Abstract
Naphthalene, a bicyclic aromatic hydrocarbon, has emerged as an important structural component in the development of antimicrobial agents. The naphthalene moiety is featured in numerous compounds that demonstrate significant activity against a wide variety of microbes, including bacteria, fungi, and viruses. This review article aims to provide an in-depth analysis of in vitro studies focusing on the antimicrobial activity of naphthalene derivatives against various pathogenic microorganisms. By reviewing the findings from recent experimental studies, this article will highlight the effectiveness of these compounds and their potential in the development of new antimicrobial therapies. The review seeks to offer a comprehensive understanding of the scope and limitations of naphthalene-based antimicrobials in addressing microbial infections, with an emphasis on in vitro results and their clinical relevance.
Keywords
Naphthalene, Marketed drug, Antimicrobial Agents, Antimicrobial Activity
Introduction
Naphthalene, the most basic bicyclic aromatic compound, is generally sourced from coal tar. It is a white solid with a strong, pungent odor. This compound was first identified by Scottish chemist Alexander Garden in 1819, and its molecular formula (C10H8) was first determined by Michael Faraday in 1826.1 Naphthalene is traditionally synthesized via the Diels-Alder reaction between maleic anhydride and 1,1-diaryl ethylene, followed by aromatization of the bis-product through decarboxylation using barium hydroxide and copper. Additionally, phenyl-substituted naphthalene can be synthesized using the Wagner-Jauregg reaction.2 Over the past few decades, p-conjugated ring systems like naphthalene have gained significant attention in both academic and industrial research. Bioactive compounds derived from naphthalene include anti-cancer agents such as podophyllotoxins (e.g., Etoposide, Teniposide).3 as well as bis-ANS 82, a tubulin polymerization inhibitor and alkylating agent.4 Furthermore, Rifampicin is used as an antitubercular drug,5 while compounds like Justiprocumin A, B, and Patentiflorin A are known for their anti-HIV properties.6 Naphthalene has cytotoxic properties, making it useful for various therapeutic applications. The reactive metabolites of naphthalene, such as naphthalene epoxides and naphthoquinones, are responsible for its cytotoxicity through covalent interactions with cysteine residues in cellular proteins. The sulfhydryl groups of cysteine react with naphthalene oxides through SN2 and SN1 mechanisms, while the naphthoquinones (1,4- and 1,2-naphthoquinone) undergo 1,4-Michael addition reactions.7 Naphthoquinone has a molecular weight of 158.156, no hydrogen bond donors, two hydrogen bond acceptors, and a LogP value of 1.71, adhering to Lipinski's Rule of 5 for drug-like molecules. Doharty MD et al. suggested that 1-naphthol is metabolized by the tyrosinase enzyme, producing 1,2-naphthoquinone and smaller amounts of 1,4-naphthoquinone, which form covalent bonds. Ethylenediamine has been shown to inhibit the covalent binding of 1,2-naphthoquinone, but not 1,4-naphthoquinone.8 According to Wilson GD et al., both 1,2- and 1,4-naphthoquinones are toxic to human colonic adenocarcinoma cell lines LoVo and COLO 206.9 Hepatic microsomal metabolism of naphthalene-containing compounds such as 1-naphthol, 1,2-, and 1,4-naphthoquinone generates reactive oxygen species. In the presence of NADPH, 1-naphthol stimulates microsomal oxygen consumption, while naphthoquinones with NADH or NADPH form superoxide spin adducts. These findings indicate that the cytotoxicity of 1-naphthol in isolated hepatocytes and other cells is due to its metabolism into naphthoquinones, followed by redox cycling and the production of reactive oxygen species, particularly superoxide radicals.10 Naphthalene derivatives exhibit a range of antagonistic activities, including anti-cancer,11–13 antimicrobial,14,15 5 anti-inflammatory,16 antiviral,17 antitubercular,18 antihypertensive,19 antidiabetic,20 anti-neurodegenerative,21–23 antipsychotic,24 anticonvulsant,25 and antidepressant properties.26 This review will focus on the chemistry and therapeutic potential of various naphthalene derivatives with an emphasis on their antimicrobial activity.
CHEMISTRY
Naphthalene, an organic molecule with the formula C10H8, is also referred to by names such as naphthene, naphthalin, camphor tar, and white tar.27 It is a crucial component of coal tar. Structurally, naphthalene consists of two fused benzene rings, making it the simplest polycyclic aromatic hydrocarbon. It is a white crystalline solid, and its planar bicyclic structure contains 10? electrons, classifying it as aromatic according to Huckel's rule. Naphthalene's stability is enhanced by resonance, with a resonance energy of 61 kcal/mol. The three resonance contributors can be derived from heats of hydrogenation or combustion.28 The molecule contains two types of equivalent hydrogen atoms: alpha positions, numbered 1, 4, 5, and 8, and beta positions, numbered 2, 3, 6, and 7. X-ray diffraction studies have shown that naphthalene does not have uniform carbon-carbon bond lengths. Bonds C1C2, C3C4, C5C6, and C7C8 are approximately 1.37 Å (137 pm), while the other carbon-carbon bonds measure around 1.42 Å (142 pm)no-substituted naphthalene compounds, two isomers can form depending on whether substitution occurs at an alpha or beta position. Bicyclo[6.2.0] decapentaene is a structural isomer of naphthalene, characterized by a fused 4-8 ring system . Naphthae reactive than benzene in electrophilic aromatic substitution reactions. Chlorination and bromination of naphthalene occur without the need for a catalyst, yielding 1-chloronaphthalene and 1-bromonaphthalene, respectively. Both benzene and naphthalene can undergo alkylation through the Friedel-Crafts reaction, and naphthalene can also be alkylated with alkenes or alcohols in the presence of sulfuric or phosphoric acid catalysts.29 When reacted with alkali metals, naphthalene forms dark blue-green radical anion salts, such as sodium naphthalenide, which serve as strong reducing agents. The compound has a melting point of approximately 80°C and a boiling point around 218°C. Its molar mass is 128.16 g/mol, and its density is 1.162 g/cm?3;. Naphthalene’s mass spectrum shows a molecular ion peak at m/z 128, which is the base peak.30 In its 1H NMR sphthalene displays two distinct peaks corresponding to eight protons, with the ring protons appearing downfield. Approximately eight protons manifest as doublets at ? 7.32 and ? 7.67. In the 13C NMR spectrum, three types of carbon atoms are identified: carbons 4a and 8a with a peak at 133.6, carbons 2, 3, 6, and 7 with a peak at 125.9, and carbons 1, 4, 5, and 8 showing a peak at ? 128 .31

