Abstract
Polymeric patches with dissolved or dispersed medication that continuously release therapeutic agents via the skin are known as transdermal drug delivery systems. Although transdermal administration has significantly advanced medical practice, it has not yet reached its full potential as a substitute for hypodermic injections and oral delivery. The idea behind TDDS is that they can deliver drugs continuously over an extended length of time, which will maintain the drug's concentration in plasma. To maintain plasma-drug levels for therapeutic efficacy, TDDS can be made to input drugs at the right rate. The capacity of the medication to penetrate the skin in enough amounts to provide the intended therapeutic effect is ultimately what determines the efficacy of the entire transdermal system.
Keywords
Polymeric patches, transdermal system, drug delivery systems.
Introduction
Transdermal patches, also known as skin patches, use a unique membrane to regulate how quickly the liquid medication in the patch's reservoir can enter the bloodstream through the skin. To be utilized as a skin patch, some medications need to be mixed with substances that improve their penetration of the skin, including alcohol. Scopolamine (for motion sickness), nicotine (for quitting smoking), estrogen (for menopause and to prevent osteoporosis after menopause), nitroglycerin (for angina), and lidocaine (for shingles pain; herpes zoster) are among the medications that are applied as skin patches. However, many chemicals, like insulin molecules, are too big to fit through the epidermis. Applying patches to the skin removes the need for pumps or syringes to gain vascular access. In the 1970s, transdermal patches were created, and the FDA authorized the first one in 1979 to cure motion sickness(1). Scopolamine was applied as a three-day patch. Clonidine, fentanyl, lidocaine, nicotine, nitroglycerin, oestradiol, oxybutinin, scopolamine, and testosterone are among the medications for which patches are currently available. In 1981, patches for nitroglycerin were approved. Combination patches for hormone replacement and contraception are also available(2)(1). The patches typically last one to seven days, depending on the medication. Improved bioavailability, more consistent plasma levels, a longer duration of action that reduces the frequency of dosing, fewer side effects, and better therapy because plasma levels are maintained until the end of the dosing interval as opposed to declining with traditional oral dosage forms are some of the main benefits of transdermal drug delivery. Transdermal patches have been helpful in lowering first-pass drug degradation effects and creating new uses for already-approved treatments(3).
Definition
A transdermal patch or skin patch is a medicated adhesive patch that is placed on the skin to deliver a specific dose of medication through the skin and into bloodstream(4)(2)
Advantages And Disadvantages
1]ADVANATAGES
i) They can prevent problems with drug absorption in the gastrointestinal tract caused by gastrointestinal pH, enzymatic activity, and drug interactions with food, beverages, and other drugs taken orally.
ii) When the oral route of pharmaceutical delivery is inappropriate, such as in cases of vomiting and diarrhea, they might be used in their place(5).
iii) To prevent transdermal nitroglycerin's first pass action. When given orally, the body metabolizes it quickly.
iv) Because they are noninvasive, parenteral therapy's drawbacks are avoided.
v) Compared to other dosage forms that need more frequent dose administration, as Tradermal clonidine day, they improved compliance by offering longer therapy with a single application.
vi) The reservoir of the drug in the therapeutic delivery system and its controlled release prolong the activity of medications with a start half life(6).
vii) By removing the application from the skin's surface, drug therapy can be quickly stopped(1)(7)
2]DISADVANTAGES
i) One or more system components may cause contact dermatitis at the application site in certain individuals, requiring stopping the treatment.
ii) Due to the inherent restrictions on drug entrance imposed by the imperability of the skin, only strong medications are appropriate candidates for transdermal patches(8).
iii) Some medications cause discomfort, such as the transdermal patch for scopolamine, which is applied behind the ear.
iv) Long-term adhesion is challenging(9)(10).
