmotic pumps were a innovation to deliver a wide range of drugs and hormones, including peptides at constant and programmed rates 3.
Table 1: History of osmotic drug delivery system

PRINCIPLE OF OSMOSIS:
Pfeffer obtained the first quantitative measurement in 1877, but the first report of an osmotic effect dates back to Abbe Nollet in 1748. In the experiment, to separate a sugar solution from pure water a membrane permeable to water but impermeable to sugar is used. Water flows into the sugar solution uncontrollably until a pressure ? is applied to the sugar solution, bringing the flow to a halt. Pfeffer showed that the osmotic pressure ? of the sugar solution is directly proportional to the solution concentration and the temperature. In just a few years, Vant Hoff demonstrated the analogy between these findings and ideal gas laws using the expression.
? = ? c r t
Where;
? = osmotic coefficient of the solution,
c = molar concentration of sugar in the solution,
r= gas constant,
t = absolute temperature.
The osmotic pressure of soluble solutes, often employed in controlled-release formulations, can be exceptionally high, varies from 30 atm for sodium phosphate to as much as 500 atm for a lactose-fructose mixture. This high osmotic pressure is attributed to their ability to induce substantial water flow across semi-permeable membranes. The equation governing the osmotic water flow through a membrane is
Dv/dt = A Q ? ?/L
Were,
dv\dt =water flow across the membrane of the area
A= thickness of L, and the permeability
Q in cm2, ? ? The osmotic pressure difference between the two solutions exists adjacent of the membrane.
This equation is strictly for a completely selective membrane that is membrane penetrable to water but completely impermeable to osmotic agent1.
ADVANTAGES:
- Delivery of drugs from osmotic pumps can be designed to follow true Zero-order Kinetics.
- Higher release rates can be obtained from osmotic systems than with conventional diffusion-based drug delivery systems.
- The rate of drug delivery is programmable and predictable4.
- The drug delivery provides in vitro and in vivo correlation.
- The drug release is independent of the physiological conditions of the body, gastric pH, gastrointestinal motility, and hydrodynamic conditions.
- The drug delivery may be delayed or pulsatile5.
- In vitro delivery rate can be accurately predicted using mathematical equations, which in turn, bears a high degree of correlation with in-vivo delivery rate.
- The drug is delivered in a readily absorbable solution form, mimicking a liquid dosage form that is prepared in situ by an osmotic pump.
- Delivery rate is almost independent of delivery orifice size within limits6.
- Increased patient compliance with reduced frequency.
- The release mechanisms are not dependent on the drug7.
- Increase margin of safety of highly potent drug8.
- The release mechanisms are independent of drug concentration.
- Reduced side effects.
- They are well-characterized and understood.
- Enhanced bioavailability of drug.
- Reduced inter-patient variability.
- They are appropriate for a wide range of drugs9.
DISADVANTAGES:
- Integrity & stability are challenging.
- The film defects result in inadequate coating process can lead to dose dumping.
- Formulating osmotic pump tablets may require the utilization of expensive machinery and inert materials10.
- Retrieval of drug is not possible in the case of adverse event11.
- Osmotic pump tablets should not be crushed or chewed as they can lead to loss of the ‘slow release’ characteristics as well as toxicity.
- Rate of drug release can be influenced by aspects such as food intake and gastric transit time, potentially leading to variations in release rates between doses3.
- Magnetic resonance imaging (MRI) of tablet elucidates that non-uniform coating leads to different patterns of drug release among the batches12.
- For making an orifice in the system special equipment is required 13.
- The dimensions of the delivery orifice is always a critical step and impacts on overall performance of the drug delivery system14.
CLASSIFICATION OF OSMOTIC DRUG DELIVERY DEVICES:
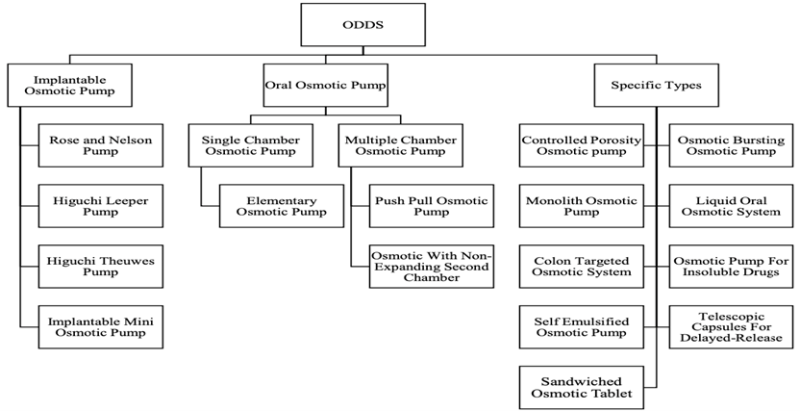
Figure 1: Classification of osmotic pumps
A. Implantable type of osmotic pumps
1. The Rose and Nelson pump:
Rose and Nelson were the first to develop the implantable osmotic pump technique in 195515. These devices consisted of 3 chambers: a salt, drug and water chamber. The flow of water from the water chamber to the salt chamber occurs due to alteration in osmotic pressure through the membrane. This water flow causes an expansion of the salt chamber, leading to the stretching of the latex diaphragm that separates the salt and drug chambers. Ultimately, this distension results in the expulsion of the drug from the delivery system through the delivery orifice16. The osmotic pressure is the driving force for the discharge of the drug from the system17. The design and mechanism of this pump are comparable to modern push-pull osmotic pump16. A significant higher osmotic pressure is generated by salt solution compared to the pressure needed for the suspension of an active agent. Consequently, the influx of water into the salt chamber maintains a constant rate as long as an ample amount of solid salt is present to sustain a saturated solution. This ensures a consistent osmotic pressure driving force18. As soon as the salt chamber meets the water osmotic flow occurs which is the key problem with the Rose-Nelson pump. Therefore, the water was loaded to the pump immediate before use, which made the procedure problematic for drug delivery. Moreover, vast research was carried out, their complex design makes them poor systems in large-scale production which makes them of limited use17.
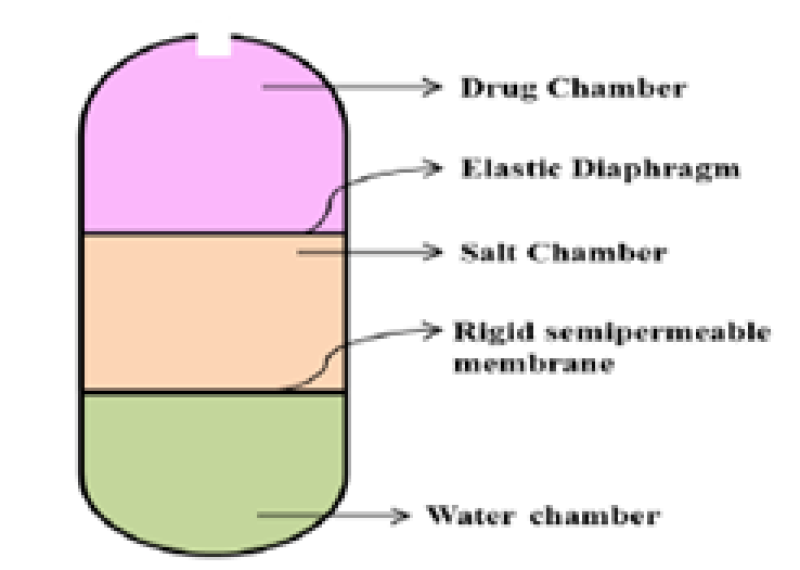
Figure 2: Rose and Nelson osmotic pump
2. Higuchi Leeper pump:
The design of the Higuchi Leeper pump described represents the first simplified version of the Rose Nelson pump introduced by the Alza Corporation in the early 19709. The Higuchi-Leeper pump is adapted iteration of the Rose-Nelson pump. The water compartment is absent in this model and begins only when water enters from the nearby atmosphere. The Higuchi-Leeper device has a strong membrane that can tolerate the pressure without rupturing. A selectively permeable membrane is supported on a porous network, and a salt compartment is present in it. When this device is made to enter the body, nearby liquid enters the device by SPM, which dissolves MgSO4. As a result, osmotic pressure is created internally, compelling the movable divider in the direction of the drug compartment and release the drug. This device is mostly used for animals when they require antibiotics and hormones. An aperture is pierced in the flexible membrane10. When the device is swallowed or implanted in the body it gets activated by imbibition of water from the surrounding environment19. When water infiltrates the device, it reaches a critical pressure point. At this juncture, apertures open, facilitating drug release. Subsequently, as the drug is discharged, the pressure decreases, leading to the closure of the apertures. Pulsatile distribution is attained by this device10.
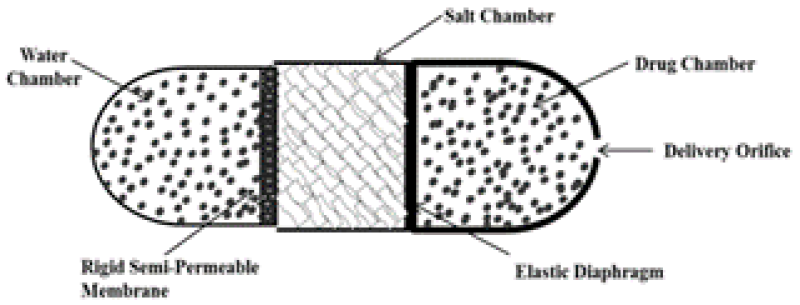
Figure 3: Higuchi Leeper pump
3. Higuchi Theeuwes pump:
In 1970s Higuchi – Theeuwes developed a similar and even simpler variant form of Rose Nelson pump9. As with the Higuchi-Leeper pump, the osmotic action of the pump was activated by water obtained from the surrounding environment16. The pump contains of an osmotic core comprising the drug surrounded by SPM with a delivery orifice10. The membrane is robust and possesses sufficient strength to endure the internal pumping pressure generated within the device. The device is loaded with the desired drug before use16. The core imbibes water osmotically at a controlled rate determined by the membrane permeability when the pump is exposed to water and by the osmotic pressure of the core formulation19. This device can be stored for a long duration because the drug is loaded only when the device has to be used. The speed of drug release depends on the salt that is used and the selectivity of the membrane10
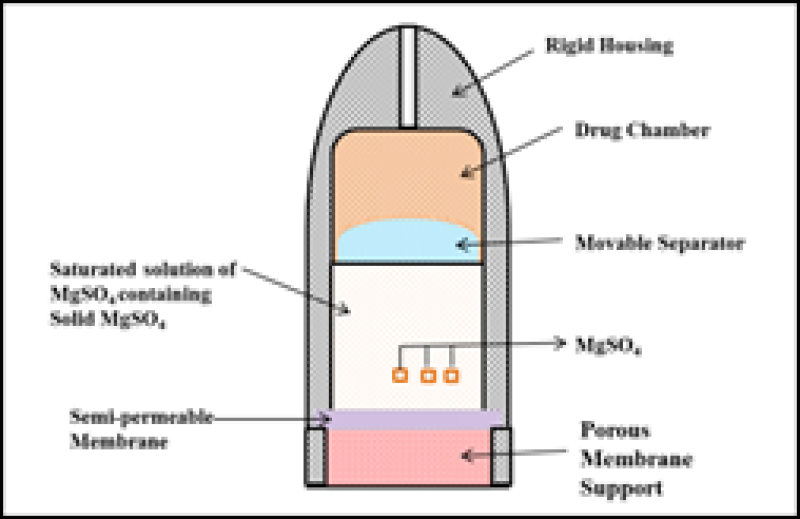
Figure 4: Higuchi Theeuwes Pump
4. Implantable Mini osmotic pump:
The illustrated implantable Mini osmotic pump consists of 3 concentric layers: the drug reservoirs, the osmotic sleeves, and the rate-controlling semi-permeable membrane. The additional component called flow moderator is inserted into the body of the osmotic. The innermost compartment of the drug reservoir is surrounded by an osmotic sleeve, a cylinder containing a high concentration of osmotic agent. When the system is exposed to water, the osmotic sleeve, covered by a semi-permeable membrane, allows water to enter. These pumps are offered with a range of delivery rates, spanning from 0.25 to 10 ml per hour, and delivery durations varying from one day to four weeks16.