Naphthalene containing marketed drugs:
Naphyrone (O-2482), a derivative of pyrovalerone, functions as a norepinephrine-dopamine reuptake inhibitor (NDRI) and exhibits stimulant properties.32 Nafcillin, a narrow-spectrum beta-lactam antibiotic from the penicillin class, is employed to treat infections caused by gram-positive bacteria.33 Tolnaftate, an antifungal thiocarbamate derivative, works by inhibiting squalene epoxidase, a key enzyme in the ergosterol biosynthesis pathway.34 Naftifine, an allylamine-based antifungal agent used topically, also exhibits antibacterial and anti-inflammatory effects, inhibiting sterol biosynthesis via squalene 2,3-epoxidase blockade.35 Terbinafine, another antifungal, disrupts ergosterol biosynthesis by inhibiting squalene epoxidase, preventing the conversion of squalene to lanosterol.36 Rifampicin, a naphthalene-based drug, is widely used as an antitubercular agent in combination therapy with other drugs such as Isoniazid, Pyrazinamide, and Ethambutol.37 Bedaquiline, also a naphthalene-containing drug approved by the FDA, is used in treating multidrug-resistant tuberculosis (MDR-TB), often in combination with other treatments.38

Pharmacological implications:
Naphthalene derivatives are linked to a wide range of activities, including anticancer, antimicrobial, anti-inflammatory, antiviral, antitubercular, antihypertensive, antidiabetic, antineurodegenerative, antipsychotic, anticonvulsant, and antidepressant effects. [fig.1]

Antimicrobial:
Antimicrobial drugs that contain naphthalene, including nafcillin, naftifine, tolnaftate, and terbinafine, are currently on the market. A wide variety of synthesized naphthalene derivatives have also shown considerable and effective antimicrobial activity. Furthermore, ?-naphthol, which is primarily used as a dye, has demonstrated powerful antimicrobial properties.39,40
H. Anke et al. investigated the antimicrobial and nematocidal properties of naphthalene derivatives 1-methoxy-8-hydroxynaphthalene (MHN) and 1,8-dimethoxynaphthalene (DMN), which are produced through the melanin biosynthetic pathway involving 1,8-dihydroxynaphthalene. Their findings indicated that both MHN and DMN (2a, 2b) exhibited minimum inhibitory concentrations (MIC) between 25 and 50 mM, with a lethal dose (LD50) of 255 µM.41

Bhawna Chopra et al. developed naphthylamine derivatives featuring an azetidinone structure to assess their antimicrobial activity. Compounds 3 and 4 demonstrated broad-spectrum effectiveness, exhibiting inhibition zones ranging from 9 to 19 mm against B. subtilis MTCC121, S. aureus MTCC96, E. coli MTCC739, and P. aeruginosa MTCC2453.42

The presence of the azetidin-2-one group in naphthylamine derivatives was thought to contribute to their antimicrobial properties. K.M. Rathod et al. assessed the activity of azo-2 naphthol 5 against five notable human pathogenic microorganisms, including Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa, and Streptococcus faecalis.43

It was proposed that the antibacterial properties of azo compounds might be linked to the resorcinol group. Azam Faizul et al. synthesized a range of Schiff bases from naphtha[1,2-d]thiazol-2-amine and created metal complexes of 2-hydroxybenzylidene aminonaphtho thiazole derivatives. The inclusion of the lipophilic naphthalene ring aimed to enhance their ability to penetrate biological membranes for antimicrobial efficacy. The synthesized compounds were tested against Staphylococcus aureus (ATCC 6571), Staphylococcus epidermidis (ATCC 155), Escherichia coli (ATCC 10418), and Pseudomonas aeruginosa (ATCC 10662) using nutrient agar. Compound 6 exhibited notable growth inhibition (6–7 mm) with a minimum inhibitory concentration (MIC) ranging from 42 to 50 µM.44

