Abstract
Niosomes are vesicles composed of non-ionic surfactants, which are biodegradable, relatively nontoxic, more stable, and inexpensive, serving as an alternative to liposomes. They are also known as vesicular nanocarriers, which are self-assembled by the hydration of a non-ionic surfactant, cholesterol, or other molecules. In novel drug delivery, it has applications in the treatment of cancer, used as a carrier in hemoglobin delivery of peptide drugs through the oral route, in the treatment of leishmaniasis, in ophthalmic delivery, and as a carrier in dermal drug delivery. This review article focuses on the composition, advantages, types of niosomes, preparation methods, characterization, and application of the vesicular system.
Keywords
Niosomes, Composition, Types, Method of preparation, Factors affecting, Application Therapy.
Introduction
Niosomes are non-ionic surfactant-based vesicles. They were originally developed as an alternative controlled drug delivery system to liposomes to overcome the problems associated with sterilization, large-scale production, and stability (Azmin et al., 1985, 1986; El Maghraby & Williams, 2009). The hydration of a film, comprising a mixture of a single or double-alkyl chain and cholesterol, leads to the formation of vesicular dispersion. These dispersions were termed niosomes (Baillie et al., 1985). These vesicles do not form spontaneously. Thermodynamically stable vesicles form only in the presence of proper mixtures of surfactants and a membrane stabilizing agent (cholesterol), at a temperature above the gel/liquid transition of the main lipid-forming vesicles (Azmin et al., 1985, 1986; Sahin, 2007). The first noisome formulations were developed and patented by L’Oreal in 1975 (Sahin, 2007). Niosomes were first utilized in drug delivery for anticancer drugs (Azmin et al., 1985, 1986). The developed niosome formulations could alter the pharmacokinetic profile, organ distribution, and metabolism of methotrexate in mice (Azmin et al., 1985, 1986). Niosomes are versatile in structure, morphology, and size; they can entrap hydrophilic drugs in aqueous compartments or lipophilic drugs by partitioning these molecules into bilayer domains. Furthermore, they can be formulated as unilamellar, oligolamellar, or multilamellar vesicles. Niosomes also possess good physical stability, are cost-effective, and are relatively straightforward for routine and large-scale production (Baillie et al., 1985; Uchegbu & Florence, 1995; Uchegbu & Vyas, 1998).
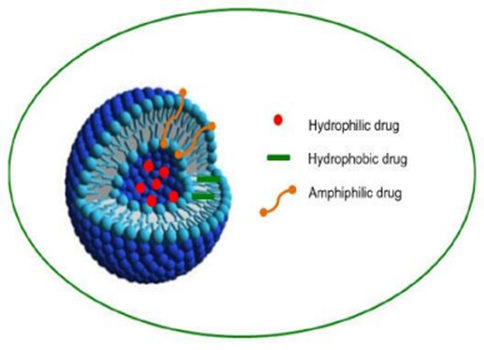
Figure 1Typical structure of niosomes
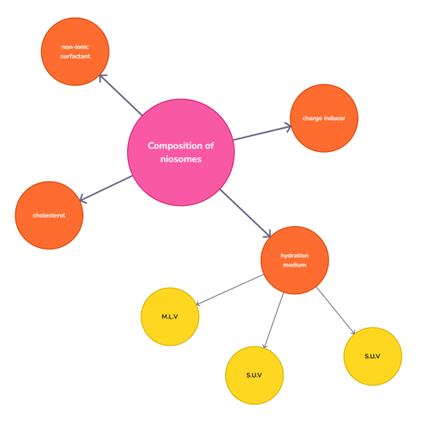
Figure 2.Composition of Niosomes
Advantages of niosomes:
1. Patient compliance is higher than with alternative administration systems.
2. Minimized adverse effects and maximal duration of action.
3. Only a tiny amount of the medication is needed to get the desired outcome.
4. The bilayer surrounding the active component of the preparation protects it from internal and external environmental influences.
5. Serve as a depot formulation to enable regulated medication release.
6. The drug is designed to resist breakdown in the gastrointestinal tract and first-pass metabolism.
7. An emulsion possesses a stable structure Even in the form of an emulsion.
8. Niosomes can be administered parenterally, topically, or orally.
- Please remember the following points:
- Surfactants do not require any special conditions for handling and storage.