Skin as site for transdermal drug administration
About one-third of the blood that circulates through the body passes through the skin, which has a surface area of two square meters on average for an adult. The skin is a complex organ with numerous histological layers. The epidermis, dermis, and hypodermis are the three main tissue layers that are typically used to characterize it (Fig 1). The stratum corneum, the outermost layer of the epidermis that is exposed to the outside world, is one of the five anatomical layers that make up the epidermis under a microscope. It is estimated that each square centimetre of human skin has between 40 and 70 hair follicles and 200 to 250 sweat ducts(11). However, in reality, these skin appendages make up a pitiful 0.1% of the entire surface of the human skin. The transappendage route of percutaneous absorption contributes very little to the overall kinetic profile of transdermal permeation at steady state, despite the fact that foreign agents, particularly those that are soluble in water, may be able to enter the skin through these skin appendages more quickly than through the intact area of the stratum corneum. Consequently, the majority of neutral chemicals' transdermal penetration can be viewed as a passive diffusion process via the intact stratum corneum in the interfollicular area(12).
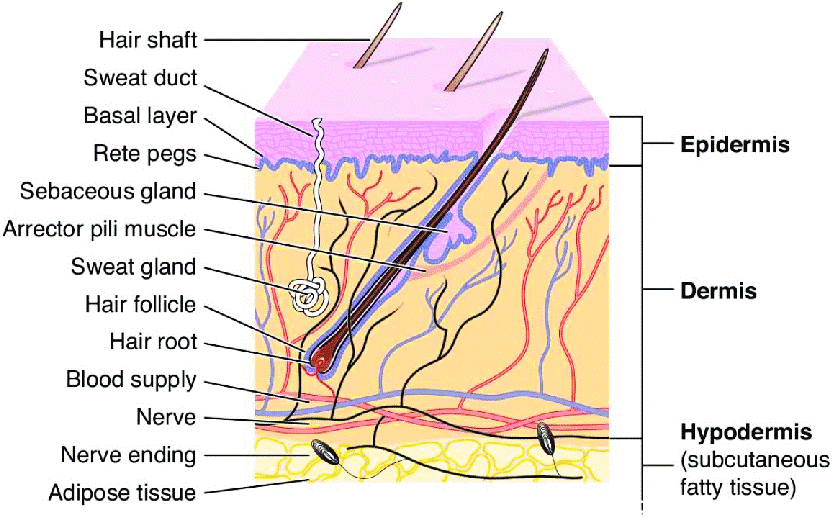
Fig 1: Cross section of skin
Skin Absorption Pathways
Skin absorption pathway can be divided into transport:
- Epidermal route (cross the intac SC)
- Trans-Follicular(shunt pathway ) Absorption (along the skin appendages)(13)
The primary determinants of the pathway selection are the drug's physicochemical characteristics and the formulation's makeup.
1]Epidermal route:(14)
1)The Transcellular (Intracellular) route
2) The Paracellular (Intercellular) route
The Transcellular (Intracellular) route:
Transport of chemicals over the epithelial cellular membrane is referred to as a transcellular route. These include endocytosis and transcytosis of macromolecules, active transport of polar and ionic substances, and passive transport of tiny molecules. Because of the extremely low permeability through the corneocytes and the requirement to partition multiple times from the more hydrophilic corneocytes into the lipid intercellular layers in the stratum corneum and vice versa, the transcellular route is not typically thought of as the preferred method of dermal invasion(15).
The Paracellular (Intercellular) route:
A paracellular pathway is a way for chemicals to move within or between cells. There are tight connections or comparable circumstances between the cells. The partition coefficient (log k) primarily determines the predominant pathway that a permeant takes. While lipophilic permeants (o/w log k >2) pass through the stratum corneum through the intracellular pathway, hydrophilic medicines preferentially partition into the intercellular domains(16). In most situations, the intercellular route is thought to be the most often employed conduit, particularly once the stratum corneum reaches steady-state conditions. The bilayer-structured continuous intercellular lipid region of the stratum corneum is where substance transfer takes place. This channel has been estimated to stretch as long as 500 µm, despite being much more convoluted than the stratum corneum's overall thickness of about 20 µm. Because the majority of medications have a high diffusion coefficient within the lipid bilayer, the intercellular route is thought to result in significantly rapid absorption(17).