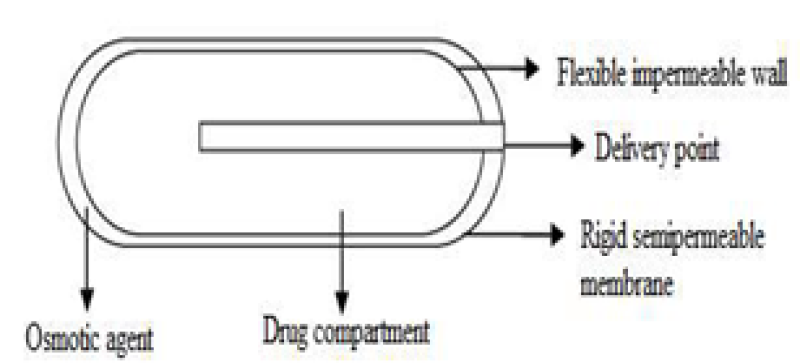
Figure 5: Implantable Mini osmotic pump
5. Alzet:
ALZET osmotic pumps are indeed miniature, implantable pumps commonly used for research in mice, rats, and other laboratory animals. These pumps operate based on the principle of osmotic displacement. These infusion pumps constantly deliver drugs from one day to six weeks in controlled manner without the need for exterior influences or frequent handling20. In this osmotic pump, drug or hormone solution to be delivered are filled in hollow reservoir of the core of the pump and is enclosed by the salt chamber with an impermeable layer between them21. The high concentration of salt induces water to enter the pump through its outer surface. This osmotic influx of water creates pressure inside the pump, leading to the release of solution from the reservoir. Alzet pumps function based on the osmotic pressure disparity between the inner chamber of the pump, known as the salt sleeves, and the surrounding tissue where the pump is implanted17. The degree of drug delivery of the pump is controlled by the water permeability of the pumps of the outer membrane19. The rate of delivery by an ALZET pump is independent of a compound's molecular weight or its physical and chemical properties21. ALZET pumps are versatile and suitable for systemic administration. Additionally, these pumps can be employed for targeted delivery, enabling the localized impact of a drug on specific tissues or organs through the use of a catheter. Their application extends to various sites of the body20. These pumps are designed for single use only because the compressed reservoir cannot be refilled is the main limitation of this system17.
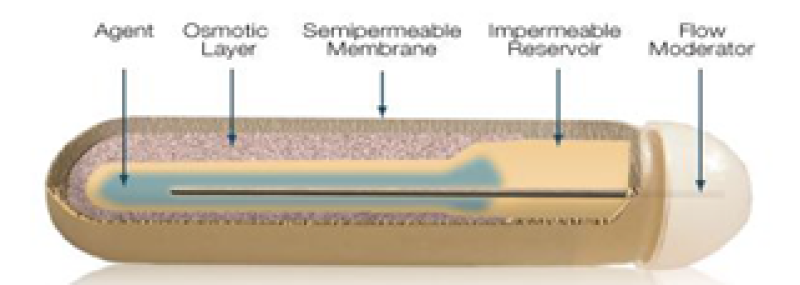
Figure 6: Alzet osmotic pump
6. DUROS mini osmotic pump:
Principles of osmosis have been implemented in human parenteral therapy, leading to the creation of the DUROS® implantable ODDS. DUROS pharmaceutical systems are miniature osmotic implants that deliver drugs for 3 months to 1 year with precise zero-order delivery kinetics. This technology is well-suited for potent drugs, capable of delivering up to 500 mg of drug from a single implant. Formulation technology has been developed to optimize drug payload, chemically and physically stabilize drugs for extended periods at body temperature, and utilize both aqueous and nonaqueous vehicles. The DUROS technology functions as a miniature drug dispensing system, operating a like to a miniature syringe, releasing minute quantities of concentrated drug formulations in a continuous and consistent flow over months or years. The system is implanted under the skin and can be as small as 4 mm OD X 44 mm L or smaller. The drug formulation is enclosed in the drug reservoir compartment. DUROS drug solutions can be both aqueous and non-aqueous in nature9. These systems delivers the drug from days to 1 year. All materials in the DUROS system were selected for their biocompatibility and suitability for implant use22. The system is made of outer cylindrical titanium alloy reservoir. This reservoir has high-impact strength and protects the drug molecules from different body elements to avoid deactivation of drug. At one end of the reservoir is positioned the membrane, constructed from a specially designed polyurethane polymer. The membrane is impermeable to ions but permeable to water. Positioned next to the membrane is the osmotic engine. Next to the engine is the piston. The piston is made from elastomeric materials and serves to separate the osmotic engine from the drug formulation. At the distal end of the titanium cylinder is the exit port. The exit port design must be coupled to the rheological properties of the drug formulation21. The osmotic agent residing in the engine compartment drives the water through the semipermeable membrane by osmosis. This enlargement of the osmotic agent displaces the piston, facilitating dispensing of small amounts of the drug through the orifice19.
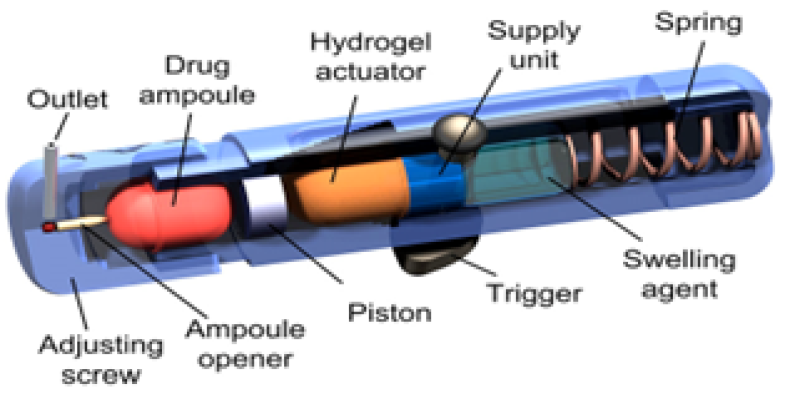
Figure 7: DUROS mini osmotic pump
B. Oral osmotic pump:
1. Single Chamber osmotic pump:
a. Elementary osmotic pump (EOP):
In 1975 Theeuwes developed the first EOP pump. EOP contains a drug of good aqueous solubility and can have an osmotic agent as well23. The key constituents of the EOP are an osmotic core which contains a drug and along or devoid of osmogens. The semipermeable coating was attained by conventional film coating techniques. The fourth component is the delivery orifice which is commonly bored by laser technique or mechanical drilling (size varies from 0.5- 1.5mm)24. The rate of release can be regulated by managing both the permeation capability of the semi-permeable membrane and the characteristics of the formulation15. When water permeates at a rate uttered by the fluid permeability of the membrane, generating osmotic pressure in the core formulation. The membrane is non-extensible, and the volume increase resulting from water imbibition elevates hydrostatic pressure within the tablet. This eventually stimuli the flow of a saturated solution of the active agent out of the device through a small orifice16. As long as there is an additional amount of solid in the device, the release of the medication persists. However, as the quantity of the drug diminishes, the delivery of the drug is no longer constant10. In general, a lag time of approximately 30-60 min is observed as the system needs to hydrate before zero-order drug delivery begins. Therefore, about 60-80% of drugs are released at a continuous rate14. This system was introduced to deliver drugs at zero order rates for prolonged periods and is negligibly affected by environmental factors such as pH or GI motility25. They allow zero-order delivery of drug26.