- They enable controlled and targeted drug delivery.
- They are stable and osmotically active.
- They enhance dermal penetration and oral bioavailability, improving drugs' therapeutic effect.
- Niosomes, being water-based, offer better patent compliance compared to greasy dosage forms.
Disadvantages Of Niosomes:
Niosomes' aqueous suspensions have a limited shelf life because they tend to fuse, aggregate, leak entrapped drugs, and undergo hydrolysis of encapsulated drugs.
1. Creating multilamellar vesicles using the extrusion and sonication method is time-consuming and requires specialized equipment for processing.
2. It is a time-consuming process and requires specialized equipment for processing.
3. Limited shelf life due to:
a) Fusion
b) Aggregation
c) Leakage of entrapped drugs
d) Hydrolysis of encapsulated drugs.
Here are different methods of preparation:
1. Thin film hydration technique for handshaking method
2. Microfluidization
3. Reverse Phase Evaporation (REV)
4. Ether Injection Method
5. Trans-membrane pH-gradient (inside acidic)
6. The Bubble Method
7. Sonication
8. Multiple extrusion method
9. Niosomes formation from proniosomes
1 Thin film hydration technique for the handshaking method: The non-ionic surfactant and cholesterol are dissolved in a volatile organic solvent (such as diethyl ether, chloroform, or methanol) in a round-bottom flask used in the hand-shaking technique. At room temperature (20°C), the organic solvent is eliminated using a rotary evaporator, leaving behind a thin coating of solid mixture that is deposited on the flask wall. When the drug-containing aqueous phase is heated to between 50 and 60 degrees Celsius, the dry surfactant film is gently stirred. Multilamellar niosomes are created in this way.
- Benefits of thin-film hydration for the handshaking technique :
- Easy to use and reasonably priced
- appropriate for both small and large-scale manufacturing.
Drawbacksof thin-film hydration for the handshaking technique :
- frequently leads to more varied and big vesicle sizes.
- requires a size-reduction step to be done after preparation, such as sonication or extrusion.
2)Micro-fluidization: The science and technology of microfluidics deals with devices that use channels of sizes ranging from one to hundreds of micro-meters to process or control tiny volumes of fluids (microliter to attoliter). This area's goal, mostly driven by technology applications, is to create whole labs inside of chips. Micro-fluidization is a technique in which unilamellar vesicles of defined size distribution are prepared. It is based on the submerged jet principle in which two fluidized streams interact at ultra-high velocities (100 ml/min), in precisely defined micro channels within the interaction chamber. The impingement of a thin liquid sheet along a common front is arranged in such a way that the energy supplied to the system remains within the area where niosomes are formed. Niosomes formed by this method have greater uniformity, smaller size, and better reproducibility
- Benefits of Micro-fluidization:
- High repeatability and scalability
- consistent and tiny vesicle sizes produced
- Drawback of micro-fluidization:
- Expensive equipment is required,
- the process is limited to specific formulas due to shear forces.
3. Reverse Phase Evaporation (REV)
The ratio of surfactant to cholesterol is 1:1 during reverse-phase evaporation. The combination mentioned above dissolves in a mixture of ether and chloroform. The drug dissolves in the water phase. Sonication is applied to both mixtures at 4-6°C. Niosomes are produced by diluting the niosome suspension in PBS at 60°C for ten minutes using a water bath. The resulting product is again mixed with PBS and sonicated at low pressure while maintaining a temperature of 40–45°C, which removes the organic phase. To produce niosomes, the resulting solution is diluted with PBS and boiled in water at 60°C for 10 minutes.
- Benefits of reverse phase evaporation:
- Large uni-lamellar vesicles may be produced.
- High encapsulation efficiency for hydrophilic pharmaceuticals in general.
- Drawbacks of reverse phase evaporation
- The process can be time-consuming.
- When using organic solvents with sensitive medications, there may be some issues.