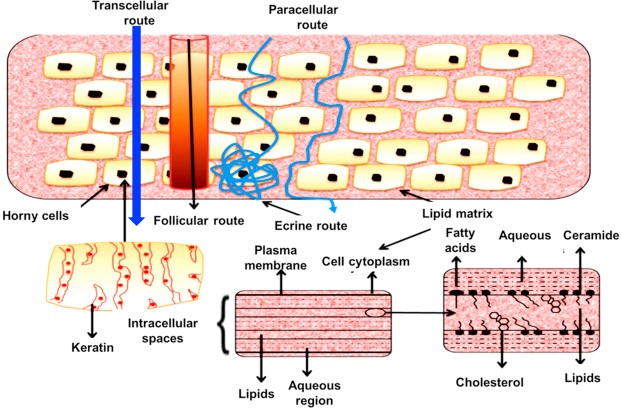
Fig 2. Possible micro routes for drug penetration across human skin
2] Trans-follicular (shunt pathway) Absorption (along the skin appendages):(18)(19)
The skin appendageal route includes transport through hair follicles and the sebaceous glands that are connected to them, as well as eccrine and apocrine sweat glands. Because they do not penetrate the stratum corneum, these routes are known as "shunt" routes.
Hair follicles : Except for the lips, palms, and soles, every part of the skin surface has hair follicles and the sebaceous glands that go along with them. Additionally, there are permeation channels deep inside the skin due to the interspersion of hair follicles down to the subcutis. Each species and bodily site has a different density of hair follicles. Sebum, which lubricates and shields the skin and helps control the pH of the skin's surface, is produced by the sebaceous glands.
Eccrine glands:
Other than the lips, external ear canal, clitoris, and labia minora, eccrine glands are present on every aspect of the human body. These glands are crucial for thermoregulation, which maintains fluid and electrolyte balance. They release a milky or oily, odorless substance that, after digestion by skin surface bacteria, gives off the distinctive body odor.
Apocrine glands: Apocrine glands are coiled tubes that are restricted to particular body parts. These glands, which are linked with hair follicles, are around ten times larger than eccrine ducts and reach as low as the subcutaneous tissues. Due to its very tiny area (about 0.1% of the total skin area), the trans-follicular (shunt pathway) route is regarded as having little significance. On the other hand, invasion through the appendages may be significant during the early phases of a skin absorption process and in the case of large hydrophilic chemicals and ions. According to recent research, the appendages route may also have a role in the absorption of cyclodextrin-inclusion complexes, liposomes, and nanoparticles.
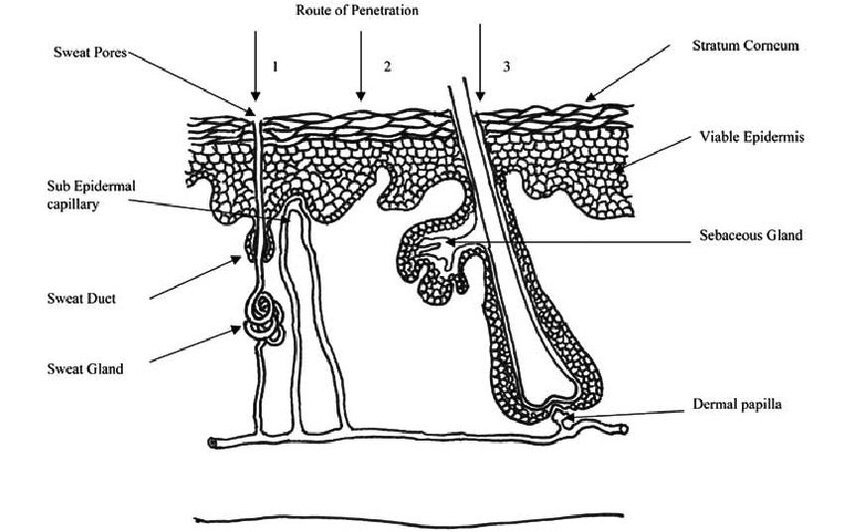
Figure 3. Absorption across the skin can occur through sweat ducts (1), intercellular regions of the stratum corneum (2) and through the hair follicles (3)
Kinetics Of Transdermal Permeation:
A systemically active medication must have certain physicochemical characteristics that allow it to sorb through the skin and enter the microcirculation in order to reach its target region. The following steps11–15 are involved in the release of a therapeutic substance from a TDDS applied to the skin's surface and its transportation to the systemic circulation:
- The formulation's internal dissolution and release
- Dividing into the skin's outermost layer, SC