Schiff bases modified with hydroxyl, halogen, and nitro groups on the phenyl ring demonstrated antibacterial properties. The presence of a hydroxyl group at the 2-position on the phenyl ring was linked to the strongest antibacterial effects, while methoxy groups at various positions had a minimal impact on inhibitory activity. D. Azarifar et al. synthesized 3,5-dinaphthyl substituted 2-pyrazoline derivatives and assessed their antimicrobial activity against a range of organisms, including E. coli, S. aureus, K. pneumoniae, P. mirabilis, S. dysenteriae, and S. typhi. The naphthalene ring substituted with chloro, hydroxy, dimethylamino-N(CH3)2, and carboxamido-CONH2 groups at the N-1 position of the 2-pyrazoline ring exhibited significant antimicrobial activity. Compound [7] emerged as the most effective, with a minimum inhibitory concentration (MIC) between 16 and 63 mM against the tested organisms.45

Among the 3,5-dinaphthyl substituted 2-pyrazoline derivatives, the antimicrobial activity was enhanced by the presence of the N(CH3)2 group in the naphthalene ring. The minimum inhibitory concentration (MIC) values revealed that hydroxyl and chloro groups at the 3-position on the naphthyl ring significantly boosted antimicrobial efficacy. Additionally, the amide group (CONH2) attached at the N-1 position of the pyrazoline ring also contributed to its strong antimicrobial properties. Naphthalene derivatives have been identified as chiral precursors for host-guest interactions.46 Amira M. reported the creation of a novel heterocyclic compound using 1-acetyl naphthalene. Compounds 8 and 9 exhibited strong activity, showing inhibition zones between 1.5 and 2.0 cm against the Gram-positive bacterium ATCC 6538-P and the Gram-negative strain NTC989.47

S. Shin et al. isolated 6-phenyl-tetrahydronaphthalene 10 from the essential oil of Styrax tonkinensis. The antifungal properties of this oil were assessed using a disk diffusion assay against Aspergillus niger and A. flavus. The essential oil and its active constituents demonstrated a minimum inhibitory concentration (MIC) of approximately 0.78 mg/ml.48

R. Kumar et al. synthesized derivatives of 4-amino-3-hydroxy-naphthalene-1-sulfonic acid, conducted quantitative structure-activity relationship (QSAR) studies, and assessed their antimicrobial properties. They found that the presence of 3,4,5-trimethoxy (11) and 2,4-dichloro (12) groups in the benzylidene amino segment was crucial for activity against a range of bacteria and fungi. The minimum bactericidal/fungicidal concentrations for compounds (11) and (12) were determined to be 0.15 mM/ml and 0.14 mM/ml, respectively.49

Zahid H. Chohan et al. investigated the antimicrobial properties of first-row d-transition metal chelates, including cobalt (II), copper (II), nickel (II), and zinc (II), derived from 2-hydroxy-1-naphthaldehyde sulfonamides. Among the synthesized metal complexes, Compound 13 exhibited moderate to significant activity against both Gram-negative and Gram-positive bacterial strains. They noted that the zinc (II) complexes of all ligands demonstrated the highest levels of activity against bacterial species. Additionally, they observed that increasing the methyl and ethyl carbon chains in the ligands positively affected bactericidal activity; as the carbon chain lengthened in the compounds, their bactericidal efficacy also improved.50

Rachel R. Butorac et al. explored the antimicrobial properties of bis iminoacenaphthene (BIAN) linked to N-heterocyclic carbene complexes of silver and gold. The most potent antimicrobial agent identified was the precursor of the imidazolium salt, which exhibited a minimum inhibitory concentration (MIC) of approximately 40 mg/mL. They assessed the antimicrobial activity of the IPr-BIAN imidazolium salt alongside AgCl and AgOAc (14, 15), noting significant antimicrobial effectiveness. The imidazolium salt showed strong activity against both Gram-positive and Gram-negative bacteria, with silver complexes such as AgCl and AgOAc contributing to the observed antimicrobial effects.51

Nasrin Fayyaz et al. analyzed the essential oil derived from Echinophora platyloba, which contained a notable quantity of naphthalene. The oil demonstrated minimum inhibitory concentrations (MIC) of 0.05 mg/ml against Staphylococcus aureus, Bacillus subtilis, and Listeria monocytogenes, and 0.1 mg/ml against Aspergillus niger.52 Yogesh Rokade et al. synthesized and assessed the antimicrobial properties of azetidinone derivatives linked to the ?-naphthol ring. Among the compounds developed, compound 16 exhibited activity against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Aspergillus niger. The presence of methyl, methoxy, and chloro substitutions on the aromatic ring contributed to significantly enhanced antimicrobial activity compared to the standard drugs ampicillin and griseofulvin.53

A. F. Zahoor et al. developed novel conjugated Schiff bases containing naphthalene, with compound 17 demonstrating significant antimicrobial activity against S. aureus (10 ± 0.856 mm) and A. alternata (10.5 ± 2.150 mm). They concluded that the delocalization of ? electrons positively influenced the antimicrobial effectiveness of these conjugated Schiff bases. This electron delocalization increased the lipophilicity of the molecules, facilitating their passage through the lipid membrane of bacteria, which contributed to the enhanced antimicrobial properties observed.54