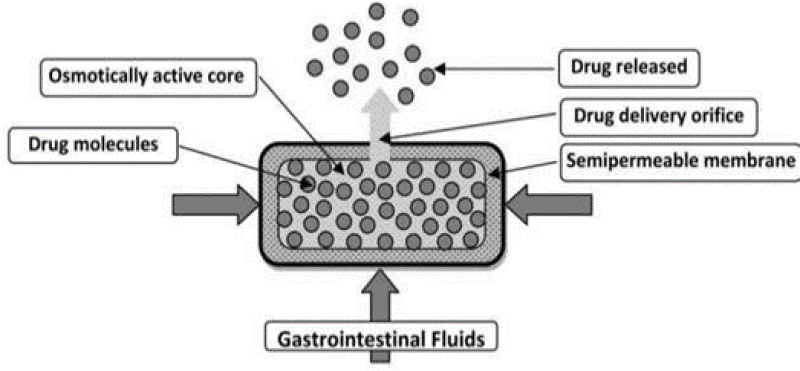
Figure 8: Elementary osmotic pump
2. Multi-chamber osmotic pump:
a. Push pull osmotic pump:
The two-layer push-pull osmotic tablet system appeared in the 1980s. A push-pull pump allows the delivery of both water-soluble and water-insoluble drugs at a continuous rate25. It is a bilayer tablet coated with a semi-permeable membrane14. The PPOP system typically comprises two compartments separated by an elastic diaphragm. A suspension of the drug can be formed by the polymeric osmotic agent in situ. The osmotic pull within the drug layer imbibes water into the compartment, leading to the in situ formation of a drug suspension. This drug suspension is pushed out of the delivery orifice by expansion of the non-drug layer27. They allow zero-order delivery of drug26. Push push-pull system is available in several modifications such as delayed push-pull system, multi-layer push-pull system and push-stick system24. The orifice is drilled through laser drilling technology16, 19, 25 and also, the drug delivered by it is released locally so these two phenomena can be called its disadvantage10
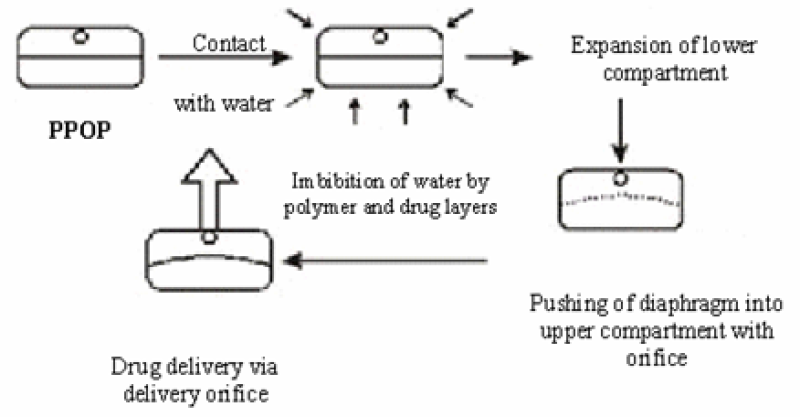
Figure 10: Push Pull Osmotic Pump
b. Osmotic pump with non-expanding second chamber:
These multi-chambered devices consist of systems that include an unexpanded second chamber. The second chamber serves the purpose of either diluting the drug solution as it exits the device or facilitating the simultaneous delivery of two drugs. This type of device is used to formulate the delivery of insoluble drugs24. This group was further classified into two subgroups that depend on the functions of the second chamber. In one type, the drug solution gets diluted in the second chamber before leaving the device. This is especially advantageous in situations where a saturated solution of the drug may cause irritation to the gastrointestinal tract. In the second type, there are two chambers, one consists of the osmotic agent and the other consists of the drug. Primarily osmotic agent solution is formed which enters the drug solution and then their mixture is released out by delivery orifice26. In another example of this type of device, one chamber contains an osmogen, and the subsequent chamber contains the drug. When the system comes in interaction with the water both chambers imbibe water through the SPM. The osmotic agent solution generated passes through the interior orifice into the drug chamber and merges with the drug before being released through the microporous membrane, which constitutes a part of the semipermeable membrane (SPM) surrounding the drug chamber. Through this device relatively insoluble drugs can be delivered14, 19, 27, 28.
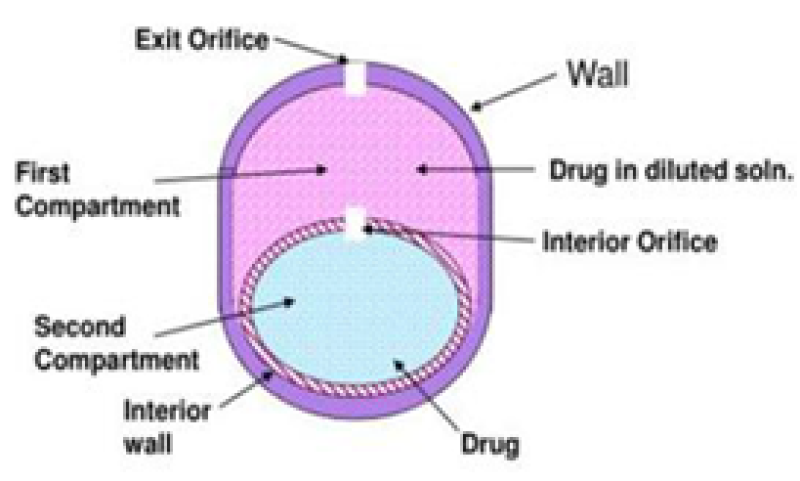
Figure 11: Osmotic pump with non-expanding second chamber
C. Specific Types:
1. Controlled porosity osmotic pump (CPOP): A CPOP comprises of drug along the osmotic core bounded by a selectively permeable membrane drilled with a delivery orifice. Various channeling agents were added to coating solution to create porous in the membrane. Generally, materials producing from 5 to 95% pores with a pore size from 10A - 100?m can be used24. The CPOP incorporates water-soluble additives within the coating membrane. Upon contact with water, the water-soluble additives dissolve, leading to the in-situ creation of a microporous membrane. Indeed, the resulting membrane is substantially permeable to both water and dissolved solutes. The mechanism of drug release from these systems is primarily osmotic, with simple diffusion playing a minor role. Drug delivery from asymmetric membrane capsules is primarily controlled by the osmotic pressure of the core formation. In-situ formed delivery orifice in the asymmetric membrane is mainly responsible for the solubilization in the core of a drug with poor water solubility16. The drug release rate from a controlled porosity osmotic pump depends on various factors, including coating thickness, the level of leachable pore-forming agent(s), solubility of the drug in the tablet core, and the osmotic pressure difference across the membrane26. The rate of drug delivery can be controlled by various factors such as:
-
- The water permeability of the semi-permeable membrane,
- Osmotic pressure of the core formulation,
- Thickness and total surface area of the coating.
As can be seen, all of the above variables are under the control of the formulator and they do not vary under different physiological conditions. Thus, they are independent of physiological conditions and thus lead to robust performance14 Nevertheless, the solubility of the agents to be delivered can be modulated, and these systems can be designed to deliver drugs having extremes of water solubility. The required modification of a controlled porosity osmotic pump depends mainly on factors such as the dose, intrinsic water solubility and osmotic pressure of the drug, and the desired release rate4. It does not depend on the pH of the gut and the mobility of the release media, speed of drug delivery, osmotic pressure, and width and covering of the total surface area. These aspects can be controlled and they do not change under any circumstance. This technique significantly reduces the problem of gut irritation caused by the drug. This is because the drug is delivered from the entire device, not from a single aperture, as is the case with other devices. In addition, a complex laser-drilling unit is not essential because the apertures are made themselves10. The CPOP offers an advantage as the drug is released from the entire surface of the device rather than from a single hole, which may help reduce stomach irritation problems. A hole is formed through a coating procedure, eliminating the need for complicated laser drilling. This allows the tablet to be made very small by using drug pills coated with an appropriate membrane. 17, 29.
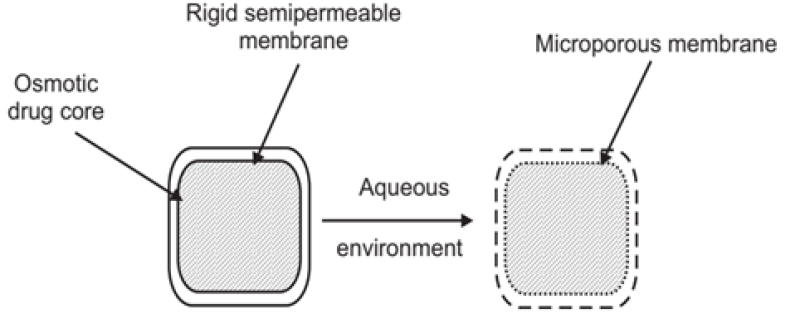
Figure 12: Controlled porosity osmotic pump
2. Osmotic bursting osmotic pump (OBOP):
This system is very alike to an EOP system. The key distinction from EOP lies in the absence of a delivery orifice, and the size of this system may be smaller in comparison. The osmotic bursting mechanism was utilized by Baker. In this procedure, no hole is present. If present then it's of very small size as compared to other devices10. The core contains drugs and osmogents. The SPM of the core is without delivery orifice19. The system imbibes water when enters into hydrated environment, leading to hydrostatic pressure buildup, until the wall ruptures, and releases the contents into the environment. The control of drug release and achieving the desired release profile can be managed by varying both the thickness and the area of the semi-permeable membrane. Pulsated drug release can be achieved through this system14, 16, 25, 27.
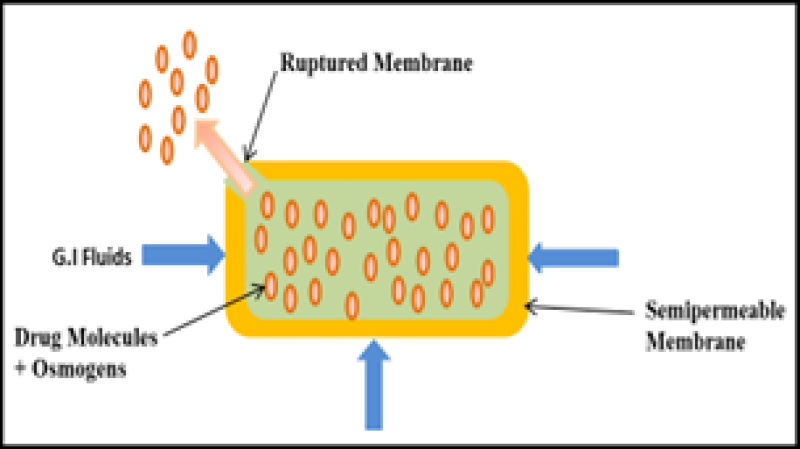
Figure 13: Osmotic bursting osmotic pump (OBOP)
3. Liquid oral osmotic system (L-OROS):
Alza developed the (L-OROS), addressing the solubility challenges. Initially, a liquid soft gelatin capsule along with drug in the dissolved state were manufactured. Subsequently, it was layered with a barrier membrane, followed by the osmotic push layer and a semi-permeable membrane with an orifice drilled. The Liquid OROS system aims to deliver drugs in liquid formulations, offering extended-release benefits along with high bioavailability. Various L-OROS systems are available to provide controlled delivery of liquid drug formulations which includes the following:
a. L- OROS hard cap,
b. L- OROS soft cap
c. Delayed liquid bolus delivery system
Delivering drugs in the form of liquid formulations and combining the benefits of extended-release with high bioavailability, is made possible by Liquid OROS14. A liquid formulation is particularly well suited for delivering insoluble drugs and macromolecules such as polysaccharides and polypeptides. Molecules with low solubility often necessitate the inclusion of external liquid components to aid in solubilization, dispersion, protection from enzymatic degradation, and promotion of gastrointestinal absorption26. The L-OROS hard or soft cap systems target for continuous drug delivery, the L-OROS delayed liquid bolus drug delivery system is specifically designed to deliver a pulse of liquid drug.24, 27.
