Abstract
The human eye is a sophisticated organ with distinctive anatomy and physiology that hinders the passage of drugs into targeted ophthalmic sites. Effective topical administration is an interest of scientists for many decades. Their difficult mission is to prolong drug residence time and guarantee an appropriate ocular permeation. Several ocular obstacles oppose effective drug delivery such as precorneal, corneal, and blood-corneal barriers. Routes for ocular delivery include topical, intravitreal, intraocular, juxtascleral, subconjunctival, intracameral, and retrobulbar. More than 95% of marketed products exists in liquid state. However, other products could be in semi-solid (ointments and gels), solid state (powder, insert and lens), or mixed (in situ gel). Nowadays, attractiveness to nanotechnology-based carries is resulted from their capabilities to entrap both hydrophilic and lipophilic drugs, enhance ocular permeability, sustain residence time, improve drug stability, and augment bioavailability. Different in vitro, ex vivo, and in vivo characterization approaches help to predict the outcomes of the constructed nanocarriers. This review aims to clarify anatomy of the eye, various ocular diseases, and obstacles to ocular delivery. Moreover, it studies the advantages and drawbacks of different ocular routes of administration and dosage forms. This review also discusses different nanostructured platforms and their characterization approaches. Strategies to enhance ocular bioavailability are also explained. Finally, recent advances in ocular delivery are described.
Keywords
Rapid drainage, physicochemical, microbiological, pharmaceutical properities, new drug delivery system, gene therapy, nano particles, hydrogels
Introduction
Occular drug delivery system: These are the special dosage forms designed to be instilled on to external surface of the eye(topical), administered inside (intra ocular) or adjacent(periocular)to the eye or used in conjuction with the devices.
The eye is a unique organ, both anatomically, and physiologically, containing several varied structures with independent physiological functions.[1] The complexity of the eye provide unique challenges to drug delivery strategies.
Ocular drug delivery is one of the most challenging tasks faced by the pharmaceutical researchers. One of the major barriers of ocular medication is to obtain and maintain a therapeutic level at the site of action for prolonged period of time.[2] Blinking, reflex lachrymation, and drainage rapidly remove drugs, from the surface of the eye.To overcome these, two approaches can be followed:
The first involves using alternate delivery routes to conventional ones allowing for more direct access to intended target site. The second approach involves development of novel drug delivery systems providing better permeability, treatability and controlled release at target site. Combination of both these approaches are being utilized and optimized in order to achieve optimal therapy with minimal adverse effects.[3]
Anatomy Of Eye:
The eye is a spherical structure with a wall layer, Ciliary body and iris and the inner section nervous tissue layer retina. The eye consists of transparent cornea, lens, and vitreous body without blood vessels. The oxygen and nutrients are transported to this non-vascular tissue by aqueous humor which is having high oxygen and same osmotic pressure asblood .[4]