- Uptake into the local capillary network and ultimately the systemic circulation;
- Diffusion across the SC;
- Partitioning from the SC into the aqueous viable epidermis;
- Diffusion from the viable epidermis and into the upper dermis
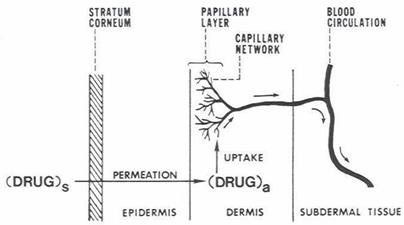
Figure 4. A multilayer skin model showing sequence of Transdermal permeation of drug for systemic delivery
For transdermal devices to be developed successfully, an understanding of skin penetration kinetics is essential. If the medication has specific physico-chemical characteristics, this penetration may be feasible. The following formula provides the rate of permeation via the skin (dQ/dt):
(dQ/dt)= Ps(Cd ? Cr)----------1
Where,
- Cd is the amount of skin penetrant present in the donar compartment, such as on the stratum corneum's surface.
- Cr is the receptor compartment's concentration (e.g., body). Correspondingly
- Ps is the skin tissue's total permeability constant to the penetrant.
PS = KSDSS/HS (2)
Where,
Ks = partition coefficient of penetrant
Dss = apperent diffusivity of penetrant
Hs = Thickness of skin
Factors affecting transdermal permeation(20)[15]
Physiochemical properties of the penetrant molecule:
- Partition Coefficient
• For the best transdermal permeability, a lipid/water partition coefficient of 1 or higher is typically needed.
• It can be changed chemically without influencing the drug's pharmacological action.
- PH condition
• Changes in pH that affect the ratio of charged and uncharged species and their transdermal permeability can impact the flux of ionisable medications;
• Applications of solutions with extremely high or extremely low pH values can be harmful to the drug.
- Penetrant concentration
- At concentrations greater than solubility, surplus solid drug acts as a reservoir and helps maintain a consistent drug composition for a long time;
- assuming membrane related transport, rising concentration of dissolved drug results in a proportionate rise in flux.
Physiochemical properties of drug delivery system(21)[16]
- Drug characteristics
• The drug's solubility in the vehicle dictates the pace of release. The following variables affect the drug release mechanism:
• Whether the drug molecules in the delivery systems are suspended or dissolved.
• The drug's interfacial partition coefficient between the skin tissue and the delivery device.
• The vehicle's pH.
- Composition of drug delivery system
In addition to influencing the rate of drug release, the composition of drug delivery systems—such as boundary layers, thickness, polymers, and vehicles—also influences the stratum corneum's permeability through hydration, skin lipid formation, or other sorption-promoting effects. For example, benzocaine permeation decreases with PEG of low molecular weight.