Z. Ates-Alagoz et al. synthesized a series of 2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalene-2-yl)-1H-benzimidazole-5-carboxamidine derivatives and assessed their antibacterial and antifungal activities against S. aureus, methicillin-resistant S. aureus (MRSA), C. albicans, and C. krusei. Among the compounds, compound 18 demonstrated the highest potency, exhibiting a minimum inhibitory concentration (MIC) of 0.78 mg/mL against both S. aureus and C. albicans. The presence of a 1-naphthyl group at the N1 position of the benzimidazole was believed to contribute to this activity.55

Goksu et al. synthesized derivatives of 5,6-dimethoxynaphthalene-2-carboxylic acid (compounds 19 and 20) and assessed their in vitro antibacterial activity against pathogenic bacteria. The compounds exhibited inhibition zones ranging from 10 to 24 mm.56

Gulay Sahin et al. developed novel derivatives of 5-(1-/2-naphthyloxymethyl)-1,3,4-oxadiazole-2(3H)-thione, 5-(1-/2-naphthyloxymethyl)-1,3,4-oxadiazole-2(3H)-one, and 2-amino-5-(1-/2-naphthyloxymethyl)-1,3,4-oxadiazole from 1 or 2-naphthol. They evaluated the antimicrobial activity of these compounds against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, C. krusei, and C. parapsilosis using the microbroth dilution method. All synthesized derivatives exhibited activity against the targeted organisms, with minimum inhibitory concentrations (MIC) ranging from 32 to 256 mg/ml. Notably, compounds 21, 22, and 23 demonstrated good activity (MIC = 64 mg/ml) against C. krusei, which was attributed to the presence of the 1,3,4-oxadiazole ring.57



Claudia G. T. Oliveira et al. synthesized derivatives of 1,4-naphthoquinone featuring a hydrazino side chain. The antimicrobial activity of diethyl 2-[(3-hydroxy-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)-hydrazono]-malonate 24 was found to be twice as effective against Staphylococcus aureus compared to Lapachol.58

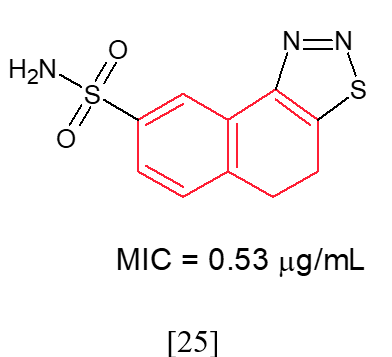
A.R. Jalilian et al. synthesized substituted 4,5-dihydronaphtho[1,2-d][1,2,3]thiadiazole derivatives, with compounds 25 and 26 demonstrating strong antifungal activity against Cryptococcus neoformans while exhibiting low toxicity. The substitution of NO2 with S and SO2NH2 led to a significant enhancement in antifungal activity against Cryptococcus neoformans, achieving a minimum inhibitory concentration (MIC) of 0.53 mg/mL, which was comparable to that of fluconazole and amphotericin B.59

Chung-Kyu Ryu et al. synthesized derivatives of 3-substituted-1,4-dioxo-1,4-dihydronaphthalen-2-ylthio-alkanoates. Compound 27, which has CH3 groups at both the R1 and R positions, exhibited significant activity against Cryptococcus neoformans, S. schenckii, and T. mentagrophytes, with minimum inhibitory concentrations (MIC) ranging from 1.56 to 12.5 mg/mL, particularly when compared to fluconazole.60
Erik Fuglseth et al. developed chiral derivatives of butenafine and terbinafine and assessed their effectiveness against Cryptococcus neoformans. The antifungal activity of both series (28, 29) was significantly influenced by the steric bulk and electronic properties of the substituents. (Fig.18). Substituting a hydrogen atom with a methyl group in the parent compounds (Butenafine and Terbinafine) resulted in a notable enhancement of antifungal activity, with minimum inhibitory concentrations (MIC) of 0.125 to 0.25 mg/mL. Conversely, ethyl substitution led to a reduction in activity, while substitutions with -CH2F, -CHF2, -CF3, or -CN completely abolished antifungal effectiveness. Additionally, the (R)-enantiomer of N-(4-tert-butylbenzyl)-N-methyl-1-(naphthalen-1-yl)ethanamine exhibited superior activity compared to the (S)-enantiomer of butenafine analogues.61



Chung-Kyu Ryu et al., (2005) synthesized a series of 2-arylamino-5-hydroxy-naphthalene-l,4-diones, 3-arylamino-5-methoxy-naphthalene-l,4-diones [30] and tested for in vitro antifungal activity against the species Candida and Aspergillus niger.62

Nagaraja et al., (2006) synthesized 2-Aryl-2,3-dihydronaphtho[2,1-b]furo[3,2-b]pyridin-4(1H) ones were synthesized from2-hydroxy-1-naphthonitrile [31]. The compounds screened for antibacterial and antifungal activity were found effective against human pathogenic Gram positive and Gram negative bacteria and fungi.63