Figure 1: Anatomy of Eye
External Structures:
1. Cornea: Transparent outer layer covering the front of the eye.
2. Sclera: White, tough outer layer covering the rest of the eye.
3. Conjunctiva: Thin membrane covering the sclera and inside of eyelids.
4. Eyelids (Upper and Lower): Protect and lubricate the eye.
5. Eyelashes: Hair follicles along the edges of eyelids.
6. Lacrimal gland: Located under the eyebrow, produces tears.[6]
Anterior Segment:
1. Iris: Coloured part of the eye, controls pupil size.
2. Pupil: Opening in the center of the iris, regulates light entry.
3. Anterior chamber: Space between cornea and iris.
4. Aqueous humour: Clear fluid filling the anterior chamber.[7]
Posterior Segment:
1. Lens: Transparent, flexible structure behind the iris.
2. Vitreous humor: Gel-like substance filling the space between lens and retina.
3. Retina: Innermost layer, converts light into electrical signals.[8]
4. Macula: Central part of retina, responsible for sharp vision.
5. Fovea: Tiny pit in the macula, provides highest visual acuity.
6. Optic disc: Point where optic nerve connects to retina.
7. Optic nerve: Carries electrical signals from retina to brain.[9]
Internal Structures:
1. Ciliary body: Muscular ring supporting the lens.
2. Ciliary muscles: Control lens shape for focus.
3. Choroid: Layer between sclera and retina, supplies blood.
4. Uvea: Collective term for iris, choroid, and ciliary body.[10]
Other Structures:
1. Canaliculi: Tiny tubes draining excess aqueous humour.
2. Trabecular meshwork: Spongy tissue filtering aqueous humour.
3. Schlemm's canal: Circular channel collecting aqueous humour.
4. Plica semilunaris: Folded membrane at the corner of the eye.[11]
Blood Supply:
1. Ophthalmic artery: Branch of internal carotid artery.
2. Central retinal artery: Supplies blood to retina.
3. Ciliary arteries: Supply blood to ciliary body and choroid.[12]
Innervation:
1. Optic nerve (CN II): Transmits visual information.
2. Oculomotor nerve (CN III): Controls eye movements.
3. Trochlear nerve (CN IV): Controls superior oblique muscle.
4. Trigeminal nerve (CN V): Supplies sensory fibers.[13]
Ocular Disorders:
Anterior Segment Disorders:
1. Cataracts: Clouding of lens, affecting vision.
2. Glaucoma: Increased intraocular pressure, damaging optic nerve.
3. Dry Eye Syndrome: Insufficient tears, causing dryness and irritation.
4. Blepharitis: Eyelid inflammation, causing redness and swelling.[14]
5. Conjunctivitis: Conjunctiva inflammation, causing redness and discharge.
6. Keratitis: Corneal inflammation, causing pain and vision loss.
7. Uveitis: Uvea inflammation, affecting vision.[15]
Posterior Segment Disorders:
1. Age-related Macular Degeneration (AMD): Vision loss in macula.
2. Diabetic Retinopathy: Damage to retina due to diabetes.
3. Retinal Detachment: Separation of retina from underlying tissue.
4. Macular Edema: Fluid accumulation in macula.[16]
5. Retinitis Pigmentosa: Progressive vision loss.
6. Stargardt Disease: Juvenile macular degeneration.
Refractive Errors:
1. Myopia (Near sightedness): Difficulty seeing distant objects.
2. Hyperopia (Farsightedness): Difficulty seeing close objects.
3. Astigmatism: Distorted vision due to irregular cornea.
4. Presbyopia: Age-related loss of near vision.[17]
Routes Of Drug Delivery:
Topical Routes:
1. Eye Drops: Most common method, drugs administered directly to the eye.
2. Eye Ointments: Thick, viscous preparations for prolonged release.
3. Gels: Semisolid preparations for sustained release.
4. Suspensions: Liquid preparations with undissolved particles.[18]
Invasive Routes:
1. Intracameral Injection: Injection into the anterior chamber.
2. Intravitreal Injection: Injection into the vitreous humor.
3. Subconjunctival Injection: Injection under the conjunctiva.
4. Periocular Injection: Injection around the eye.
5. Intraocular Implants: Surgical implantation of drug-releasing devices.[19]
Non-Invasive Routes:
1. Trans corneal Route: Drugs diffuse through the cornea.
2. Transscleral Route: Drugs diffuse through the sclera.
3. Conjunctival Route: Drugs diffuse through the conjunctiva.
4. Nasolacrimal Route: Drugs administered through the nasal cavity.
Semi-Invasive Routes:
Iontophoresis: Electrical current enhances drug penetration.
Electroporation: Electrical pulses increase membrane permeability.
Microneedle-Based Delivery: Tiny needles enhance drug penetration.
Ocular Inserts: Drug-releasing devices placed in the eye.[20]
Systemic Routes:
1. Oral Administration: Drugs taken orally, reaching the eye through the bloodstream.
2. Intravenous Administration: Drugs injected into the bloodstream.
3. Intramuscular Administration: Drugs injected into muscles.
Targeted Routes:
1. Intravitreal Implants: Drug-releasing devices implanted in the vitreous humor.
2. Suprachoroidal Space Injection: Injection into the space between sclera and choroid.
3. Subretinal Injection: Injection under the retina.
Factors Influencing Route Selection:
1. Drug properties (solubility, molecular weight, charge)
2. Disease target (anterior vs. posterior segment)
3. Patient compliance
4. Duration of treatment
5. Side effects and toxicity[21]
Barriers of Drug permeation:
• 1. Ocular surface barriers
• 2. Ocular wall barriers
• 3. Retinal barriers
• 4. Viterous body
• 5. The lachrymal fluid
• 6. Solubility of the drug
• 7. lipophilisity of the drug
• 8. Mol.wt and size of the drug
1.Occular surface barriers:
? The corneal and conjuctival superficial layers form the ocular surface i.e., in contact with the tear film
? These create a defence barrier against permeation from undesired molecules
? Corneal surface 5%, Conjuntival surface 95%
? Out of corneal five layers only the outermost squamous epithelial layer forms a barrier
2.Ocular wall barriers:
Sclera Choroid
? Sclera
• It contain stroma made of bundles of collagen and fibroblasts covered by vascular episclera -occupies 80%of eye globe thickness is 0.3 to 1.0mm
? Choroid
beneath sclera - highly vascular tissue thickness is 0.25mm
Microscopic structure
Histologically, sclera consist of following three layers[22]