- L-OROS hard cap:
In this system, the liquid formulation is enclosed within a hard gelatin capsule, surrounded by the barrier layer, osmotic layer, and the rate-controlling membrane. One delivery orifice is designed through these three layers. Water penetrates across the selective permeable membrane when the system is exposed to the fluid environment and triggers the osmotic layer. The expansion of the osmotic layer generates hydrostatic pressure within the system, compelling the delivery of the liquid formulation through the delivery orifice19.

Figure 14: L-OROS hard cap
- L-OROS soft cap:
The liquid drug formulation is enclosed within a soft gelatin capsule, which is surrounded by the barrier layer and the osmotic layer and SPM.A delivery orifice is formed through these three layers. When the system comes into contact with an aqueous surroundings, water is imbibed. This pressure forces the liquid formulation to break through the hydrated gelatin capsule shell at the delivery orifice19.

Figure 15: L-OROS soft cap
c. Delayed liquid bolus delivery system:
This system is comprised of 3 compartments: a placebo delay layer, liquid drug layer, and an osmotic engine, all surrounded by a SPM. The orifice is pierced on the placebo layer end of the capsule-shaped device. When the system comes into contact with an fluid environment, water permeates across the rate-controlling membrane and activates the osmotic layer. The enlargement of the osmotic layer results in the development of hydrostatic pressure inside the system, and the placebo is released first, postponing the release of the drug layer from the delivery orifice. The LOROS delayed liquid bolus drug delivery system is formulated to administer a liquid drug pulse, with the release of the drug being adjustable within a range of 1 to 10 hours. This variability is contingent on the permeability of the rate-controlling membrane and the thickness of the placebo layer19, 27, 29. Alza has also made its name in this system by making devices to deliver liquid medicaments10
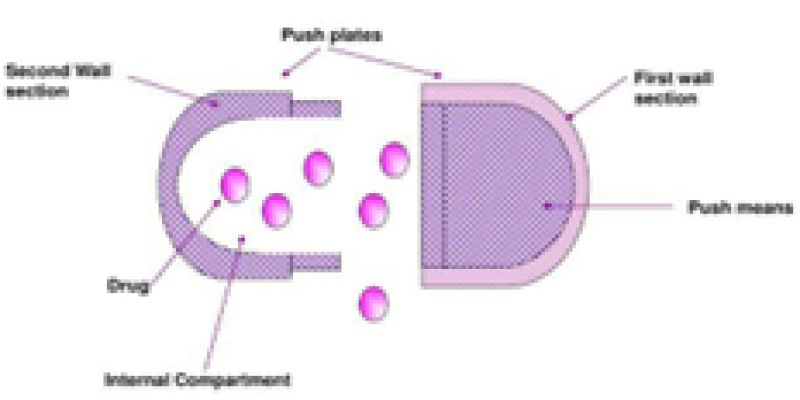
Figure 16: Delayed liquid bolus delivery system
Delayed Delivery Osmotic Device:
As the name suggests, the osmotic device inherently shows the lag time before drug delivery begins because of the semi-permeable walls. The existence of a lag time is often considered a drawback; however, it can be useful in cases where the delayed release of specific drugs may be beneficial14.
4. Effervescent Osmotic Tablet (EOT):
Poorly soluble drugs at low pH may precipitate when exposed to gastric pH, potentially leading to blockage of the delivery orifice and affecting the functionality of the osmotic pump. An effervescent compound, such as potassium bicarbonate or sodium bicarbonate can be incorporated to overcome the problem. The bicarbonate reacts with the acid in the exterior environment generating carbon dioxide. The increase in the gas dispenses the precipitated drug and aids in the rapid absorption of the drug and preventing the blockage of the orifice. Sodium chloride and sodium hydrogen carbonate are the most frequently used osmogen in EOT15, 22.
5. Telescopic capsule:
This device comprises 2 compartments: The initial chamber houses the drug along with an exit port, while the second chamber encompasses an osmotic engine. A layer of wax-like material separates the two chambers. The bilayer capsule, featuring the osmotic engine, is inserted into a fully assembled cap part of the capsule. The convex osmotic layer is oriented towards the closed end of the cap, while the barrier layer is exposed towards the cap opening. The open end of the filled vessel is inserted into the open end of the cap, and the two components are compressed together until the cap, osmotic bilayer tablet, and vessel tightly fit together. As fluid is absorbed into the device, the osmotic engine expands and applies pressure on the slidable connected first and second wall sections. Throughout the delay period, the volume of a reservoir containing the active agent remains constant, resulting in a negligible pressure gradient between the environment of use and the interior of the reservoir10, 14, 19, 26, 27.
6. Osmotic controlled release oral delivery system - Colon targeting (OROS CT):
The OROS-CT TM system was designed for colon-targeted drug delivery. It can consist of either a single or as many as five to six enteric-coated push-pull osmotic units enclosed in a hard gelatin capsule, ensuring specific delivery to the colon. This ensures that the drug delivery is targeted and activated in the colon. In the OROS-CT system, when it enters a higher pH environment (pH>7) of the small intestine, the enteric coating dissolves. Water is then absorbed into the core, initiating the push compartment to swell and release the drug through the orifice. Simultaneously, a flowable gel is formed in the drug compartment, which is pushed out of the orifice at a rate precisely controlled by the rate of water transport across the semi-permeable membrane. About 80% of drug was delivered to the large intestine by OROS-CT10,14,19,24,26,27.
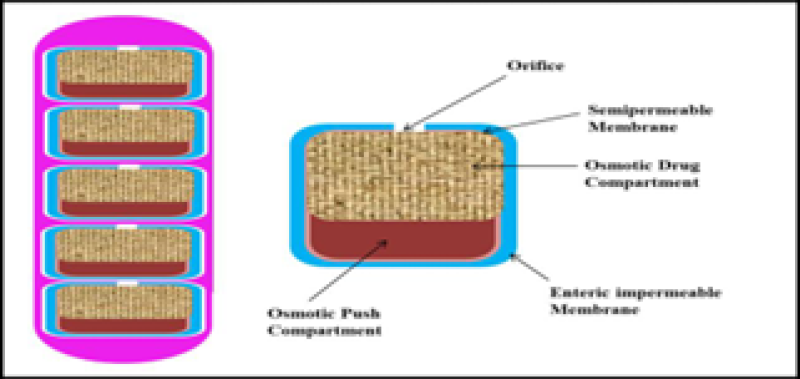
Figure 17: Osmotic controlled release oral delivery system - Colon targeting (OROS CT)
7. Sandwiched osmotic tablets (SOTS):
This system features a tablet core comprising a polymeric push layer positioned among two layers, each equipped by two delivery orifices. When exposed to fluid environment, the central part of the push layer, undergoes swelling, facilitating drug release through the two orifices located on opposing sides of the tablet16, 19, 24, 25, 26.

Figure 18: Sandwiched osmotic tablets (SOTS)
8. Monolithic osmotic system:
In this system the polymer matrix constitutes a simple dispersion of water-soluble agents. When the system encounters the aqueous environment, imbibition of water by the active agent occurs. This leads to the rupture of the polymer matrix capsule that encloses the drug. Therefore, drug release occurs in the external environment14. This process initially occurs in the outer environment of the polymer matrix but progressively proceeds towards the innermost layer of the matrix in a consecutive way. Nonetheless, this system becomes ineffective when the active agent's volume exceeds 20 to 30%, as beyond this point, there is a substantial contribution from the straightforward leaching of the substance24, 25, 26, 29.
9. Osmotic matrix tablets (Osmat):
Osmat is an innovative in-situ matrix system. Its operation relies on a straightforward principle: when introduced into an aqueous medium, hydrophilic polymers undergo swelling, leads to the development of a semi-permeable membrane in situ, subsequently facilitating drug release from a matrix system comprising an osmogen. Osmat combines the properties of both matrix and osmotic characteristics, leading to a significant advancement in drug delivery from a swellable matrix system. Thus, coating a semi-permeable membrane and drilling a delivery orifice. It is a low-cost technology and can be adapted to a wide variety of drugs6, 14, 24. The system produces osmotically driven controlled drug release from a swellable matrix system19.
10. Multi particulate delayed release systems (MPDRS):
In the multi-particulate delayed-release system, pellets containing drugs with or without osmotic agents are coated with an SPM-like cellulose acetate. Upon exposure to fluid environment, water infuses the core, creating a saturated solution of soluble components. The osmotic pressure disparity initiates water influx, causing a swift enlargement of the membrane and subsequent formation of apertures. The osmotic ingredient and the drug are released through these apertures according to zero-order kinetics. Furthermore, dissolution characteristics was influenced by such membrane components as incorporation of plasticizer and its concentration and lipophilicity 10, 19, 24, 27, 28.
11. Pulsatile delivery based on expandable orifice:
This system contains in the form of a capsule, and the drug was distributed through the osmotic infusion of moisture from the body. The orifice in the capsule wall opens intermittently, creating a pulsatile delivery effect. An orifice is created in the capsule wall, constructed from an elastic material. As osmotic infusion advances, pressure within the capsule rises, leading to the stretching of the wall. The orifice is designed to be sufficiently small, ensuring that when the elastic wall returns to its original state, the flow of the drug through the orifice essentially comes to a halt. However, when the elastic wall stretches beyond a certain threshold due to elevated pressure, the orifice enlarges adequately to permit the release of the drug at the desired rate. Elastomers such as Styrene-Butadiene copolymer can be used for this purpose19, 27.

Figure 19: Pulsatile delivery based on expandable orifice
12. Pulsatile delivery by a series of stops:
The capsule is composed of a drug and an absorptive osmotic agent engine, both positioned in sections divided by a movable partition. Pulsatile delivery is accomplished through a sequence of stops sideways the inner wall of the capsule19. These stops act as barriers to the movement of the partition, yet they are successively overcome as the osmotic pressure surpasses the threshold level. The number of stops and their longitudinal arrangement along the length of the capsule determine the quantity and frequency of pulses. Reports document that Porcine somatotropin was delivered by this system19, 27.
13. Lipid osmotic pump:
The core contains of water-insoluble active ingredients which are lipid soluble or lipid wettable. An adequate amount of water-insoluble lipid carrier is used for the pump. The wall, which is microporous, is wetted by a lipid carrier. The device is created by rapidly dissolving the drug in the lipid vehicle. The osmogent is melted in the lipid and then cooled to form a lump that is fragmented and made into a tablet. The microporous is coated at the moderate flow of ambient air19.