Zeynep et al., (2006) studied the antimicrobial activity of certain chemically synthesized compounds. The compound containing naphthalene moiety. The compound 2-hydroxy-1-napthalene with 6,7-dihydro-13H dibenzo [e,n] [1,4] doxomin-2,11 diamine [32] were studied on the Gram-negative bacteria like Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853), the Gram-positive bacteria like S. aureus (ATCC 25923), MRSA (clinical isolate), Enterococcus faecalis (ATCC 29212) and fungi like Candida krusei (ATTC 6258) and Candida albicans (ATCC 10231). The compound was found to have potent antibacterial and antifungal activity.64

S. Sari et al., (2020) synthesized Naphthalene- Azole derivatives and tested against Candida species. The synthesized compounds [33-35] outperformed standard drug fluconazole in antifungal activity.65

M. Nagy et al., (2020) synthesized original amino-isocyanonaphthalene compounds [36] showing promising antifungal activity against Candida species.66

Derya Osmaniye et al., ten new naphthalene-chalcone derivatives were synthesized and evaluated for their anticancer, antibacterial, and antifungal properties. Notably, compound 37 demonstrated significant activity against the A549 cancer cell line with an IC50 of 7.835 ± 0.598 ?M. It also exhibited antibacterial and antifungal effects. Flow cytometry analysis revealed that compound 37 induced apoptosis at a rate of 14.230% and maintained 58.870% mitochondrial membrane potential. Additionally, it inhibited the VEGFR-2 enzyme with an IC50 of 0.098 ± 0.005 ?M, highlighting its potential as a therapeutic agent.67


Research conducted by Sara Firas Jasim et al., a new series of naphthalene-based derivatives was synthesized and characterized using various spectral methods, including UV–Vis, FTIR, ?1;?3;C-NMR, and ?1;H-NMR, to validate their chemical structures. An ADME study was performed to assess their pharmacokinetic properties and drug-like characteristics. The potential of these derivatives as anti-infective agents was evaluated against pathogenic microorganisms through a broth microdilution assay, which included six aerobic gram-negative bacteria, four anaerobic bacteria, and two fungal strains.68

Research conducted by Rajesh Bhosale et al., novel amide-coupled naphthalene scaffolds (4a-l) were synthesized through an acid-amine coupling reaction of 2-(naphthalen-1-yloxy)acetic acid with various amines. These derivatives were tested for antibacterial, antifungal, and anti-malarial properties. Notably, compound 39 demonstrated impressive antibacterial activity with minimum inhibitory concentration (MIC) values between 12.5 and 100 ?g/mL against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pyogenes. Additionally, compounds 39c, 39f, and 39i exhibited significant antifungal efficacy (MIC - 250 ?g/mL) against Candida albicans, outperforming the standard drug griseofulvin.69

Nikita A. Frolova et al., the focus was on exploring the structure-activity relationship (SAR) of a class of effective biocides, specifically bis-quaternary ammonium compounds (QACs) derived from dihydroxynaphthalene. Twenty gemini-QACs were tested against ESKAPE pathogens, revealing that several compounds demonstrated superior bacteriostatic and bactericidal properties compared to standard mono-QACs and were on par with leading gemini-QAC antiseptics. The SAR analysis suggested that while the linker conformation had minimal impact on efficacy, compound symmetry and lipophilicity were significant factors influencing antibacterial performance. Additional investigations, including time-kill assays and cytotoxicity tests, highlighted compound 40 as particularly promising, exhibiting 2 to 3 times lower cytotoxicity and hemotoxicity than commercial QACs. Scanning electron microscopy (SEM) images showed that these gemini-QACs caused considerable membrane damage in strains of S. aureus and P. aeruginosa, underscoring their potential as effective antiseptics and disinfectants.70