4. Ether Injection Method:
The niosomes are made using the ether injection process, which involves adding a surfactant solution mixed in volatile organic solvent diethyl ether to warm water kept at 60°C. Using a 14-gauge needle, the surfactant combination in ether is injected into an aqueous solution of the substance. Ether vapourization produces single-layered vesicles (volatile organic solvent)
- Benefits of ether injection method:
- Good control over vesicle size
- produces smaller vesicles than other techniques.
- Drawbacks of the ether injection method:
- the use of volatile organic solvents
- the danger of solvent contamination from leftovers.
5)Trans-membrane pH-gradient (inside acidic)
This process involves mixing or blending the cholesterol and surfactant in a round-bottom flask and then dissolving them in chloroform. The chloroform evaporates at low pressure, leaving behind a thin layer on the flask wall. The film is hydrated by vortex mixing 300 mM (pH 4.0) citric acid. An aqueous solution containing 10 mg/ml of the medication is added to the niosomal suspension mentioned earlier, and it is vortexed. After adding 1M disodium phosphate, the sample's pH is brought to 7.0–7.2. The mixture is then heated for 10 minutes at 60°C. Using this technique, multilamellar vesicles are created.
- Benefits of trans-membrane pH-gradient:
- Excellent drug loading performance.
The capacity to regulate medication release in response to pH variations.
- Drawbacks of trans-membrane pH-gradient:
- Needs certain tools and circumstances.
- Some sensitive medications may be deteriorated by a pH gradient.
6. The Bubble Method
An innovative approach for producing niosomes without the use of organic solvents is the bubble method. This approach use the bubbling unit. The temperature is regulated by three necks on a round-bottom flask that is submerged in a water bath. First neck is used for water-cooled reflux; the second neck is used for thermometers; and third neck is used for nitrogen passage. In a buffer solution (pH-7.4), cholesterol and surfactant are combined at 70 degrees Celsius. After mixing the mixture for 15 seconds with a high shear homogenizer, nitrogen gas is utilized to instantly cause the mixture to bubble at 70°C.
- Benefits of the bubble method:
- Doesn't need the use of organic solvents.
- Suitable for substances that are sensitive to temperature.
- drawback of the bubble method:
- the low encapsulation efficiency.
- often produces a wide range of sizes.
7. Sonication One of the traditional methods for niosome preparation is sonication. This approach involves dissolving the medication in the buffer to create the drug solution. Next, the non-ionic surfactant combination is added to this buffer drug solution at an ideal ratio. Sonicating the combination at a certain frequency, temperature, and duration yields the required niosomes. It's one of the simplest ways to regulate the niosomes' particle size. Niosomes having a limited size distribution can have their diameters reduced using this technique. Probe sonicators are another option, although they require a lot of energy. As a result, there is an abrupt rise in temperature and titanium ejection.
- Benefits of sonication:
- Generates homogeneous, tiny vesicles.
- Easy and efficient way to reduce size.
- Drawbacks of sonication:
- Heat generation may cause sensitive medications to degrade.
- Post-processing is required to eliminate any metal contamination on the probe.
8)Multiple extrusion method
This process involves mixing diacetyl phosphate, cholesterol, and surfactant in chloroform. To create a thin film, this chloroform combination is then evaporated. A polycarbonate drug membrane that is aqueous is used to hydrate thin films. The solution and the resulting suspension are forced through the eight channels in this membrane. This approach also yields the requisite size of the niosomes.
- Benefits of multiple extrusion method:
- Generates vesicles with a limited range of sizes.
- Ideal for both tiny and big quantities.
Drawbacks of multiple extrusion method:
- Takes a lot of time.
- needs certain equipment
9. Niosomes formation from proniosomes
The generation of niosomes occurs with brief agitation at a temperature higher than the surfactant's typical transition phase temperature.
T m where Tm = Mean Phase Transition Temperature and T = Temperature
Preniosomes based on maltodextrin were used to formulate niosomes, as reported by Blazek-Walsh A.I. et al. With this formulation, niosomes may be quickly reconstituted with the least amount of leftover carrier. Drying the mixture of maltodextrin and surfactant produced a free-flowing powder that could be rehydrated by adding warm water.
- BenefitsNiosomes formation from proteasomes:
- Easy storage and excellent stability.