Figure 2: Microscopic Structure of Sclera
3.Retinal barriers:
The blood-retinal barrier (BRB) is a physiological barrier that regulates the flow of substances into and out of the retina. It's made up of two barriers: the inner blood-retinal barrier (iBRB) and the outer blood-retinal barrier (OBRB):

Figure 3: Retinal Barrier
4.Vitreous body
It occupies a vol about 4.5 ml and is the largest single structure in the eye Drugs are rapidly eliminated from the vitreous by first order kinetics
5.The lachrymal fluid
It is an aqueous fluid which maintain Isotonicity and contain proteins [lysozyme)and

6. Solubility of the drug
Solubility is dependent on the pKa of the drug and pH of the solution
-Usually unionised drugs permeate rapidly than ionised drug As the corneal epithelium bears negetive charge cationic species penetrate fast[23]
7. Lipophilisity of the drug
Outermost corneal epethlium is tend to permeate lipophilic drugs where as inner stromal layer of cornea permeate hydrophobic drugs
Partition coefficient value ranging from 2-4 is found to be optimum
8. Molecular weight and size of Drug
M.wt less than 500 daltons can permeate readily in corneal epithilium
Conjunctva has larger paracellular pore diameter thus allow permeation of larger molecules such as small and medium size peptides[5000-10000d]
Permeation through sclera is by aquous pores
Sclera permeability is half of conjunctiva but much higher than[24]
Methods to overcome Intraocular Barriers
1. Microneedle drug delivery
2. Ultrasound mediated Drug Delivery
3. Iontophorosis
4. Periocuar route DDS
5. Intravitreal injection[25]
1.Microneedle
It is an non invasive method to deliver drugs to intraocular regions
Researchers have developed drug coated microneedles with a length of 500-750 ?m
Drug to be delivered can be coated on a solid metal
• On administration coated molecules dissolve rapidly and
subsequently microneedles are removed from the tissue
Similarly intrascleral hollow microneedles have also developed.
This delivery system is able to deliver microparticles, nanoparticles and drugs in a solution with minimal invasion[26]

Figure 4: Microneedle
2. Ultrasound-Mediated Drug Delivery (UMDD):
Principle: Low-intensity ultrasound enhances drug penetration and absorption.
Mechanisms:
1. Cavitation: Microbubbles increase permeability.
2. Thermal effects: Increased blood flow and temperature.
3. Mechanical effects: Disrupted cell membranes

Figure 5: UMDD
Delivery of beta blockers such as Atenelol, timolol and beraxalol was attempted[27]
3.Iontophorosis:
Iontophoresis is a non-invasive technique
using low-level electrical currents to enhance
transcorneal or transscleral drug delivery.
Principle:
Electric field facilitates movement of charged molecules across ocular tissues.
Mechanism:
1. Electromigration: Charged molecules move towards oppositely charged electrode.
2. Electroosmosis: Neutral molecules move through tissues due to fluid flow
Disadvantages:
No sustained half life
Requires repeated administration
Mild pain in some cases
Low patient compliance becuase of frequent administration that may needed[28]

Figure 6: Iontophorosis
4.Periocular Route
• It has been considered as most promising and efficient route for administering drugs to posterior eye segment
• Periocular refers to region surrounding the eye
Drug solution placed in close proximity to sclera, which results in high retinal and vitreal concentration
It has an advantage like improved drug absorption over systemically and topically and more safety towards posterior segment of eye
Intravitreal Injection (IVI):
Direct injection of medication into the vitreous humor, the gel like substance inside the eyeball.
Purpose: Treat various retinal diseases, including:
1. Age-related macular degeneration (AMD)
2. Diabetic macular edema (DME)
3. Retinal vein occlusion (RVO)
4. Macular hole
5. Retinal detachment
Procedure:
1. Topical anesthesia
2. Pupil dilation
3. Sterile preparation
4. Injection through pars plana (superotemporal or superonasal)
5. Medication delivery to vitreous humor