CONCLUSION
In recent years, drug discovery and development with significant biological profiling have gained lot of importance in research. Even though, there is considerable adverse effects, the medicinal chemists have always tried to design drug molecules possessing maximum therapeutic activity and minimal toxicity. Naphthalene nucleus, whether from the natural origin or synthesized in the laboratory, has been explored widely for diverse biological activities viz. anticancer, antimicrobial, anti-inflammatory, antitubercular, antiviral, neurological disorders, cardiovascular disorders etc. Many naphthalene-based molecules have been approved by FDA and are being marketed as therapeutics. The potential of naphthalene scaffold should be more explored through extensive research. This review incorporates the pharmacological aspects of chemically modified naphthalene-based molecules along with their activity profile. We also tried to include structural significance of different naphthalene containing molecules for the design and development of clinically relevant drug candidates.
REFERENCES
1. Thomas, J. M. The Genius of Michael Faraday. Engineering and Science 55, 21–27 (1992).
2. Bergmann, F., Szmuszkowicz, J. & Fawaz, G. The Condensation of 1,1-Diarylethylenes with Maleic Anhydride. J. Am. Chem. Soc. 69, 1773–1777 (1947).
3. Vogel, K., Sterling, J., Herzig, Y. & Nudelman, A. ?-1-Tributyltin-O-2,3-bisacetyl-4,6-ethylidene-glucose as a convenient glycosidation reagent: An efficient synthesis of etoposide. Tetrahedron 52, 3049–3056 (1996).
4. Luduena, R. F., Roach, M. C. & Horowitz, P. The effects of the anilinonaphthalenesulfonates on the alkylation of tubulin: correlation between the appearance of sulfhydryl groups and apolar binding sites. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology 873, 143–146 (1986).
5. Asif, M. Rifampin and their analogs: a development of antitubercular drugs. World Journal of Organic Chemistry 1, 14–19 (2013).
6. ZHANG, H., Soejarto, D., Rong, L., Fong, H. H. S. & Rumschlag-Booms, E. Aryl naphthalide lignans as anti-hiv agents. (2014).
7. Zheng, J., Cho, M., Jones, A. D. & Hammock, B. D. Evidence of quinone metabolites of naphthalene covalently bound to sulfur nucleophiles of proteins of murine Clara cells after exposure to naphthalene. Chem Res Toxicol 10, 1008–1014 (1997).
8. Doherty, M. D., Cohen, G. M., Gant, T. W., Naish, S. & Riley, P. A. Metabolism of 1-naphthol by tyrosinase. Biochemical Pharmacology 34, 3167–3172 (1985).
9. Wilson, G. D., d’Arcy Doherty, M. & Cohen, G. M. Selective toxicity of 1-naphthol to human colorectal tumour tissue. Br J Cancer 51, 853–863 (1985).
10. Thornalley, P. J., d’Arcy Doherty, M., Smith, M. T., Bannister, J. V. & Cohen, G. M. The formation of active oxygen species following activation of 1-naphthol, 1,2- and 1,4-naphthoquinone by rat liver microsomes. Chemico-Biological Interactions 48, 195–206 (1984).
11. de Groot, F. M. et al. Elongated multiple electronic cascade and cyclization spacer systems in activatible anticancer prodrugs for enhanced drug release. J Org Chem 66, 8815–8830 (2001).
12. Valente, S. et al. 1,3,4-Oxadiazole-Containing Histone Deacetylase Inhibitors: Anticancer Activities in Cancer Cells. J. Med. Chem. 57, 6259–6265 (2014).
13. Abate, C. et al. Analogues of ? Receptor Ligand 1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine (PB28) with Added Polar Functionality and Reduced Lipophilicity for Potential Use as Positron Emission Tomography Radiotracers. J. Med. Chem. 54, 1022–1032 (2011).
14. Laverty, G. et al. Ultrashort Cationic Naphthalene-Derived Self-Assembled Peptides as Antimicrobial Nanomaterials. Biomacromolecules 15, 3429–3439 (2014).
15. Chen, Y.-Y. et al. Novel Naphthalimide Aminothiazoles as Potential Multitargeting Antimicrobial Agents. ACS Med. Chem. Lett. 8, 1331–1335 (2017).
16. Goudie, A. C. et al. 4-(6-Methoxy-2-naphthyl)butan-2-one and related analogs, a novel structural class of antiinflammatory compounds. J. Med. Chem. 21, 1260–1264 (1978).
17. Debnath, A. K., Radigan, L. & Jiang, S. Structure-based identification of small molecule antiviral compounds targeted to the gp41 core structure of the human immunodeficiency virus type 1. J Med Chem 42, 3203–3209 (1999).
18. Wilkinson, R. G., Shepherd, R. G., Thomas, J. P. & Baughn, C. STEREOSPECIFICITY IN A NEW TYPE OF SYNTHETIC ANTITUBERCULOUS AGENT1,2. J. Am. Chem. Soc. 83, 2212–2213 (1961).
19. Atwal, K. S. et al. Substituted 1,2,3,4-tetrahydroaminonaphthols: antihypertensive agents, calcium channel blockers, and adrenergic receptor blockers with catecholamine-depleting effects. J. Med. Chem. 30, 627–635 (1987).
20. Bokor, É. et al. C-Glycopyranosyl Arenes and Hetarenes: Synthetic Methods and Bioactivity Focused on Antidiabetic Potential. Chem. Rev. 117, 1687–1764 (2017).