- A straightforward hydration procedure forms niosomes
- drawbacks ofNiosomes formation from proteasomes :
- Needs extra steps to complete the conversion.
- restricted to specific combinations.
Distance From Unentrapped Drug:
Dialysis: Phosphate buffer, glucose solution, or regular saline are used to dialyze the aqueous niosomal suspension in dialysis tubing.
2) The process of gel filtration
Phosphate buffered saline or regular saline is used for elution after gel filtration employing a Sephadex-G-50 column to extract the medicine that was not encapsulated in the niosomal solution.
3)Centrifugation
The niosomal suspension is centrifuged and the liquid supernatant is separated during the centrifugation process. Washing the particle and the resuspended solution yields a niosomal suspension devoid of unentrapped medication.
Liposomes and niosomes are nearly identical in their molecular makeup. If niosomes are composed of non-ionic surfactants, they are stable in nature, but the phospholipids employed in liposomes are not. Liposomes are made from double-chain phospholipids, while niosomes are made from unaltered single-chain non-ionic surfactants. Liposomes are less than 10-300 nm, while niosomes are between 10 and 100 nm in size. When compared to liposomes, niosomes are more cost-effective.
The following variables affect niosomal formulation:
- surfactant nature;
- surfactant structure;
- membrane composition;
- nature of encapsulated medication;
- temperature of hydration Charge;
- Cholesterol content;
- Adaptability to osmotic stress;
1. Surfactant Nature
A single alkyl hydrophobic tail on an ether-type surfactant makes it more hazardous than a dialkyl ether chain. Due to the increased likelihood of esterases breaking down ester linkage into fatty acids and triglycerides, ester-based surfactants are chemically less stable than ether-type surfactants. Compared to ester-based surfactants, ether-type surfactants are more hazardous. The mean size of niosomes increases when the HLB value of surfactants increases because the hydrophobicity of the surfactant increases and surface free energy decreases. Niosome bilayers can be in a liquid or gel condition at any one time. It is dependent on the surfactant type, temperature, and cholesterol. In the gel state, alkyl chains are well-organized, while in the liquid state, they are disorganized.
For example, span 60 with a higher TC shows superior entrapment. Surfactants with an HLB value of 14–17 are not appropriate for use in niosomal preparations. The entrapment efficiency is reduced when the HLB value of surfactants drops from 8.6 to 1.7; the maximum entrapment efficiency is achieved when the HLB value is 8.6. The alkyl chain lengths of C12–C18 surfactants are appropriate for niosome production.
2.Surfactant Structure
The vesicle's shape is influenced by the critical packing parameter of the surfactant structure. Critical packing parameters (CPP) provide a prediction for the vesicle's shape. 37. If the CPP is less than ½, then round micelles ½ CPP < 1> dual-layer micelles
Inverted Micelles: CPP > 1 To find the critical packing parameter (CPP), use the following formula. CPP is equal to v/lc*ao. Where Critical packing parameters, or CPP Critical hydrophobic group length (lc) equals V, the hydrophobic group volume. Area of the hydrophilic head group, or ao
3: Membrane Composition: To stabilize the niosomes, several chemicals are added to the medication and surfactant. The addition of cholesterol increases the membrane's stiffness and decreases medication leakage. The polyhedral niosomes generated from C16G2 do not aggregate when a small quantity of solution C24 (cholesteryl poly-24-oxy ethylene ether) is added. This is because steric hindrance is prevented from developing.
4. Nature Of Encapsulated Medication: The physicochemical characteristics of the medication contained have a significant impact on the charge and stiffness of the niosomal bilayer. Entrapment of medication happens by interacting with the surfactant head groups leading to the increased charge and producing mutual repulsion of the surfactant bilayer and therefore increasing vesicle size 38. The level of entrapment is influenced by the drug's HLB.