Figure 7: Intravitreal Injection
Factors affecting intraocular bioavailability:
• Inflow and outflow of lachrimal fluids.
• Efficient nasolachrimal drainage.
• Interaction of drug with proteins of lachrimal fluids.
• Corneal barriers.
• Dilution with tears.
• Physiochemical properties of a drug.[29]
Ocular formulations and ocuserts:
1. Eye drops
2. Ophthalmic solutions
3. Microemulsions
4. In situ gels
5. Eye ointments
6. Contact lenses coated with drugs
7. Liposomes
8. Niosomes and Discosomes
9. Ocuserts
1. Eye drops:
Eye drops are in the form of water and oil solitions, emulsions or suspensions of one or more active ingredients and may contain preservatives if stored in multidose packaging.These are sterile and isotonic dosage forms.The optimum pH for eye drops should be equal to pH of tear fluid which is about 7.4.If pH value gets outside the range, it is intolerated to the eye which leads to decrease in drug bioavalibility

Figure 8: Eye drops
2. Ophthalmic solutions:
Sterile liquid preparations for topical application to the eye, designed to diagnose, treat, or prevent various ocular conditions.
3.Microemulsion:
A clear, stable, and isotropic mixture of oil, water, and surfactant, with droplet size typically <100>
Advantages:
1. Enhanced solubility
2. Improved bioavailability
3. Increased stability
4. Reduced toxicity
5. Targeted delivery
Examples:
1. Restasis (cyclosporine microemulsion)
2. Vigamox (moxifloxacin microemulsion)
3. Systane Ultra (artificial tears microemulsiomicroemulsion) [30]

Figure 9: Microemulsion
In Situ Gels:
Liquid formulations that
transform into gel-like structures upon contact with physiological fluids or specific conditions.
Advantages:
1. Easy administration
2. Improved patient compliance
3. Enhanced bioavailability
4. Reduced dosing frequency
Examples:
1. Timoptic-XE (timolol maleate in situ gel)
2. Pilopine HS (pilocarpine hydrochloride in situ gel)
3. Gattex (teduglutide in situ gel)

Figure10: In Situ Gels
5.Eye Ointments:
Semi-solid, viscous preparations applied topically to the eye surface to provide localized therapeutic effects, protective benefits, or lubrication.
Advantages:
1. Prolonged contact time with eye surface
2. Enhanced corneal penetration
3. Reduced systemic absorption
4. Targeted delivery
5. Easy administration
6. Moisturizing properties
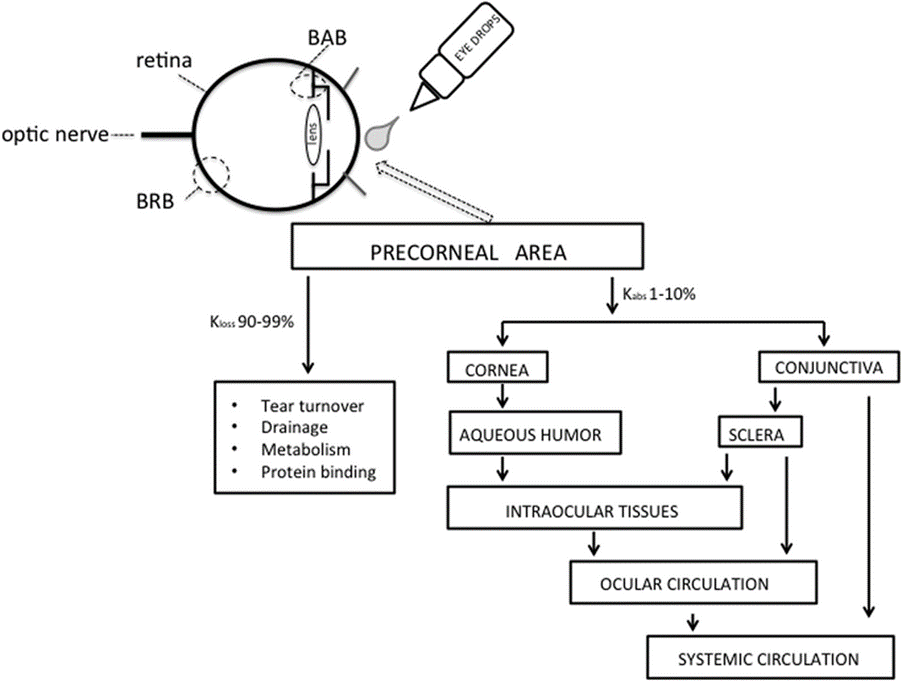
Figure 11: Eye ointments
5.Contact Lenses Coated with Drugs:
Contact lenses designed to release medication directly to the eye surface, combining vision correction with therapeutic benefits.
Applications:
1. Glaucoma management
2. Dry eye syndrome treatment
3. Post-surgical care
4. Ocular inflammation management
5. Infectious disease treatment
Examples:
1. Medennium's OcuMed contact lenses(timolol)
2. Johnson & Johnson's Acuvue Theravision (levocabastine)
3. Sensimed's Triggerfish (timolol)[31]