Basic component of transdermal patch
- Polymer matrix / Drug reservoir
- Drug
- Permeation enhancers
- Pressure sensitive adhesive (PSA
- Backing laminates
- Release liner and other excipients like plasticizers and solvents
*Polymer(22)[17]
The key component of TDDS that regulates the medication's release from the device is polymers. Medicate can be dispersed throughout a fluid or strong state engineered polymer base to create a polymer framework. Businesses involved in the transdermal delivery space focus on specific polymeric structures. For example, Searle Pharmacia focuses on silicon elastic, while Alza Organization primarily focuses on microporous polypropylene or ethylene vinyl acetic acid derivation (EVA) copolymers. The polymers used in TDDS fall into the following categories:
Natural polymers :- include things like shellac, zein, gelatin, gums, waxes, and cellulose derivatives.
synthetic elastomers : - Polybutadiene, hydrin rubber, polyisobutylene, silicon acrylonitrile, neoprene, butylrubber.
synthetic polymers:- system Polyvinyl alcohol, polyvinyl chloride, polyethylene, polyacrylate, polyamide, polyurea, polyvinylpyrrolidone .
*Drug(23)[18]
The medication selection process is crucial for the successful development of a TDDS. Some desirable characteristics of a medication for transdermal delivery include the following.
Physiochemical characteristics
1. The drug's molecular weight should be less than about 1000 Dalton.
2. The medication should be able to bind to both hydrophilic and lipophilic phases.
3. The drug's melting point should be low.
The biological characteristics
1. The medication should be strong, with a daily dosage of a few milligrams.
2. The half-life ought to be brief.
3. The medication must not cause an allergic reaction or cutaneous irritation.
4. Transdermal administration is appropriate for drugs that break down in the gastrointestinal tract.
* Permeation enhancers (24)
Enhancers interact with the structural elements of the stratum corneum, such as proteins and lipids, to increase the permeability of the stratum corneum and achieve greater therapeutic levels of the drug penetration enhancer. The partial leaching of the epidermal lipids by chemical enhancers appears to be the cause of the improved absorption of oil-soluble medications, which improves skin condition for wetting and transepithelial and transfollicular penetration.
1. Chemical enhancer: Accelerants, absorption promoters, or penetration enhancers are terms used to describe chemicals that facilitate the uptake of medications administered topically(25).
Chemical enhancer classification
1. Terpenes, such as carvone, menthol, etc.
2. Pyrollidones, including azone and N-methyl-2-pyrollidone, among others.
3. Fatty acids, such as lauric acid and oleic acid, among others.
4. Sulfoxides: dimethyl sulfoxide, for example.
5. Alcohols, such as ethanol and octyl alcohol, among others.
6. Other enhancers, such as amino derivatives, phospholipids, and cyclodextrin.
2. Physical enhancers
Iontophoresis and ultrasound, also known as phonophoresis or sonophoresis, are physical methods used to improve the percutaneous penetration and absorption of different therapeutic agents.
* Adhesives
The pressure-sensitive adhesive ensures a close connection between the patch and the skin. For example, adhesive types include polyacrylates, polyisobutylene, and silicon-based adhesives. The adhesive system must meet the following criteria:
- It is important for the product not to cause any irritation or sensitization to the skin.
- It's important for the patch to stay firmly on the skin throughout the dosing period, remaining in place even during activities like bathing or exercise.
- It should be simple to take out.
- It is essential that it does not leave any lingering residue on the skin.
- It is important for the fabric to have a superior feel against the skin.
* Backing laminate
The main purpose is to ensure a strong bond with the drug reservoir and prevent the drug from escaping the dosage forms through the top. It acts as a barrier to shield the product while it is applied on the skin, examples include materials like metallic plastic laminate, occlusive base plates (such as aluminum foil), and adhesive foam pads (made of flexible polyurethane).
- Types Of Transdermal Patches(26)(18)(27)[20,21,22]
Single Layer Drug -In- Adhesive
The incorporation of the drug directly within the skin-contacting adhesive is what distinguishes the single-layer drug-in-adhesive system. In this transdermal system design, the adhesive serves as the foundation for the formulation and keeps the medication and all of the excipients together under a single backing sheet.
Multi Layer Drug In Adhesive
A semisolid matrix containing a medication solution or suspension that comes into direct touch with the release liner is what defines the matrix system design. The skin-adhesive component is integrated into an overlay and surrounds the semisolid matrix in a concentric pattern.