5. Temperature Of Hydration Charge: The noisome's size and shape are influenced by the hydration temperature. The gel liquid phase transition temperature should be higher than the hydration temperature. Temperature variations have an impact on surfactant vesicle assembly and vesicle shape change. The alteration is also explained by the hydration medium's amount and duration. Fragile niosomes and drug leakage issues may occur from choosing the hydration temperature, duration, and medium volume incorrectly.
viii. Cholesterol content: The entrapment effectiveness and hydro-dynamic diameter of niosomes are enhanced by the incorporation of cholesterol. Cholesterol functions in two ways 39: it either increases or decreases the chain order of bilayers in the liquid state.
An increase in cholesterol content results in stiffer bilayers and a slower rate of encapsulated substance release.
viii. Adaptability to osmotic stress
Vesicle diameter decreases upon the addition of hypertonic solution. Because of the mechanical vesicle structural loosening brought on by osmotic stress, inhibition of fluid eluting from vesicles in hypotonic solution first causes a sluggish release, which is then followed by a rapid release.
CONCLUSION: The creation of innovative medication delivery systems has undergone a significant revolution in the last several decades. Niosome technology as a viable medication delivery mechanism is still in its early stages of development. Niosomes have demonstrated a strong effect in focusing on specific tissues and organs. Niosomes can be used as more effective transdermal, nasal, ocular, and vaccine delivery methods, as well as superior diagnostic and tumor-targeting agents. Niosomal compositions that are sold commercially need a great deal of research.
REFERENCES
- Jeganath S, Nitish B, Khalifa FKA. Niosomes as target drug delivery system: A Review. Int. J. Res. Pharm. Sci, 2020; 11(3): 3198-3203.
- Baillie AJ, Florence AT, Hume LR, Muirhead GT, Rogerson A. The preparation and properties of niosomes-non-ionic surfactant vesicles. Journal of Pharmacy and Pharmacology, 1985; 37(12): 863–868.
- Madhav NVS, Saini A. Niosomes: a novel drug delivery system. International Journal of Research in Pharmacy and Chemistry, 2011; 1(3): 498– 511.
- Allen TM. Liposomal drug formulations: Rationale for development and what we can expect for the future. Drugs, 1998; 56(5): 747–756.
- Handjani-Vila RM, Ribier A, Rondot B and Vanlerberghie G. Dispersions of lamellar phases of nonionic lipids in cosmetic products. Int. J. Cos. Sci, 1979; 1:303-314.
- Kemps J and Crommelin DA. Hydrolyse van fosfolipiden in watering milieu. Pharm Weekbl, 1998; 123: 355-363.
- Rai AK, Alam G, Singh AP and Verma NK. Niosomes: An approach to current drug delivery-a Review. International Journal of Advances in Pharmaceutics, 2017; 6(2): 41-48.
- Kaur D, Kumar S. Niosomes: present scenario and future aspects. Journal of Drug Delivery & Therapeutics, 2018; 8(5): 35-43.
- Syeda SF, Shireen B, Talath F, Madiha J. Niosomes as nanoparticular drug carriers. Ijppr.Human, 2017; 9(3): 117-133.
- Keshavshetti GG, Shirsand SB. Recent advances in niosomal drug delivery - a review. Research Journal of Life Sciences, Bioinformatics, Pharmaceutical and Chemical Sciences, 2019; 5(3): 514-531.
- Sanklecha VM, Pande VV, Pawar SS, Pagar OB and Jadhav AC. Review on Niosomes. Austin Pharmacol Pharm., 2018; 3(2): 1-7.
- Gurjar P, Naik N, Chouksey S. Niosome: a promising pharmaceutical drug delivery. Int. J. Pharm. Anal., 2014; 2(5): 425-431.
- Kalra N, Jeyabalan G. Niosomes: A versatile drug delivery system. Research Journal of Life Sciences, Bioinformatics, Pharmaceutical and Chemical Sciences, 2016; 2(4): 44-54.
- Bhat MI, Ganesh NS, Majeed T and Chandy V. Niosomes a controlled and novel drug delivery system: A brief review. World journal of Pharmaceutical sciences, 2019; 3(8): 481-497.
- Usman MRM, Ghuge PR and Jain BV. Niosomes: a novel trend of drug delivery. European Journal of Biomedical and Pharmaceutical Sciences, 2017; 4(7): 436-442.