14. Asymmetric membrane capsule:
This system involve a core containing core enclosed through a membrane that has an asymmetric structure i.e., it has a relatively thin, dense region supported on a denser, porous region. The capsule wall is made from a water-insoluble polymer such as cellulose acetate unlike a conventional gelatin capsule; because gelatin shell dissolves instantly in water, and provides prolonged release of the active ingredient integrated in the capsule10, 22. Water is imbibed through the capsule wall, causing the dissolution of soluble constituents within it and subsequent release from the same wall8, 19.
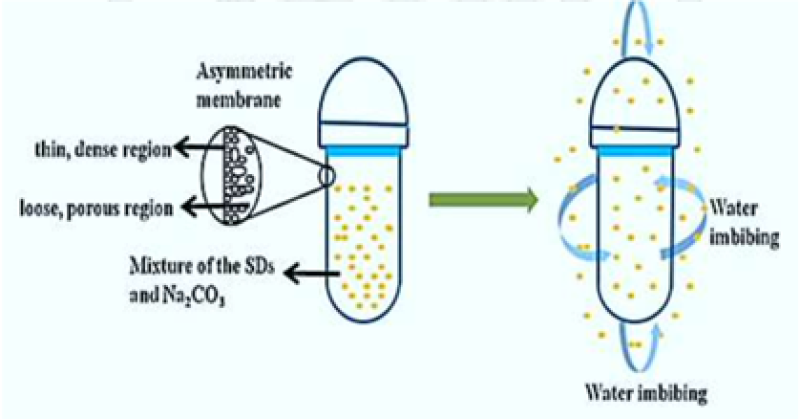
Figure 20: Asymmetric membrane capsule
15. Self-Emulsified Osmotic Tablet:
Insoluble or nearly insoluble medicaments are added with self-emulsifying agents. Self-emulsifying mechanism rises the convenience of medicaments and their release rate is controlled and their plasma concentrations are constant. It emulsifies the water-insoluble drugs. Distinctive surfactants include polyoxyethylene glyceryl ricinoleate, polyoxyethylene castor oil etc10, 30.
16. Longitudinally compressed tablet (LCT) multilayer formulation:
The LCT multilayer formulation is the advanced design. It comprises of an push layer and several drug layers. The multiple drug layers provides improved flexibility and control over the arrangement of release of medication from the system, which can deliver a drug only in a zero-order fashion. For instance, you may design two drug layers with dissimilar drug concentrations to achieve modulation in the release rate profile. Similar to the push-pull system, water absorption through the exposed semi-permeable tablet shell expands the push compartment, releasing the drug primarily through the first compartment at a predetermined controlled rate. The LCT multilayer formulation can also incorporate different drugs in various layers, allowing for combination therapy. The LCT multilayer formulation can be unaffected by gastric pH, gut motility, and the presence of food, depending on where in the GI tract the drug is released, providing greater flexibility to achieve the target release profile25, 31.
17. Osmotically Rupturable Pumps:
The system was established by Baker and comprises of an osmotic core bounded by an SPM. When comes in contact with water, osmotic components imbibe water into the systems resulting in swelling, swelling continues until the membrane ruptures and releases the active compound to the outside environment22. This process continues until the internal pressure inside the device becomes greater than the cohesive force of the membrane and the membrane breaks down into a weak area, usually around the edges. When the membrane is ruptured, the device turn into an EOP and the drug is withdrawn from the cracked area with the mechanism of osmotic pumping. The rupture time of SPM can be controlled by.
- Varying type, area or thickness of SPM
- Changing the osmotic agents embedded in the osmotic core17.
18. Ruminal osmotic bolus pump:
The RUTS push-melt osmotic system comprises of an injection-moulded semi-permeable membrane that encapsulates an osmotic tablet, a partition layer, drug formulation and an iron densifier. The system can vary in size from 2 to 3 cm in diameter and up to 10 cm in length, with an overall drug loading capacity of up to 10gm in the aqueous environment of the rumen, water is imbibed into the SPM into the osmotic tablet, which swells and pushes against the partition layer. The semipermeable membrane controls the degree of water imbition and therefore the inflating rate of the system. This membrane is composed of cellulosic esters and plasticizers and must be rigid enough to ensure device integrity. The osmotic tablet consists of a swelling hydrogel (e.g. sodium carbomer), which provides a high osmotic gradient (more than 300atm) across the membrane, between the osmotic tablet and drug formulation layer is the partition layer, which acts like a plunger in the syringe to ensure a smooth response to the swelling of the osmotic tablet. It contains a compound with a higher melting point or a higher viscosity than the drug formulation layer. Site of administration – ruts push-melt technology was developed to meet the need for drug –a dedicated osmotic system for use in ruminant production animals. Ruminants such as cattle and sheep have a complex digestive system that includes a large four-chambered stomach; the chamber is the rumen, the reticulum, the omasum and the abomasum. In the rumen, ingested cellulose is broken down by microorganisms into simple mono- and disaccharides suitable for digestion. Orally administered sustained release delivery systems are typically limited by the target animal git transit time, but in ruminants (cattle, goats and sheep) the transit time can be controlled by using a device with sufficient density or a geometrical configuration that keeps it in the rumen if for an indefinite period. RUTS Push-melt system can be designed for a variety of drug delivery profiles such as zero order, pulsatile ascending, or descending. These systems are generally engineered for zero-order drug delivery, enabling the release of up to 5 grams per day, with a duration spanning from 1 day to 1 year21.
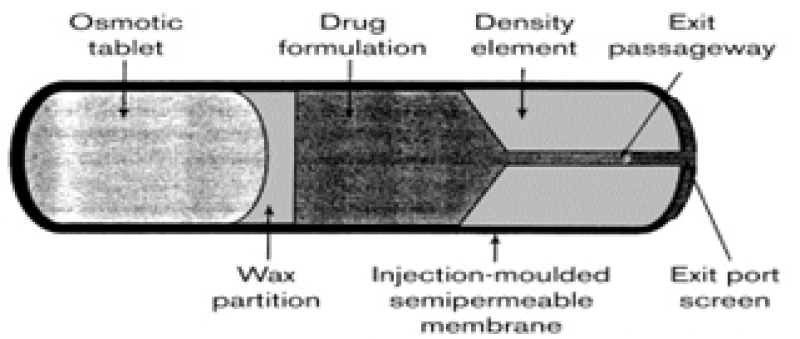
Figure 21: Ruminal osmotic bolus pump
19. Osmotic Pellet:
The osmotic pellet represents a novel drug release involving both osmotic pumping and diffusion mechanisms. Pellets coated with a semipermeable film experience the formation of pores, initiated by the leaching of water-soluble compounds initially present in the coating. The model dynamically describes the key processes in the release, including solvent inflow driven by osmotic pressure differences through the coating film, drug dissolution, pellet swelling due to mass buildup, the development of hydrostatic pressure inside the pellet, and the outflow of dissolved drug through the pores. Release from a coated formulation may occur via diffusion through the film and its pores or by convection through pores or micro-cracks present in the film. Drug release by diffusion from coated pellets along with osmotic pressure was broadly studied22.
Newer Technologies:13
- Ensotrol Technology
The drug is combined with wicking agents, enhancing its surface area and creating a network of channels within the core.
- Portab System (Andrx Pharmaceuticals)
PORTAB is a drug delivery system featuring an osmotic core, typically containing a water-soluble drug which is expanded by a soluble agent to create microporous channels for drug release.
- Zeros Tablet Technology (ADD Drug Delivery Technologies AG, Switzerland)
The technology is utilized for lipophilic drugs, involving drugs, excipients, and gel-forming agents, which form a drug suspension when fluid imbibes and gels with specific viscosity.
- The Port® System (Therapeutic system research laboratory Ann Arbor, MI, USA)
This capsule consists of an active drug with osmotic agents, a sliding partition, and an immediate-release drug. These are encased in a semi-permeable membrane, and the drug along with an osmotic agent expands, creating osmotic pressure, which is then pushed by a non-swellable polymer plug, causing immediate drug release after a lag time. For example suitable for continuous drug delivery, such as methylphenidate for attention deficit hyperactivity disorder treatment.
- DURIN Technology
DURIN technology uses biodegradable polyester co-polymer as an excipient mixed with drug in implantable drug formulations, allowing up to 70-80% loading of drugs. This method can be used for hydrophobic and hydrophilic drugs and is degraded by hydrolysis after release. The drug and excipients are mixed into rods, fibres, or tablets, such as Zoladex-goserelin implants used for prostate cancer were developed. The membrane includes a pore-forming agent that leaches out upon exposure to an aqueous environment, creating a microporous membrane. DURIN technology has achieved controlled, zero-order drug release for up to 6 months in vivo13.
Basic components of osmotic pumps:
- Semipermeable Membrane (SPM):
SPM is a membrane that allows solvent and certain molecules or ions to pass through it by diffusion or specialized facilitated diffusion3. Hence, the selection of the polymeric membrane is crucial for the formulation of the osmotic delivery system. ODDS contains SPM as an outer layer19. Discrimination is the significant measure for the selection of selectively permeable polymers10. The semipermeable membrane is typically 200-300?m thick to endure the pressure within the device19. Cellulose acetate is frequently used as a semipermeable polymer in the preparation of osmotic pumps. The membrane must have sufficient rigidity to maintain its dimensional integrity throughout the operational lifespan of the device2, 19. The material must exhibit adequate wet strength and wet modulus to maintain its dimensional integrity throughout the operational lifespan of the device. Additionally, it should be biocompatible, rigid, and non-swelling. The membrane exhibits sufficient water permeability to retain a water flux rate at preferred range. Indeed, water vapor transmission rates can be employed to estimate water flux rates. The reflection coefficient and leakiness of the osmotic agent should approach the limiting value of unity19. Unfortunately, polymer membranes that are more permeable to water are generally also more permeable to the osmotic agent. Additionally, the membrane should be biocompatible. 8, 9, 16, 26. Examples of materials are cellulose acylate, diacylate and triacylate, etc. Cellulose acetate, cellulose di&triacetate are usually used. Other polymers like agar acetate, amylose triacetate, beta-glucan acetate, poly (vinyl methyl) ether copolymers, poly (orthoesters). The other SPM-forming polymers are groups consisting of acetaldehyde dimethyl cellulose acetate, cellulose acetate ethyl carbamate, cellulose dimethylamino acetate, polyamides, polyurethanes etc.