Figure 12: contact Lenses Coated with Drugs
6.Liposomes:
Tiny, spherical vesicles made of lipids, used to deliver drugs, vaccines, or genetic material to cells.
Characteristics:
1. Bilayer membrane structure
2. 50-500 nm diameter
3. Can be neutral, cationic, or anionic
4. Biodegradable and biocompatible
Advantages:
1. Improved drug solubility
2. Enhanced bioavailability
3. Targeted delivery
4. Reduced toxicity
5. Controlled release
Applications:
1. Cancer therapy
2. Gene therapy
3. Vaccine delivery
4. Ophthalmic diseases (e.g., macular degeneration)
5. Dermatological conditions (e.g., skin cancer)[32]

Figure13:Structure Of Liposome
7.Niosomes:
Non-ionic, vesicular systems made from non-ionic surfactants
Used for drug delivery and encapsulation
Characteristics:
Bilayer membrane structure
50-500 nm diameter
Non-ionic, biodegradable, and biocompatible
Advantages:
Enhanced bioavailability
Improved solubility
Targeted delivery
Controlled release
Reduced toxicity

Discomes:
Disc-shaped, vesicular systems made from phospholipids and surfactants
Used for drug delivery and encapsulation
Characteristics:
Discoidal shape
100-500 nm diameter
Improved stability and solubility
Advantages:
Enhanced bioavailability
Targeted delivery
Controlled release
Reduced toxicity
Improved pharmacokinetics[33]

Figure 14: Structure of Niosomes and Discomes
8.Ocuserts:
Ocuserts [ocular inserts] are defined as ste preparations, multilayered, solid or semisolid devices placed in cul-de-sac or conjunctival s and whose size and shape are designed espe for ophthalmic application
• Deliveres at constant rate by diffusion mechanism
Ocuserts increase corneal contact time, prolongs
duration of action, improve bioavailability, reduces the frequency of administration and thus acheive patient compliance
Ocusert®, pilocarpine ocular therapeutic system is the firstproduct by Alza incorporationUSA from this catogary Generally all types of ocuserts consist of 3 components namely:
1.A central drug reservoir
2. Rate controlling membrane
3.An outer annular ring meant for easy handling[34]