21. Biswas, S. et al. Further Structure–Activity Relationships Study of Hybrid 7-{[2-(4-Phenylpiperazin-1-yl)ethyl]propylamino}-5,6,7,8-tetrahydronaphthalen-2-ol Analogues: Identification of a High-Affinity D3-Preferring Agonist with Potent in Vivo Activity with Long Duration of Action. J. Med. Chem. 51, 101–117 (2008).
22. Ermolieff, J., Loy, J. A., Koelsch, G. & Tang, J. Proteolytic activation of recombinant pro-memapsin 2 (pro-beta-secretase) studied with new fluorogenic substrates. Biochemistry 39, 12450–12456 (2000).
23. Mao, G.-J. et al. High-Sensitivity Naphthalene-Based Two-Photon Fluorescent Probe Suitable for Direct Bioimaging of H2S in Living Cells. Anal. Chem. 85, 7875–7881 (2013).
24. Jele?, M. et al. Synthesis of quinoline/naphthalene-containing azaphenothiazines and their potent in vitro antioxidant properties. Med Chem Res 24, 1725–1732 (2015).
25. Walker, K. A. M., Wallach, M. B. & Hirschfeld, D. R. 1-(Naphthylalkyl)-1H-imidazole derivatives, a new class of anticonvulsant agents. J. Med. Chem. 24, 67–74 (1981).
26. Ang, W. et al. Synthesis and biological evaluation of novel naphthalene compounds as potential antidepressant agents. European Journal of Medicinal Chemistry 82, 263–273 (2014).
27. Borwitzky, H. & Schomburg, G. Separation and identification of polynuclear aromatic compounds in coal tar by using glass capillary chromatography including combined gas chromatography-mass spectrometry. Journal of Chromatography A 170, 99–124 (1979).
28. Cruickshank, D. W. J., Sparks, R. A. & Cox, E. G. Experimental and theoretical determinations of bond lengths in naphthalene, anthracene and other hydrocarbons. Proceedings of the Royal Society of London. Series A. Mathematical and Physical Sciences 258, 270–285 (1997).
29. Cremer, D., Schmidt, T. & Bock, C. W. Theoretical determination of molecular structure and conformation. 14. Is bicyclo[6.2.0]decapentaene aromatic or antiaromatic? J. Org. Chem. 50, 2684–2688 (1985).
30. Dougherty, R. C. & Weisenberger, C. R. Negative ion mass spectra of benzene, naphthalene, and anthracene. A new technique for obtaining relatively intense and reproducible negative ion mass spectra. J. Am. Chem. Soc. 90, 6570–6571 (1968).
31. Desai, N. C. et al. Synthesis, characterization, and antimicrobial evaluation of novel naphthalene-based 1,2,4-triazoles. Med Chem Res 21, 2981–2989 (2012).
32. Iversen, L. et al. Neurochemical profiles of some novel psychoactive substances. Eur J Pharmacol 700, 147–151 (2013).
33. Chang, F.-Y. et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 82, 333–339 (2003).
34. Ryder, N. S., Frank, I. & Dupont, M. C. Ergosterol biosynthesis inhibition by the thiocarbamate antifungal agents tolnaftate and tolciclate. Antimicrob Agents Chemother 29, 858–860 (1986).
35. Mühlbacher, J. M. Naftifine: A topical allylamine antifungal agent. Clinics in Dermatology 9, 479–485 (1991).
36. Petranyi, G., Meingassner, J. G. & Mieth, H. Antifungal activity of the allylamine derivative terbinafine in vitro. Antimicrob Agents Chemother 31, 1365–1368 (1987).
37. Calvori, C., Frontali, L., Leoni, L. & Tecce, G. Effect of Rifamycin on Protein Synthesis. Nature 207, 417–418 (1965).
38. Yadav, S., Rawal, G. & Baxi, M. Bedaquiline: A Novel Antitubercular Agent for the Treatment of Multidrug-Resistant Tuberculosis. Journal of Clinical and Diagnostic Research?: JCDR 10, FM01 (2016).
39. Wilson, C. O., Gisvold, O., Delgado, J. N. & Remers, 1932, William A. (William Alan). Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry. (Lippincott-Raven, 1998).
40. Rokade, Y. B. & Sayyed, R. Z. Naphthalene derivatives: A new range of antimicrobials with high therapeutic value. Rasayan J. Chem 2, 972–980 (2009).
41. Anke, H., Stadler, M., Mayer, A. & Sterner, O. Secondary metabolites with nematicidal and antimicrobial activity from nematophagous fungi and Ascomycetes. Can. J. Bot. 73, 932–939 (1995).
42. Chopra, B., Dhingra, A. K., Kapoor, R. P. & Parsad, D. N. Synthesis and Antimicrobial Activity of Naphthylamine Analogs Having Azetidinone and Thiazolidinone Moiety. Journal of Exploratory Research in Pharmacology 2, 105–112 (2017).
43. Rathod, K. M. Synthesis and Antimicrobial Activity of AZO Compounds Containing Resorcinol Moiety. Asian Journal of Research in Chemistry 4, 734–736 (2011).
44. Azam, F., Singh, S., Khokhra, S. L. & Prakash, O. Synthesis of Schiff bases of naphtha[1,2-d]thiazol-2-amine and metal complexes of 2-(2’-hydroxy)benzylideneaminonaphthothiazole as potential antimicrobial agents. J Zhejiang Univ Sci B 8, 446–452 (2007).
45. Azarifar, D. & Shaebanzadeh, M. Synthesis and Characterization of New 3,5-Dinaphthyl Substituted 2-Pyrazolines and Study of Their Antimicrobial Activity. Molecules 7, 885–895 (2002).