Table 2: Polymers Used As Semipermeable Membrane and Their Water Vapour
Transmission Rates (WVTR)

2. Hydrophilic and Hydrophobic Polymers:
Polymers play a crucial role in the development of osmotic systems, where they are used to create the drug-containing matrix core. The selection of the polymer is influenced by considerations such as the drug's solubility, the required quantity, and the desired rate of drug release from the pump. Polymers can be of either swellable or non-swellable nature9. The highly water-soluble compounds can be added in hydrophobic matrices and moderately water-soluble compounds can be entrapped in hydrophilic matrices to gain controlled release. Swellable polymers are utilized in pumps comprising water-soluble drugs, as they contribute to an increase in hydrostatic pressure inside the pump due to their swelling nature. For achieving a more controlled release of drugs from the osmotic tablet, ionic hydrogels like sodium carboxymethyl cellulose have been utilized due to their osmogenic nature8. Hydrophilic polymers such as hydroxyl ethyl cellulose, carboxy methyl cellulose, hydroxyl propyl methyl cellulose, and polyvinyl pyrrolidone can be used for osmotic systems. Hydrophobic polymers like ethyl cellulose and wax materials can be used for this purpose19, 26.
3. Wicking Agents.
This agent have the capability to absorb water into the porous network of the delivery device. The wicking agent may be swellable or non-swellable. This agent is chosen based on its nature, whether swellable or non-swellable, and its capacity to undergo physisorption with solvent molecules15. They can undergo physisorption with water. The main function of the agent is to transport water to the surfaces inside the core of the device and create channels or a web of enlarged surface area. The wicking agent employed in the formulation of the osmotic tablet serves to increase the contact surface area of the drug with the incoming aqueous fluid. Additionally, it aids in enhancing the rate of drug release from the orifice of the osmotic system8. Examples include colloidal silicon dioxide, kaolin, titanium dioxide, alumina, niacinamide, PVP k, bentonite, SLS etc 3, 19.
4. Solubilizing Agents:
The incorporation of solubilizing agents into the core tablet significantly enhances the solubility of the drug26. Drugs that are extremely soluble in water and follow zero order are the main targets of the osmotic system. Thus, the drugs which have very low water solubility cannot be used for osmotic devices. Indeed, it is essential to improve the solubility of highly water-soluble drugs to optimize their performance. It is probable to increase the drug solubility by adding solubilizing agents. Non-swellable solubilizing agents have three types,
- Materials that prevent the formation of crystals e.g., PVP, poly (ethylene glycol) and ?cyclodextrin
- A solubilizing agent which can form micelle. They have high HLB values. Examples include Tweens, poly ethylene and others like SLS.
- Citrate esters (e.g., alkyl esters) The blends of complexing agents like polyvinyl pyrrolidone and poly (ethylene glycol) with positively charged surfactants are typically considered10.
5. Surface Active Agents:
Surfactants are highly beneficial when incorporated into wall-forming material, as they contribute to the formation of an integral composite essential for the functionality of the device's wall. Surfactants play a role in regulating the surface energy of materials, and preserving their integrity in the atmosphere of use during the drug release period26. Distinctive surface active agents like poly oxyethylene glyceryl ricinoleate, polyoxyethylene castor oil having ethylene oxide, glyceryl laurates, and glycerol (sorbiton oleate, stearate, or laurate) are merged into the formulations10.
6. Coating Solvents:
Coating solvent is another important component. Its selection depends upon the polymers that are used in the formulations because polymeric solution forms a semipermeable membrane8. Coating solvents are appropriate for creating polymeric solutions used in the manufacturing of the wall of the osmotic device. The primary function of the solvent system is to dissolve or disperse the polymer and other additives and convey them to the substrate surface16. It includes inert organic and inorganic solvents. They should not affect the core and other additives. The solvents used for coating solvents are methylene chloride, acetone, methanol, ethanol, Isopropyl alcohol, butyl alcohol, ethyl acetate, cyclohexane, carbon tetrachloride, water etc. The mixtures of solvents like acetone-methanol (80:20), acetone-ethanol (80:20), acetone-water (90:10), methylene chloride-methanol (79:21), methylene chloride-methanol-water (75:22:3) etc19.
7. Plasticizers:
The role of the plasticizer in the osmotic system is crucial for modifying the physical properties and enhancing the film-forming characteristics of the polymer9. Plasticizer is used to lower the temperature in the phase transition of the wall and also enhance the workability, flexibility and penetrability of the fluids. The range of plasticizers or mixtures of plasticizers is between 0.01 parts to 50 parts which is integrated into 100 parts of wall-forming materials2. Plasticizers can alter the viscoelastic behavior of polymers, and these changes may impact the permeability of the polymeric films3. To improve the membrane properties low molecular weight diluents or plasticizers are used. They alter the elastic behaviour of materials considerably10. Indeed, plasticizers have the capability to transform a hard and brittle polymer into a softer, more pliable material, potentially enhancing its resistance to mechanical stress. Examples of plasticizers are phthalates (dibenzyl, dihexyl, butyl, octyl), triacetin, epoxidized tallate, triisoctyl trimellitate, alkyl adipates, citrates, acetates, propionates, glycolates, myristates, benzoates, halogenated phenyls etc19. Polyethylene glycols, Ethylene glycol monoacetate, for low permeability, Tri ethyl citrate, Diethyl tartrate or Diacetone for more permeable films3.
8. Pore-Formers:
Pore-forming agents induce the creation of microporous membranes. The microporous wall may be formed in situ by a pore-former through its leaching during the operation of the system. These agents are generally used in pump development for very low water-soluble drugs and the development of controlled porosity and multi-particulate osmotic pumps3. The pore-forming agents create microporous structures in the membrane through their leaching action during the operation of the system. The pores may be formed in the wall before the operation of the system by gas formation by volatilization of components or by chemical reactions in polymer solution which creates pores in the wall19. They can be inorganic, organic, solid or liquid. Alkaline metal salts such as sodium chloride, sodium bromide, potassium chloride, potassium sulphate, potassium phosphate, and alkaline earth metals like calcium chloride and calcium nitrate are used as pore formers. Carbohydrates like sucrose, glucose, fructose, mannose, lactose, sorbitol, and mannitol, along with diols and polyols such as polyhydric alcohols, polyethylene glycols, and polyvinyl pyrrolidone, serve as pore formers as well. Triethyl citrate (TEC) and triacetin (TA) are also employed to create apertures in the membrane. Film selectivity to the drug is further enhanced by adding HPMC or sucrose10.
9. Drug
All drugs are not ideal candidates for the osmotic system as prolonged action medication is needed. Drugs those which has a biological half-life of more than 12 hr e.g.: Diazepam and drugs that have very short half-life i.e., less than 1 hr e.g., Penicillin G, furosemide are not appropriate candidates for osmotic controlled release8. Drugs that are intended for prolonged treatment of diseases with a biological half-life in the range of 1–6 hours are well-suited for osmotic systems. The drug's short half-life is advantageous for sustaining or maintaining its presence in plasma, aligning with the requirement for prolonged release15. Various drug candidates such as venlafaxine hydrochloride, diltiazem hydrochloride, carbamazepine, Glipizide, Metoprolol, Nifedipine, Verapamil, oxprenolol are suitable to deliver through the osmotic system8.
Characteristics of the drug that are suitable for formulation
- It should have a short half-life
- Prolonged release of drugs should be desired.
- It should be potent in nature.
- Solubility of the drug should not be very high or very low16, 19.
10. Osmotic agent
These substances are commonly referred to as osmogens or osmagents, and their purpose is to generate osmotic pressure within the system. Osmogenes are fundamental ingredients of the osmotic formulation. These maintain concentration gradient across the membrane. The drug uniformity is maintained in the hydrated formulation. Osmogents are used in the fabrication of osmotically controlled drug delivery systems and modified devices for the controlled release of relatively low water-insoluble drugs. To enhance the release rate osmotic agent is added in the formulation. Osmogens create osmotic pressure in the concentrated solution varying from 8 atm to 500atm19. For the selection of osmogen, the two most critical properties to be considered are osmotic movement and aqueous solubility2. The most common used osmogens are mannitol, sodium chloride, and potassium chloride8.

Table 3: Osmotic pressures of saturated solution of commonly used osmogents
11. Emulsifying agents:
Emulsifying agents are included in the wall-forming material to create an integral composition that is useful in forming the wall of the device for its operation. Excipients are utilized to modify the surface energy of materials, improving their integration into the composite and maintaining their integrity in the usage environment throughout the drug release period.. Examples of emulsifying agents are polyoxyethylene glyceryl ricinoleate, polyoxyethylene castor oil having ethylene oxide, glyceryl laureates, glycerol (sorbitan oleate, stearate or laurate) etc19.
12. Flux regulating agents:
Flux regulating, flux enhancing, or flux decreasing agents are incorporated into wall-forming materials to control the fluid permeability of flux through the wall. These agents may be hydrophilic or hydrophobic substances. Hydrophilic substances increase the flux, but hydrophobic materials tend to decrease the flux. Insoluble salts or insoluble oxides, characterized by being substantially water-impermeable materials, can also be utilized for this purpose26. The hydrophilic ingredients such as polyethene glycols (300 to 6000Da), polyhydric alcohols, and polyethene glycols where hydrophobic substances like phthalates substituted with alkyl or alkoxy (diethyl phthalate, ordimethoxethyl phthalate) decrease the flux19.
13. Barrier layer formers:
The role of the barrier former is to inhibit water entry into specific sections of the delivery system and to create a separation between the drug layer and the osmotic layer. Examples of barrier layer formers are high-density polyethylene, wax, rubber etc19.
Factors influencing release of the drug:24, 25
- Drug Solubility:
For the release of the drug a drug solubility is a crucial factor, as solubility is directly proportional to the release kinetics from the osmotic system. Drugs with moderate solubility levels are often preferable candidates for osmotic delivery compared to drugs with either very high or very low solubilities. The following equation can give drug release with zero-order kinetics -
F (z) = 1-S/P
Where,
F(z) = fraction release of zero order
S= drug solubility in g/cm3
P= density of core tablet23.
The drug having a solubility of ?0.05 g/cm2 would release ?95% drug. Drugs with density > 0.3 g / cm 3 solubility would demonstrate with higher release rate of > 70 % by zero order. The inherent solubility in water of many drugs may inhibit them from fusion in an osmotic system of EOP. A scale of 50-300 mg/ml is considered suitable for an osmotic system10.