Figure 15:Ocuserts
Emerging Drug Delivery Systems for Ocular Diseases Advances and Marketable Solutions:
Millions of people around the world suffer from eye diseases such as age-related macular degeneration (AMD), diabetic retinopathy (DR), glaucoma, and uveitis, which cause impaired vision or blindness. The eye's distinct anatomy, particularly its protective barriers such as the cornea, conjunctiva, and blood-retinal barrier, complicates drug delivery to the posterior segment of the eye. Traditional treatment methods frequently involve frequent intravitreal injections or topical formulations, which present challenges such as low patient compliance and potential side effects. To address these limitations, novel drug delivery systems (NDDS) are being developed that improve drug penetration, maintain drug release, and improve patient convenience. This article investigates the most recent advances in ocular drug delivery systems and evaluates marketed drugs that use these technologies to treat eye diseases.
Nanoparticle-Based Drug Delivery:
Nanoparticles have emerged as a promising option for ocular drug delivery due to their ability to encapsulate drugs and protect them from degradation. Nanoparticles can be designed to target specific areas of the eye, increasing drug bioavailability while limiting systemic exposure.
Polymeric Nanoparticles: Polymers such as poly(lactic-co-glycolic acid) (PLGA) are frequently used to create nanoparticles for long-term drug release in the eye. Nanoparticles can pass through ocular barriers, allowing for controlled drug release over time.
Example drug: Lucentis (Ranibizumab), which is used to treat AMD and DR, has been studied in nanoparticle formulations to improve drug delivery to the retina. Preclinical studies suggest that nanoparticle-based Ranibizumab can improve drug retention in the retina, reducing the need for intravitreal injections.
Lipid-Based Nanoparticles: For the delivery of drugs into the eyes, liposomes, solid lipid nanoparticles (SLNs), and niosomes have all been investigated. Drug formulation is flexible because liposomes can encapsulate both hydrophilic and hydrophobic medications.
Example Drug: To enhance drug absorption through the cornea, lipid nanoparticle formulations of the dry eye medication restasis (cyclosporine A) have been investigated.
Obstacles: Although drug delivery systems based on nanoparticles have higher efficacy, issues like stability, scalability in manufacturing, and long-term safety need to be resolved before these technologies are widely used in clinical settings.[35][
2. Implants and Inserts Intraocular:
Drug-eluting implants and intraocular implants have been developed to deliver controlled and sustained drug release into the eye. One benefit of these systems is that they can improve patient adherence by minimising the need for frequent dosing. Implants may be divided into two categories: Biodegradable (PLGA-based, for example) and non-biodegradable systems. While non-biodegradable implants offer long-term drug delivery but need to be surgically removed when the drug is exhausted, biodegradable implants release the drug gradually as they break down.
Example Medication: The biodegradable implant Ozurdex (dexamethasone intravitreal implant) is used to treat non-infectious uveitis and retinal vein occlusion (RVO). Over a few months, it directly administers dexamethasone to the retina, lowering macular oedema and inflammation.
Example Medication: A non-biodegradable implant called Iluvien (fluocinolone acetonide intravitreal implant) is authorised for the management of diabetic macular oedema (DME). It reduces the need for repeated injections by offering continuous drug release for up to three years.
Benefits: Long-term medication release via intraocular implants lessens the need for ongoing treatment for eye disorders. Additionally, they administer medications straight to the intended location, increasing effectiveness and reducing systemic side effects.
Obstacles: Implant insertion surgery can be intrusive, and there is a chance of consequences such implant dislocation, infection, or the need for non-biodegradable implant removal surgery.[36]
3. Gene Therapy for Disorders of the Eye:
An innovative method of treating inherited retinal disorders (IRDs) and other hereditary problems affecting the eyes is gene therapy. Gene therapy presents a viable means of treating previously incurable illnesses over the long term or possibly curing them by directly transferring corrected genes to retinal cells. Adeno-Associated Virus (AAV) Vectors: Because of its safety profile and capacity to transduce non-dividing cells, AAV vectors are frequently utilised for gene delivery to the retina.
Example Therapy: The first gene therapy for a hereditary retinal illness is Luxturna (Voretigene Neparvovec), which is approved for the treatment of RPE65 mutation-associated retinal degeneration. It restores the production of the vital protein required for vision by delivering a functioning copy of the RPE65 gene to retinal cells.