Drug Reservoir-in-Adhesive
A liquid compartment with a medication solution or suspension that is isolated from the release liner by an adhesive and semi-permeable membrane defines the Reservoir transdermal system design. The product's adhesive ingredient that promotes skin adhesion can be integrated in a concentric pattern around the membrane or as a continuous layer between the membrane and the release liner.
Drug Matrix-in-Adhesive
A semisolid matrix containing a medication solution or suspension that comes into direct touch with the release liner is what defines the matrix system design. The skin-adhesive component is integrated into an overlay and surrounds the semisolid matrix in a concentric pattern.
Approches used in development of TDDS
Several technologies have been successfully developed to provide for rate control over transdermal penetration and medicine release.
- Membrane permeation – controlled systems
- Adhesive dispersion – type systems.
- Matrix diffusion – controlled systems.
- Micro reservoir type or micro sealed dissolution controlled systems.
- Membrane permeation – controlled systems
A shallow compartment made of a drug-impermeable metallic plastic laminate and a rate-controlling polymeric membrane—which can be either microporous or non-porous, as seen in Figure —encloses the drug reservoir in this kind of device. Only through the rate-controlling polymeric membrane are the medication molecules allowed to release. The drug particles are either uniformly distributed in a solid polymer matrix (like polyisobutylene adhesive) or suspended in a viscous, unbleachable liquid medium (like silicon fluids) to create a paste-like suspension in the drug reservoir compartment.
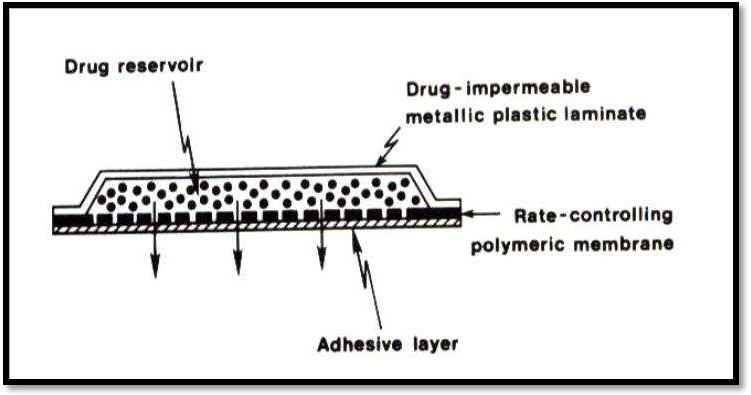
Membrane permeation controlled system
The main benefit of a membrane permeation regulated system is the drug's steady release rate. However, there is also a rare chance that dose dumping or the quick release of the full drug content could occur from an unintentional rupture of the rate-controlling membrane. Transderm-nitro, Transderm-scop, Catapres, and Estraderm are a few examples of this system.
-
- Adhesive Dispersion – Type Systems
A streamlined version of the membrane-permeation regulated system is this one. In order to create a thin layer of drug reservoir, the drug is first dispersed in an adhesive polymer, such as poly (isobutylene) or poly (acrylate) adhesive, and then the medicated adhesive is applied, either by solvent casting or hot melt, to a flat sheet of drug-impermeable metallic plastic backing, as illustrated in fig.
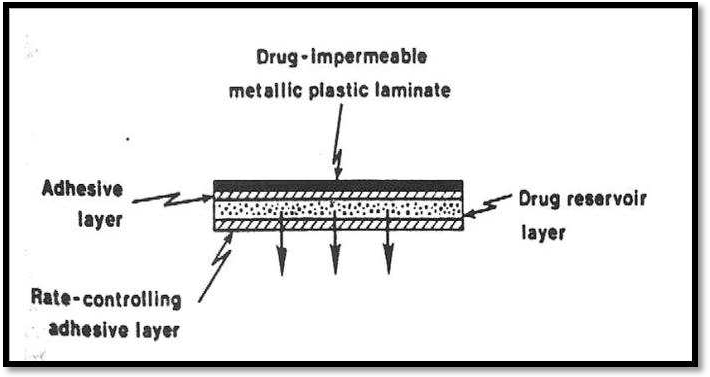
Adhesive dispersion type system
Matrix Diffusion- Controlled Systems
By uniformly spreading the drug particles in a hydrophilic or lipophillic polymer matrix, this method creates the drug reservoir. A medicated disc with a specified surface area and regulated thickness is then formed from the resulting medicated polymer. A compartment made of a drug-impermeable plastic backing membrane is then used to adhere a drug reservoir with a polymer disc onto an occlusive foundation plate (fig.). For instance. Nitro-Dur: Treats angina pectoris by delivering nitroglycerin.