Some approaches are designed to deliver drugs having extremes of solubility are given below.
a. Use of wicking agents:
The wicking agents in osmotic formulations were added for low water-soluble drugs. The agent is uniformly dispersed throughout the composition, enhancing the contact surface area of the drug with aqueous fluids. Examples, colloidal silicon dioxide, sodium lauryl sulfate, etc. Therefore, the drug release primarily occurs in a soluble form through the delivery orifice in the membrane.
b. Resin modulation approach:
Ion-exchange resin approaches are generally used to adjust the solubility of drugs. Citric acid and adipic acids were used to maintain a low core pH to ensure that both the drug and resin carry a positive charge. Pentaerythritol was employed as an osmotic agent, resulting in the controlled release of diltiazem hydrochloride over an extended period, with a release profile following pH-independent zero-order kinetics19.
c. Use of swellable polymers:
In ODDS with poor aqueous solubility swellable polymers were employed. The formulation design involves a compartment containing the drug, a swelling agent, and osmogents encapsulated with a rate-controlling membrane. Examples of swelling agents include vinyl acetate copolymer, vinylpyrrolidone, and polyethylene oxide.
d. Use of effervescent mixtures:
This mixture can be engaged to deliver poorly water-soluble drugs within the framework of an ODDS. When this type of system is administered, this mixture containing the drug is delivered under pressure. A mixture of citric acid and sodium bicarbonate is used for the delivery of aspirin. This process dispenses the drug in a suspension form.
e. Use of cyclodextrin derivatives:
This method is utilized to deliver poorly water-soluble drugs. In the CPOP type of osmotic system, the solubility of testosterone is enhanced by forming a complex with cyclodextrin. Testosterone's solubility at 37°C is 0.039 mg/ml, and this was improved to 76.5 mg/ml through complexation with sulfobutyl ether ?-cyclodextrin sodium salt, (SBE)7m?-CD.
f. Use of alternative salt form:
Alteration in the salt form of the drug might change solubility. The hydrochloride salt of oxprenolol was observed to be highly soluble in water (70% w/v), posing challenges in achieving prolonged zero-order delivery from the osmotic dosage form. Hence it was replaced by less soluble succinate salt19.
g. Use of crystal habit modifiers:
It is beneficial to include a crystal modifying agent if the drug has more crystal form having different aqueous solubility. For slightly soluble drugs such as carbamazepine, the addition of crystal-modifying agents (hydroxyethyl cellulose and hydroxymethyl cellulose) along with other excipients results in zero-order drug release when formulated as an osmotic system.
h. Co- compression of drugs along excipients: The incorporation of excipients plays a crucial role in controlling the drug's solubility within the core, thereby influencing drug release in osmotic systems. Various excipients can be employed to adjust the solubility of drugs using mechanisms such as saturation solubility and pH-dependent solubility..
- Use of lyotropic crystals:
To assist osmotic delivery of poorly water-soluble drugs the lyotropic liquid crystals are used. Lyotropic liquid crystals are non-polymeric compounds with molecular weights ranging from 200 to 1500 Da. It is also known as amphipathic compounds. These crystals form mesophases and undergo swelling in the presence of water. phosphatidylcholine (lecithin), phosphatidyl ethanolamine, phosphatidyl glycerol are few examples. Alcolec lecithin and a combination of soybean phospholipids were used for osmotic delivery of dual insoluble drugs such as glipizide and prazosin and gave extended drug release up to 24h19.
2. Delivery Orifice:
The majority of the osmotic systems comprises minimum one opening for drug release23. The dimensions of the orifice need to be optimized to regulate the release of drug from the osmotic system. The size of orifice during the delivery should be smaller than a maximum size to reduce drug delivery through diffusion. Additionally, the area must be adequately large, exceeding a minimum size (Smin), to mitigate hydrostatic pressure buildup in the system, or else, the hydrostatic pressure can rupture the membrane and disturb the zero-order delivery rate. Hence, it is essential to keep the cross-sectional area of the orifice within the specified range of minimum and maximum values32. The ideal diameter for an aperture is considered to be 0.075-0.274 mm. The medicament released will not be regulated if the dimension exceeds 0.368 mm or greater10. The delivery orifice is made by either laser or mechanical drilling23. All the devices do not require puncturing of the wall. Some can make their apertures themselves. In these devices, pore formers are added which are soluble in water. On contacting water, they dissolve and an aperture is generated10.
Methods for creating a delivery orifice in coated osmotic tablet include:
- Mechanical drill:
In this method, we make an aperture by mechanical means. The hole dimension and the film width are taken by a microscope when all the material is dissolved leaving behind the hollow shell10.
- Laser drill:
In the laser method, the laser is fired on the device surface, it absorbs the energy from the laser and is heated and an aperture is created. By changing the power of laser rays, we can alter the aperture sizes. For the production of very small-size apertures, this technology is used. Typically, CO2 laser ray (wavelength of 10.6?) is helpful for us, through which outstanding trustworthiness features at small expenditures are obtained. As the wall only permits water, when water enters, it liquefies the outer portion of the device. Due to this, pressure is generated across the membrane10.
- Modified pumches:
In this process, the dosage form undergoes perforation through the utilization of a piercing device initially enclosed in a secured position. It becomes exposed when compression force is applied. The coating powder, designed for compression, is filled into the die mold, and an unpierced tablet core is placed on top of it. An additional quantity of coating powder is introduced, followed by the simultaneous application of compression and piercing.19.
- Use of pore formers:
The integration of water-soluble ingredients into the membrane wall is widely employed to enhance the Controlled Porosity Osmotic Pump (CPOP) system. When exposed to water, these water-soluble additives dissolve, forming pores within the membrane through which drug release takes place. These substances generate one or more passages with diverse geometric shapes.19.
3. Coating Membrane:
A main aspect by which water entry is regulated is the width of the selective membrane. By altering the composition of the film, the release of the drug can be regulated and varied up to 1000 times10. A rate-controlling membrane is an important aspect of the Osmotic System. Semipermeable polymers are preferred for osmotic systems having permeability for water but impermeability for solutes. Thickness of the coating membrane is mostly kept in range of 200-300 ?m to endure pressure in the osmotic system23.
Membrane type:
Some of the membrane variables that are important in the design of the oral osmotic system are:
a. Nature and type of polymer:
Any polymer permeable to water but impermeable to solute can be selected. Examples of polymers that were used for the above purpose include Hydroxyl ethyl cellulose, carboxy methyl cellulose, and hydroxyl propyl methylcellulose.
b. Membrane thickness:
The width of the membrane significantly influences the drug release from the osmotic system, and these two factors are inversely proportional to each other.
c. Type and amount of plasticizer:
The plasticizers or low molecular weight diluents are incorporated to alter the features and enhance the film-forming characteristics of polymers. Plasticizers can change the viscoelastic behaviour of polymers significantly. Specifically, plasticizers have the capability to transform a rigid and fragile polymer into a softer one, potentially enhancing its resistance to mechanical stress. These changes also affect the porousness of polymer films32.
4. Osmotic pressure:
The drug release rate from an osmotic system is proportional to the core osmotic pressure. The most straightforward and predictable method to ensure a constant osmotic pressure is to uphold a saturated solution of an osmotic agent within the compartment. To achieve the desired osmotic pressure, it is necessary to supplement the drug solution with an additional osmogen within the core formulation19, 32.
Evaluation of ODDS: 5, 33
A. Disintegration test:
In this test apparatus, the disintegration time of tablets is measured by placing tablets in each tube, and the basket rack assembly is positioned in a 1litre beaker of the water or simulated gastric or intestinal fluid at 37?C ± 2?C as the tablet remains 2.5 cm away from the bottom of the beaker. A conventional motor operates by moving the basket up and down within a range of 5 to 6 cm at a frequency of 28 to 32 cycles per minute (cpm). USP disintegration test will be passed if all the tablets disintegrate and the particles are passed through the #10 mesh screen within the specified time.
B. In-vitro dissolution studies:
In-vitro dissolution study is performed by using a suitable USP Type Apparatus. The tablet is kept in 900 ml of dissolution fluid suitable buffer of suitable pH or simulated gastric fluid with a stirrer rotating at a specified r.p.m and maintaining the temperature at 37± 0.5?C of dissolution media. 5 ml of the samples withdrawn at different time intervals were replaced with fresh medium and analyzed in a UV-visible spectrophotometer for estimation of the absorbance by taking a suitable blank solution. At the end, drug release rate is calculated using a suitable equation.
C. Scanning electron microscopy (SEM)
SEM is used to study drug release from the formulations of surface coated tablets before and after dissolution studies were examined. The membranes were dehydrated at 45? for 12 hours and then preserved between sheets of wax paper in a desiccator. The samples were fixed using double-sided tape on a brass stub and gold coated in a vacuum by a sputter coater. Scans were taken. The surface morphology of the coated membrane from the optimized formulation film was examined both begining and after dissolution. By comparing the porous morphology, the effectiveness of the porogen and drug release capability can be evaluated.
IN-VIVO EVALUATION:
Beagle canines are commonly used to assess the in vivo drug delivery rate from OODS and to establish the in vitro/in vivo correlation (IVIVC). Dogs have been utilized extensively for in vivo drug delivery rate evaluation from oral osmotic drug delivery devices because their digestive tracts pH and motility are quite similar to those of humans. Healthy human volunteers are also empolyed for in vivo assessment. AUC, MRT, Tmax, Cmax, and other pharmacokinetic parameters are computed together with relative bioavailability 24, 32.