Benefits: Gene therapy offers a one-time cure for hereditary illnesses and can have long-lasting therapeutic effects following a single treatment.
Difficulties: Since gene therapy is still in its infancy, more research is required to determine its long-term safety and efficacy. Furthermore, many individuals are unable to get gene therapy therapies due to their exorbitant cost.[37]
4. Ocular Drug Delivery Systems with Sustained Release:
Drug-eluting contact lenses, hydrogels, and microspheres are a few examples of sustained-release drug delivery technologies that are being developed to decrease the frequency of administration and lengthen the duration of medication action.
Hydrogels: Hydrogels are systems made of polymers that have the capacity to hold a lot of water and expand to create gels. They can be applied topically or injected intravenously to administer medications over prolonged periods of time.
Example Drug: Ocusert (Pilocarpine) is a silicone-based device used to administer pilocarpine for the treatment of glaucoma. It is an early example of a sustained-release ocular drug delivery system. It lowers intraocular pressure by delivering continuous medication release over a few days (IOP). Drug-eluting contact lenses are being investigated as a cutting-edge approach to administering medications to the eyes, providing an alternative to eye drops.
Example Drug: Antibiotic- or anti-inflammatory-loaded drug-eluting contact lenses are being developed for the treatment of dry eye disease and as a post-operative measure. By delivering medications to the ocular surface in a continuous manner, these lenses improve bioavailability.
Obstacles: Accurate regulation of drug release rates and ocular tissue biocompatibility are necessary for the development of sustained-release ocular devices. It's also crucial to guarantee device stability and patient comfort.[38]
5. Drugs Sold with Advanced Delivery Mechanisms:
Novel drug delivery systems are being used by a number of marketed medications for eye illnesses in an effort to enhance patient adherence and therapeutic results.
Lucentis (Ranibizumab): used intravitreally, Lucentis is approved to treat AMD and DR. Investigations are underway to improve drug delivery to the retina and decrease injection frequency using ranibizumab nanoparticle compositions. Dexamethasone intravitreal implant, or Ozurdex: This biodegradable implant reduces inflammation and macular oedema by delivering dexamethasone over a period of several months, treating uveitis and retinal vein blockage.
The fluocinolone acetonide intravitreal implant, or Iluvien, is approved to treat diabetic macular oedema. It reduces the need for repeated intravitreal injections by offering sustained medication release for up to three years. Retinal dystrophy linked to RPE65 mutations is treated with Luxturna (Voretigene Neparvovec), the first gene therapy authorised for an inherited retinal illness. Luxturna provides a functional copy of the RPE65 gene.
In summary:
Innovative medication delivery approaches are changing how ocular conditions like glaucoma, diabetic retinopathy, and AMD are treated. These technologies show significant promise for improving treatment results by increasing patient compliance, sustaining release, and enhancing drug bioavailability. These cutting-edge technologies are already being used by commercially available medications like Lucentis, Ozurdex, and Luxturna to treat complicated eye conditions. Future developments in gene therapy, sustained-release methods, and nanoparticle-based administration will probably enhance the effectiveness and practicality of eye treatments even further.[39]
CONCLUSIONS:
The eye is one of the most complex and sophisticate organ as previously discussed in this
review. Many successes in anterior DDSs for prolonging retention time and reducing administration frequency have been achieved, but Additional requirement is needed in this field might be to improve for patient and compliance. On the other side, A few new products of ophthalmic delivery system have been commercialized as a result of the research. The
performance of these new products, however, is still far from level of satisfaction. An ideal ophthalmic drug delivery system should be able to achieve minimum effective drug concentration at the target tissue of eye for prolonged period with minimizing systemic exposure and these systems should be comfortable to use. More research required in each of the technologies discussed in this review. For ophthalmic delivery system some formulations are relatively easy to manufacture, but limited in their ability to provide sustain and
controlled drug release for prolong time period. Other approaches are promising with regard to sustained and controlled drug release, but are difficult to manufacture, use and for achieving Stability especially in case of particulates, liposomes, oligonucleotide therapy, aptamer and other novel advanced delivery system. The novel advanced delivery systems