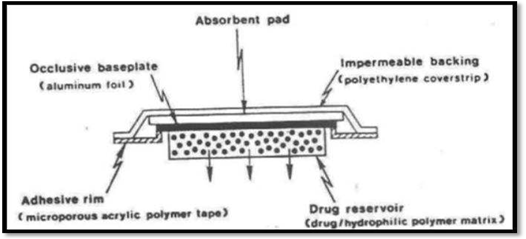
Matrix diffusion-controlled system
Micro reservoir type or Micro sealed Dissolution
The reservoir and matrix diffusion types of drug delivery systems can be combined to create the micro reservoir type. A number of distinct, impermeable microspheres of drug reservoirs are created by first suspending the drug solids in an aqueous solution of a water-soluble liquid polymer (such as polyethylene glycol) and then uniformly dispersing the drug suspension in a lipophillic polymer, such as silicone elastomers, using a high energy dispersion technique(28).
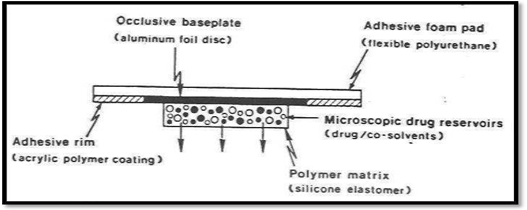
Micro reservoir type controlled system
Applications of Transdermal Patches (29)[23]
- The nicotine patch, which helps people quit smoking tobacco by releasing nicotine in controlled dosages, is the most popular transdermal patch in the US.
- Fentanyl (marketed as Duragesic) and buprenorphine (marketed as BuTrans) are two opioid drugs that are frequently administered in patch form to treat extreme pain 24/7.
- Postmenopausal osteoporosis and menopausal symptoms can both be treated with oestrogen patches. The contraceptive patch (marketed as Ortho Evra or Evra) is another type of transdermal patch used to distribute hormones.
- Angina pectoris can occasionally be treated with nitroglycerin patches.
- Clonidine, an antihypertensive medication, comes as transdermal patches.
- The first transdermal antidepressant delivery system was the transdermal version of the MAOI selegiline(21). [24]
CONCLUSION
Transdermal drug delivery devices are a useful advancement in medicine administration, especially for individuals who have trouble swallowing or remembering to take their prescriptions. To guarantee the best possible outcomes for patients and the safety of everyone who comes into contact with patients who utilise transdermal delivery systems, clinicians and other allied health professionals should be knowledgeable about the proper administration practices. More control over treatment plans and the ongoing growth of the number of medications available for use are probably going to be the main focusses of future TDDS advances. Transdermal dose forms might give doctors the chance to give their patients more treatment choices so that their care is optimised.
REFERENCES
- Bathe R, Patil M. An updated review on transdermal drug delivery systems * Correspondence Info?: 2015;1(06):244–54.
- Design and Evaluation of Matrix T ype and Membrane Type Controlled T ransdermal Delivery Systems of Transdermal Nicotine Suitable for use in Smoking Cessation. :179–84.
- Review On Approaches Of Computer. 2022;12(1):771–81.
- Hanbali OAAL. Transdermal patches?: Design and current approaches to painless drug delivery. 2019;69:197–215.
- Gaikwad AP, Parhad MB, Sanap GS. REVIEW?: HERBAL ANTIDIABETIC DRUG. 2023;12(5):1920–31.