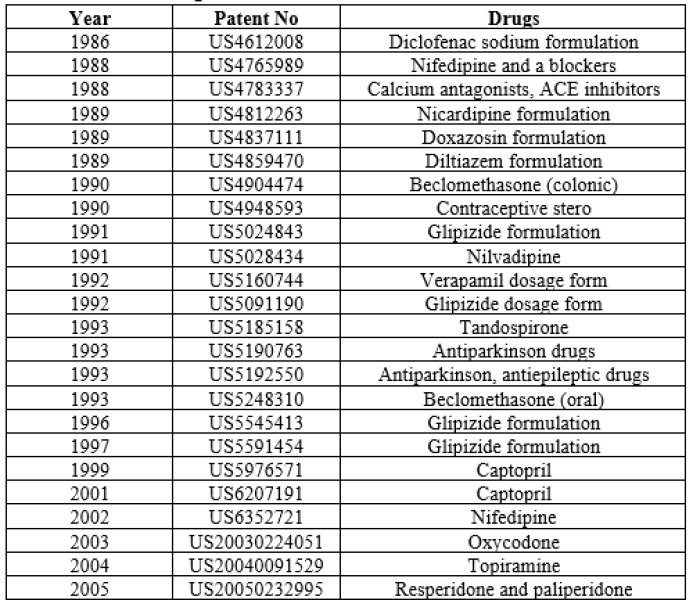
Table 4: Patents on Some Drugs Formulated in the Form of Elementary Osmotic Pump34

Table 5: Patents of Some Drugs Formulated in the Form of Multichamber Osmotic Pumps20

Table 6: Patents of Some Drugs Formulated in the Form of other Osmotic Pumps34,35,36
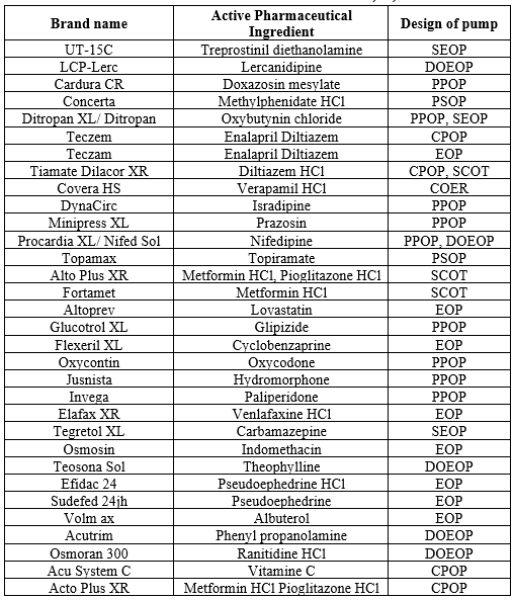
Table 7: Marketed Products available 17,18, 20:
CONCLUSION:
Osmotic drug delivery provides rate-controlled targeted drug delivery of drugs by shorter half-lives that must be consumed more frequently in zero-order kinetics. In addition, this system is unaltered by gastric pH and other physiological factors. In this system, osmotic pressure provides the force for drug release. The rise in pressure within the dosage form due to water infiltration triggers the release of the drug from the system. The osmotic drug delivery is a slightly expensive drug delivery system still it is used because it provides a good amount of drug release which tends to raise its acceptance in the pharmaceutical world. Therefore, this system improves patient compliance for the treatment of chronic illnesses because these disorders require long-term treatment and doses are given more frequently, but by using an osmotic system dosing frequency can be reduced. This system uses osmotic pressure, which swells when comes in contact with water, generates osmotic pressure, and selectively releases Osmotic drugs delivery system is observed to provide a better delivery system of drugs intended for better therapeutic efficacy.
REFERENCES
- Venkateswarlu BS, Pasupathi C, Pasupathi A, Jaykar B, Margret C.R, Palanisamy P, Osmotic drug delivery system: A review, Journal of Drug Delivery and Therapeutics (2019), 9(2):611-616.
- C.M. Dhage, D.M. Shankar, V.T.Pathan, A.G.Jadhav; A Review on Osmotic Drug Delivery System, International Journal of Pharmaceutical Sciences Review and Research (2020), 62(1):160-167
- Bhagat Babasaheb, Hapse Sandip, Darkunde Sachin; Osmotic drug delivery system: An overview, International Journal of Pharmacy and Pharmaceutical Research (2014), 2(1):29-44.
- K. Raja Rao, V. Vasu Naik, A. Anka Rao and A. Rajesh: Osmotic Controlled Drug Delivery System: A Review, International Journal of Pharma And Chemical Research (2015), 1(1):31-37.
- Chinmaya Keshari Sahoo, Nalini Kanta Sahoo, S Ram Mohan Rao, Muvvala S D, K Satyanarayana: A Review on Controlled Porosity Osmotic Pump Tablets and its Evaluation, Bulletin Faculty Pharmacy Cairo Univ (2015), 1-10.
- Gupta Roop N, Gupta Rakesh, Basniwal Pawan K, Rathore Garvendra S: Osmotically Controlled Oral Drug Delivery Systems: A Review, International Journal of Pharmaceutical Sciences (2009), 1(2):269-275.
- Nikhil P. Mahalpure, Sheetal B. Gondkar, Ravindra B. Saudagar: A Review on Controlled Porosity Osmotic Pump Tablet; Asian Journal of Research in Pharmaceutical Sciences (2016), 6(2):101-106.
- Dhole Akshay V, Savita Deokar, Dr. Sonia Singh: A Comprehensive Review On Osmotically Controlled Oral Drug Delivery System, Journal of Emerging Technologies and Innovative Research (2020). 7(10):3819-3837.
- Patil P. B., Uphade K. B., Saudagar R. B: A Review: Osmotic Drug Delivery System, An International Journal Of Pharmaceutical Sciences (2018), 9(2):283-300.
- Nayab A: Review Article On Osmotic Drug Delivery System, Research Gate (2021); 1-33.
- Urvashi Radheshyam S, Shubham Manta F, Neha Bhaurao D, Dr. Rashmi Vipinchandra T and Dr. Millind Janraoji U: Osmotic Controlled Drug Delivery System: A Novel Approach For Diabetic Care, World Journal of Pharmaceutical Research (2022), 11(13):388-403.
- Neetu Khatri, Sarika Nikam and Ajay Bilandi: Oral Osmotic Drug Delivery System: A Review, International Journal of Pharmaceutical Sciences and Research (2016), 7(6):2302-2312.
- Sadhana Shahi, Nityanand Zadbuke, Ajit Jadhav, Santosh Borde: Osmotic Controlled Drug Delivery Systems: An Overview, Asian Journal of Pharmaceutical Technology & Innovation (2015), 3 (15):32-49
- Hiren Patel, Vaishnavi P Parikh: An overview of Osmotic Drug Delivery System: An updated review, International Journal of Bioassays (2017), 6(7):5426-5436.
- Almoshari Y: Osmotic Pump Drug Delivery Systems—A Comprehensive Review, Pharmaceuticals (2022), 15:1430.
- Bansode AS, Saravanan K: Review on Novel Osmotic Drug Delivery System, Journal of Drug Delivery and Therapeutics (2018), 8(5):87-93.
- G. Sandhya Vani, Ch. Kiranmai, B. Hema Latha, K. Padmalatha: A Review on Osmotic Drug Delivery System, Asian Journal of Pharmacy and Technology (2023), 13(1):70-76.
- Rajesh A. Keraliya, Chirag Patel, Pranav Patel, Vipul Keraliya, Tejal G. Soni, Rajnikant C. Patel andM.M. Patel: Osmotic Drug Delivery System as a Part of Modified Release Dosage Form, International Scholarly Research Network Pharmaceutics (2012), 1-9.
- Chinmaya Keshari Sahoo, Surepalli Rammohan Rao, Muvvala Sudhakar, D. Venkata Ramana, Kanhu Charan Panda: Formulation Techniques for Designing of Osmotic Controlled Drug Delivery Systems: A Review, Research Journal of Pharmaceutical Dosage Forms and Technology (2017), 9(4):147-157.
- Ravikant S. Murkute, R. S. Wanare, Krishna J.D: A Review: Recent Scenario on Osmotic Controlled Drug Delivery System, Research Journal of Pharmacy and Technology (2012), 5(5):580-581.
- Thorat Mangesh S, P. Sapkale Anita, Vir Prasad R and C. Singh Meera: Overview of Past and Current Osmotic Drug Delivery Systems, International Journal Of Pharmaceutical and Chemical Sciences (2012), 1(3):1092-1102.
- Pradnya M. Khandagale, Bhushan Bhairav, R.B. Saudagar: Osmotically Controlled Drug Delivery System-A Novel Approach, Asian Journal of Research in Pharmaceutical Sciences (2017), 7(2):68-76.
- Kanchan A.L, Vikram N. Sancheti: Review on Osmotically Controlled Drug Delivery System, International Journal of Trend in Scientific Research and Development (2020), 5(1):961-965.
- Nihar Shah, Nishith Patel, Dr. Kanubhai R.Patel, Dipen Patel: A Review on Osmotically Controlled Oral Drug Delivery Systems, Journal of Pharmaceutical Science and Bioscientific Research (2012), 5(12):230-237.
- Gawai Mamata N, Aher Smita S, Saudager Ravindra B: A Review on Oral Osmotically Controlled Release Drug Delivery System, Asian Journal of Pharmaceutical Research (2016), 6(1):49-55.
- Mijba Shaikh, Ruchita Parmar, Priyanka Rohit, Hitesh Jain, D. B. Meshram: Oral Osmotic System: A Review, International Journal of Pharmaceutical Research and Applications (2021) 6(1):397-403.
- Amitava Ghosh, Arnab Majumder: Drug Delivery Through Osmotic Systems-An Overview, Asian Journal of Experimental Sciences (2009), 23(3):01-19.
- R. K. Verma, B. Mishra, S. Garg: Osmotically Controlled Oral Drug Delivery, Drug Development and Industrial Pharmacy (2000), 26(7):695–708
- Pandey Shivanand, Viral Devmurari: Osmotic Pump Drug Delivery Devices: From Implant to Sandwiched Oral Therapeutic System, International Journal of PharmTech Research (2010), 2(1):693-699.
- Rapol Swathi, Dr M. Sunitha Reddy: A Review on Osmotic Drug Delivery System; International Journal of All Research Education and Scientific Methods (2022), 10(4):718-736
- Brahma P Gupta, Navneet Thakur, Nishi P Jain, Jitendra Banweer, Surendra Jain: Osmotically Controlled Drug Delivery System with Associated Drugs, Journal of Pharmacy and Pharmaceutical Sciences (2010), 13(3)571 - 588.
- Sharma Amit: Formulation and Evaluation of Sublingual Tablet of Losartan Potassium, Asian Journal of Pharmaceutical Research and Development (2018), 6 (4): 101-109.
- Harsha P L, K. Senthil Kumar: Oral Osmotic Drug Delivery System-An Overview, International Journal of Pharmacy and Pharmaceutical Research (2019), 16 (2): 449-467.
- Gundu R, Pekamwar S: An Overview on Osmotically Driven Systems, International Journal of Pharmaceutical Sciences Review and Research (2015), 31(2):96-106
- Dudhat K: An Insight to Modified Release Dosage Form with Osmotic Drug Delivery System, Bioequivalence & Bioavailability International Journal (2023), 7(1):1-4.
- Chinmaya K. S, Surepalli R M R, M Sudhakar and Nalini K S: Advances in osmotic drug delivery system, Journal of Chemical and Pharmaceutical Research (2015), 7(7):252-273


 Prasanna N.* 1
Prasanna N.* 1


























 10.5281/zenodo.10810393
10.5281/zenodo.10810393