- Sharma D, Ali AAE, Aate JR. Niosomes as novel drug delivery system: review article. PharmaTutor, 2018; 6(3): 58-65.
- Sudheer P, Kaushik K. Review on niosomes – a novel approach for drug targeting. Journal of Pharmaceutical Research, 2015; 14(1): 20-25.
- Hadjizadeh A, Moghassemi S. Nano-niosomes as nanoscale drug delivery systems: an illustrated review. Journal of Controlled Release, 2014; 185: 22–36.
- Zhang S, Morris ME. Efflux transporters in drug excretion. In: Wang B, Siahaan T, Soltero R, editors. Drug Delivery: Principles and Applications, New Jersey, John Wiley &Sons publishers, 2005 .p. 381–398.
- Bhatt P, Lalani R, Vhora I, Patil S, Amrutiya J, Misra A, et al. Liposomes encapsulating native and cyclodextrin enclosed paclitaxel: Enhanced loading efficiency and its pharmacokinetic evaluation. International journal of pharmaceutics. 2018;536(1):95-107.
- Shtil AA, Grinchuk TM, Tee L, Mechenter EB, and Ignatova TN. Overexpression of P-glycoprotein is associated with a decreased mitochondrial transmembrane potential in doxorubixin selected K562 human leukemia cells. International Journal of Oncology, 2000; 17: 387–392.
- Kumar SD, Agarwal S, Sheikh MM, Raveendran S, Rochani AK, Maekawa T. Formulation, characterization and evaluation of morusin loaded niosomes for potentiation of anticancer therapy. Royal Society of Chemistry Advances, 2018; 8: 32621-32636.
- Amoabediny G, Naderinezhad S, Haghiralsadat F. Co-delivery of hydrophilic and hydrophobic anticancer drugs using biocompatible pH-sensitive lipid-based nano-carriers for multidrug-resistant cancers. Royal Society of Chemistry Advances, 2017; 7: 30008–300019.
- Ueda K, Okamura N, Hirai M, Tanigawara Y, Saeki T,Kioka N, Komano T and Hori R. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. The Journal of Biological Chemistry, 1992; 267(34): 24248-24252.
- Greenblatt DJ, Perloff MD, Moltke LLV, Marchand JE. Ritonavir induces P-glycoprotein expression, multidrug resistance associated protein (MRP1) expression, and drug transporter mediated activity in a human intestinal cell line. Journal of Pharmaceutical Sciences, 2001; 90(11): 1829-1837.
- Kumar GP, Rajeshwarrao P. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta Pharmaceutica Sinica B, 2011; 1(4):208-219.
- Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. International Journal of Pharmaceutics, 1998; 172: 33-70.
- Uchegbu IF, Florence AT. Non-ionic surfactant vesicles (niosomes): physical and pharmaceutical chemistry. Advances in Colloid Interface Science, 1995; 58:1-55.
- Florence AT, Arunothayanun P, Bernard MS, Craig DQM, Uchegbu IF.The effect of processing variables on the physical characteristics of non-ionic surfactant vesicles (niosomes) formed from a hexadecyl diglycerol ether. International Journal of Pharmaceutics, 2000; 201:7–14.
- Tripathi P, Bhardwaj P, Gupta R, Pandey S. Niosomes: A review on niosomal research in the last decade. Journal of Drug Delivery Science and Technology, 2020; 56:1-17.
- Nasir A, Harikumar SL and Kaur A. Niosomes: An excellent tool for drug delivery. International Journal of Research in Pharmacy and Chemistrys, 2012; 2(2): 479-487.
- De S, Girigoswami A, Das S. Fluorescence and dynamic light scattering studies of niosomes membrane mimetic systems. Spectrochimica Acta Part A, 2006; 64: 859-866.
- Rogerson A, Cummings J, Florence AT. Adriamycin-loaded niosomes-drug entrapment, stability and release. Journal of Microencapsulation, 1987; 4(4): 321– 328.
- Cummings J, Rogerson A, Florence AT, Willmott N. The distribution of doxorubicin in mice following administration in niosomes. J. Pharm. Pharmacol., 1988; 40: 337-342.