- Article R, Ghadge RN, Parhad MB. REVIEW?: NIOSOMAL DRUG DELIVERY SYSTEMS. 2023;12(5):680–91.
- Aher TR, Mokle BA, Sanap GS. REVIEW?: INTRODUCTION TO 3D BIOPRINTING. 2023;12(5):724–40.
- On R, Dioica M. World Journal of Pharmaceutical Research. 2023;12(5):2295–308.
- Reddy KR, Mutalik S, Reddy S. Once-Daily Sustained-Release Matrix Tablets of Nicorandil?: Formulation and In Vitro Evaluation. 2003;4(4):1–9.
- Yu Y, Yang X, Wu X, Fan Y, Fan Y. Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocarriers?: Novel Strategies for Effective Transdermal Applications. 2021;9(March):1–17.
- Shaping The Future Of Bone Health?: A Review Of. 2024;13(1):320–48.
- Neupane R, Boddu SHS, Renukuntla J, Babu RJ. Alternatives to Biological Skin in Permeation Studies?: Current Trends and Possibilities.
- Asian Journal of Pharmaceutical Research and Development. 2024;12(3):66–74.
- Prausnitz MR, Mitragotri S, Langer R. CURRENT STATUS AND FUTURE POTENTIAL OF TRANSDERMAL. 2004;3(February).
- Bolkar PE, Shelke PA, Sanap GS. Review?: Effect of medicinal herbs on dysmenorrheoa. 2023;12(2):150–9.
- Science In. International Journal Of Review on Fennel ( Foeniculum Vulgare ) is an Universal Medicine. 2023;6(12).
- Sanap GS, Mohanta GP. Solid lipid nanoparticles (SLN) -based hydrogel formulation for topical delivery of miconazole nitrate. Int J Pharma Bio Sci. 2013;4(2):21–33.
- Shamma RN, Basha M. Soluplus ®?: A novel polymeric solubilizer for optimization of Carvedilol solid dispersions?: Formulation design and effect of method of preparation. 2013;237:406–14.
- Benson HAE. Transdermal Drug Delivery?: Penetration Enhancement Techniques. 2018;23–33.
- Nair A, Jacob S, Al-dhubiab B, Attimarad M, Harsha S. Basic considerations in the dermatokinetics of topical formulations. 2013;49.
- John L. Review on Transdermal Drug Delivery System. 2014;2(4):261–72.
- Vargason AM, Anselmo AC, Mitragotri S. technologies. Nat Biomed Eng [Internet]. 2021;5(September). Available from: http://dx.doi.org/10.1038/s41551-021-00698-w
- Aldawood FK, Andar A, Desai S. A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications. 2021;1–34.
- Parivesh S, Sumeet D, Abhishek D. Available online through Design , Evaluation , Parameters and Marketed Products of transdermal patches?: A Review. 2010;3(2):235–40.
- Thorat AN, Kathar NP, Sanap GS, Bodkhe Ms. Review?: Bilayer Tablet Is A New Approach. 2023;12(5):1028–47.
- Iii V, Micro T. BioMEMS and Biomedical Nanotechnology.
- Bathe R, Kapoor R, Bathe RS. Transdermal drug delivery system?: formulation , development and evaluation-An overview * Correspondence Info?: 2015;6(01):1–10.
- Murkute PS, Raut RS, Kathar NP, Sanap GS, Gaikwad RG. Brief Introduction to In-Silico Drug Discovery Process and Virtual Screening Method?; Ubiquitination Regulator in Cancer?: A Review. Int J Res Trends Innov. 2022;7(3):31–40.
- Lingayat VJ, Zarekar NS, Shendge RS. Solid Lipid Nanoparticles?: A Review. 2017;4(2):67–72.


 Sachin Pawar*
Sachin Pawar*
 Nitin Sawle
Nitin Sawle









 10.5281/zenodo.14628391
10.5281/zenodo.14628391