offer more protective and effective means of the therapy for the nearly inaccessible diseases of eyes. The latest available targeted drug delivery systems focus on the safe and easily localized delivery of the drugs and certain macromolecular substances like DNA, siRNA, and protein to the internal parts of the eye.
REFERENCES
- Thakur RR and Kashiv M. modern delivery systems for ocular drug formulation: A comparative overview W.R.T convetional dosage form. Int J Res Pharm and Bio Sci, 2011; 2(1): 8-18.
- Patel V and Agrawal YK. Current status and advanced approaches in ocular drug delivery system. Journal of global trends in pharmaceutical sciences, 2011; 2(2): 131-148
- Rathore KS, Nema RK and Sisodia SS. An overview and advancement in ocular drug delivery systems. Int J Pharm Sci and Res, 2010; 1(10): 11-23
- Jitendra, Sharma PK, Banik A and Dixit S. A new trend: ocular drug delivery system. Int J of Pharm Sci, 2011; 2(3): 1-25.
- Tangri P, and Khurana S. basics of ocular drug delivery systems. Int J Res Pharm and Biomed Sci, 2011; 2(4): 1541-1552.
- Haders DJ. New controlled release technologies broaden opportunities for ophthalmic therapies. Drug delivery technology, 2008; 8(7): 48-53.
- Lallemand F, Daull P, Benita S, Buggage R and Garrigue JS. Successfully improving ocular delivery using the cationic nano- emulsion, novasorb. Journal of drug delivery, 2012; Article ID 604202:1-16.
- Sireesha DS, Suriaprabha K and Prasanna PM. Advanced approaches and evaluation of ocular drug delivery system. Ame J Pharmatech Res, 2011; 1(4): 72-92.
- Short BG. Safety evaluation of ocular drug delivery formulation: techniques and practical considerations. Toxicologic pathology, 2008; 36: 49-62.
- Sikandar MK, Sharma PK and Visht S. ocural drug delivery system: An overview. Int J Pharm Sci and Res, 2011; 2(5): 1168- 75.
- Ratnam VG, Madhavi S and Rajesh P. ocular drug delivery: An update review. Int J Pharm Bio Sci, 2011; 1(4): 437-46.
- Willoughby CE, Ponzin D, Ferrari S, Lobo A, Landau K and Omidi Y. anatomy and physiology of the human eye: effects of mucopolysaccharidoses disease on structure and function a review. Clinical and experimental ophthalmology, 2010; 38: 2-11.
- McCaa CS. The eye and visual nervous system: anatomy, physiology and toxicology. Environmental health perspectives, 1982; 44: 1-8.
- Rathore KS and Nema PK. An insight into ophthalmic drug delivery system. Int J Pharm Sci Drug Res, 2009; 1(1): 1-5
- Visser L. common eye disorders in the elderly- a short review. SA Fam Pract, 2006;48(7): 34-8.
- Pandey H, Sharma UK and Pandey AC. Eudragit- based nanostructures: A potential approach for ocular drug delivery. Int J Res Dev Pharm L Sci, 2012; 1(2): 40-3.
- Bodos N and Buchwald P. Ophthalmic drug design based on the metabolic activity of the eye: soft drugs and chemical delivery systems. AAPS J, 2005;7(4): E820-33.
- Kumar DP and Arnab D. Pharmacosomes: A potential vesicular drug delivery system.Int Res J Pharm,2012;3(3):102-5.
- Arulkumaran KSG, Karthika K and Padmapreetha J. Comparative review on conventional and advanced ocular drug delivery formulation. Int J Pharm and Pharm Sci, 2010; 2(4): 1-5.
- Singh VK and Tripathi P. Gene therapy in ocular diseases. Indian J Ophthalmol, 2002;50: 173-81.
- Vemuganti GK, Sangwan VS and Rao GN. The promise of stem cell therapy for eye diseases. Clin Exp Optom, 2007; 90(5): 315-16.
- Song KM, Lee S and Ban C. Aptamers and their biological applications. Sensors, 2012; 12: 612-31.
- Degim T and Celebi N. controlled delivery of peptides and proteins. Current pharmaceutical design, 2007; 13: 99-117.
- Games Dos Santos AL, Bochot A and Fattal E. intraocular delivery of oligonucleotides. Current pharmaceutical bio technology, 2005; 6: 7-15.
- Tuchinovich A, Zoidl G and Dermietzel R. Non- viral si RNA delivery into the mouse retina invivo.BMC Opthimology,2010;(25):2-5.
- Patel NM, et al. Dry eye syndrome: a review. Journal of Clinical Ophthalmology. 2020; 14(2): 129-136. doi: 10.1007/s40592-020-00243-4
- Singh RP, et al. Diabetic retinopathy: current concepts and management. Indian Journal of Ophthalmology. 2019; 67(10): 1530-1538. doi: 10.4103/ijo.IJO_645_19
- Lee SS, et al. Glaucoma: diagnosis and treatment. Journal of Glaucoma. 2020; 29(6): 541-548.
- Kuppermann BD, et al.Sustained-release ocular implants. Expert Opinion on Drug Delivery. 2018; 15(10): 931-941.
- Del Amo EM, et al. Ocular drug delivery systems. J Control Release. 2020; 321: 1-15.
- Kuno N, Fujii S. Intravitreal ranibizumab for diabetic macular edema. N Engl J Med. 2019; 380(23):2229-2239.
- Zhang W, et al. Biodegradable intraocular implants. Biomaterials. 2020; 230: 119673.
- Nguyen QD, et al. Ranibizumab for diabetic macular edema. Ophthalmology. 2019; 126(3): 385-393.
- Campochiaro PA, et al.Sustained delivery of aflibercept. Ophthalmology. 2017; 124(12): 1715-1723


 D. Reshma banu*
D. Reshma banu*

















 10.5281/zenodo.14267024
10.5281/zenodo.14267024