46. Chen, P. C., Patil, V., Guerrant, W., Green, P. & Oyelere, A. K. Synthesis and structure–activity relationship of histone deacetylase (HDAC) inhibitors with triazole-linked cap group. Bioorganic & Medicinal Chemistry 16, 4839–4853 (2008).
47. El-Metwally, A. M. Synthesis and biological evaluation of some new naphthyl derivatives as anti-microbial activity. Egyptian Journal of Chemistry 54, 129–139 (2011).
48. Shin, S. Anti-Aspergillus activities of plant essential oils and their combination effects with ketoconazole or amphotericin b. Arch Pharm Res 26, 389–393 (2003).
49. Kumar, R., Kumar, P., Kumar, M. & Narasimhan, B. Synthesis, anti-microbial evaluation, and QSAR studies of 4-amino-3-hydroxy-naphthalene-1-sulfonic acid derivatives. Med Chem Res 21, 4301–4310 (2012).
50. Chohan, Z. H. & Shad, H. A. Structural elucidation and biological significance of 2-hydroxy-1-naphthaldehyde derived sulfonamides and their first row d-transition metal chelates. Journal of Enzyme Inhibition and Medicinal Chemistry 23, 369–379 (2008).
51. Butorac, R. R., Al-Deyab, S. S. & Cowley, A. H. Antimicrobial Properties of Some Bis(Iminoacenaphthene (BIAN)-Supported N-Heterocyclic Carbene Complexes of Silver and Gold. Molecules 16, 2285–2292 (2011).
52. Fayyaz, N., Mohamadi Sani, A. & Najaf Najafi, M. Antimicrobial Activity and Composition of Essential Oil from Echinophora platyloba. Journal of Essential Oil Bearing Plants 18, 1157–1164 (2015).
53. Rokade, Y. & Dongare, N. Synthesis and antimicrobial activity of some azetidinone derivatives with the ?-naphthol. Rasayan Journal of Chemistry 3, (2010).
54. Zahoor, A. F. et al. Synthesis, Characterization and Antimicrobial Potential of Novel Conjugated Schiff Bases. ajc 26, 6159–6162 (2014).
55. Ates?Alagöz, Z. et al. Synthesis and Potent Antimicrobial Activities of Some Novel Retinoidal Monocationic Benzimidazoles. Archiv der Pharmazie 339, 74–80 (2006).
56. Göksu, S., Uguz, M., Özdemir, H. & Secen, H. A. A concise synthesis and the antibacterial activity of 5,6- dimethoxynaphthalene-2-carboxylic acid. Turkish Journal of Chemistry 29, 199–205 (2005).
57. ?ahin, G., Palaska, E., Ekizo?lu, M. & Özalp, M. Synthesis and antimicrobial activity of some 1,3,4-oxadiazole derivatives. Il Farmaco 57, 539–542 (2002).
58. Oliveira, C. G. T. et al. Synthesis and antimicrobial evaluation of 3-hydrazino-naphthoquinones as analogs of lapachol. J. Braz. Chem. Soc. 12, 339–345 (2001).
59. Jalilian, A. R., Sattari, S., Bineshmarvasti, M., Daneshtalab, M. & Shafiee, A. Synthesis and in vitro antifungal and cytotoxicity evaluation of substituted 4,5-dihydronaphtho[1,2-d][1,2,3]thia(or selena)diazoles. Il Farmaco 58, 63–68 (2003).
60. Kathiravan, M. K. et al. The biology and chemistry of antifungal agents: A review. Bioorganic & Medicinal Chemistry 20, 5678–5698 (2012).
61. Fuglseth, E. et al. Chiral derivatives of Butenafine and Terbinafine: synthesis and antifungal activity. Tetrahedron 65, 9807–9813 (2009).
62. Ryu, C.-K. & Chae, M. J. Synthesis and antifungal activity of naphthalene-1,4-diones modified at positions 2, 3, and 5. Arch Pharm Res 28, 750–755 (2005).
63. Kathiravan, M. K. et al. The biology and chemistry of antifungal agents: A review. Bioorganic & Medicinal Chemistry 20, 5678–5698 (2012).
64. Ali, S. S. & Elliott, W. H. Bile acids. XLVII. 12alpha-Hydroxylation of precursors of allo bile acids by rabbit liver microsomes. Biochim Biophys Acta 409, 249–257 (1975).
65. Sari, S. et al. Azole derivatives with naphthalene showing potent antifungal effects against planktonic and biofilm forms of Candida spp.: an in vitro and in silico study. Int Microbiol 24, 93–102 (2021).
66. Nagy, M. et al. Antifungal Activity of an Original Amino-Isocyanonaphthalene (ICAN) Compound Family: Promising Broad Spectrum Antifungals. Molecules 25, 903 (2020).
67. Osmaniye, D. et al. Design, Synthesis, and Biological Evaluation Studies of Novel Naphthalene-Chalcone Hybrids As Antimicrobial, Anticandidal, Anticancer, and VEGFR-2 Inhibitors. ACS Omega 8, 6669–6678 (2023).
68. Jasim, S. F. F. & Mustafa, Y. F. Synthesis, ADME Study, and Antimicrobial Evaluation of Novel Naphthalene-Based Derivatives. Journal of Medicinal and Chemical Sciences 5, 793–807 (2022).
69. Kalariya, R. et al. Synthesis, biological evaluation and molecular docking study of novel amide-coupled naphthalene scaffolds as potent inhibitors of bacterial recombinase A. European Journal of Medicinal Chemistry Reports 6, 100078 (2022).
70. Frolov, N. A. et al. Development of Naphtalene-Derivative Gemini Qacs as Potent Antimicrobials: Unraveling Structure-Activity Relationship and Microbiological Properties. SSRN Scholarly Paper at https://papers.ssrn.com/abstract=4919346 (2024).


 Shivam Yadav *
Shivam Yadav *
 Chetana Mayekar
Chetana Mayekar
 Nikita Pagare
Nikita Pagare
 Alnaj Thange
Alnaj Thange
 Sonal Yadav
Sonal Yadav




































 10.5281/zenodo.14565869
10.5281/zenodo.14565869