- Louis D, Mahmoud K, Mohamed M and Ibrahim A. An overview on niosomes: a drug nanocarrier. Drug Designing and Intellectual Properties International Journal, 2018; 1(5): 143-151.
- Uchegbu IF, Lalatsa A, Wong D (2013). Polymeric nanoparticles. In: Uchegbu IF, Schatzlein AG, Cheng WP and Lalatsa A editors. Fundamentals of pharmaceutical nanoscience, Springer, New York, USA Publishers, .p. 211-234.
- Khan R, Irchhaiya R. Niosomes: a potential tool for novel drug delivery. Journal of Pharmaceutical Investigation, 2016; 46(3): 195-204.
- Dwivedi C, Kumar B, Tiwari SP, Satapathy T, Yadav R, Sahu G, Roy A. Niosomes: an excellent tool for drug delivery. Int.J.of Res. in Pharmacology and Pharmacotherapeutics, 2014; 3(3): 192-204.
- Cetinel S, Zarrabi A, Durak S, Rad ME, Yetisgin AA, Sutova HE and Kutlu O. Niosomal drug delivery systems for ocular disease—recent advances and future prospects. Nanomaterials, 2020; 10(6): 1-29.
- More VV, Gilhotra RM, Manoj M. Nitalikar, Prajakta K. Khule. Niosomal drug delivery - a comprehensive review. Asian Journal of Pharmaceutics, 2018; 12(4): 1159-1164.
- Baillie AJ, Coombs GH, Dolan TF, Laurie J. Non-ionic surfactant vesicles, niosomes, as a delivery system for the anti-leishmanial drug, sodium stibogluconate. J. Pharm Pharmacol., 1986; 38: 502-505.
- Chavda VP. Niosome: a vesicular weapon for targeted and controlled drug delivery. Indian Journal of Novel Drug Delivery, 2016; 8(3): 133-156.
- Frank LS and Huang L. Large scale production of DC-Chol cationic liposomes by microfluidization. International Journal of Pharmaceutics, 1996; 144(2): 131–139.
- Gowda DV, Jain SC, Gupta NV, Kulkarni PK. A brief review on niosomes. Journal of Pharmacy Research, 2017; 11(5): 450-458.
- .Raja NR, Pillai GK, Udupa N, Chandrashekar G. Antiinflammatory activity of niosome encapsulated diclofenac sodium in arthritic rats. Indian Journal of Pharmacology, 1994; 26(1): 46-48.
- Sunilkumar MR, AdlinJinoNesalin J, Man TT. Niosome as a novel drug delivery system-review. Internationa Research Journal of Pharmaceutical and Applied Siences, 2015; 5(3): 1-7.
- Lohumi A, Rawat S, Sarkar Si, Sipai AB, Yadav MV. A novel drug delivery system: niosomes review. Journal of Drug Delivery & Therapeutics, 2012; 2(5): 129-135.
- Mayer LD, Bally MB, Hope MJ, Cullis PR. Uptake of antineoplastic agents into large unilamellar vesicles in response to a membrane potential. Biochem Biophys Acta, 1985; 816(2): 294-302.
- Kuotsu K, Karim MK, Mandal AS, Biswas N, Guha A, Chatterjee S, Behera M. Niosome: a future of targeted drug delivery systems. J Adv Pharm Tech Res., 2010; 4(1): 374-380.
- Navya MN, Parthiban S, Neasalin JAJ, Vikneswari A. Niosomes as novel vesicular drug delivery system- a review. Asian Journal of Research in Biological and Pharmaceutical Sciences, 2014; 2(2): 62-68.
- Chauhan S, Luorence MJ. The preparation of polyoxyethylene containing non-ionic surfactant vesicles. J Pharm Pharmacol., 1989; 41(6).
- Yuan WE, Xuemei Ge, Minyan Wei, Suna H. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics, 2019; 11(2): 1-16.
- Yadav JD, Kulkarni PR, Vaidya KA, Shelke GT. Niosomes: a review. Journal of Pharmacy Research, 2011; 4(3): 632-636.
- Khanam N, Alam MI, Sachan AK, Gangwar SS, Sharma R. Recent trends in drug delivery by niosomes: a review. Asian Journal of Pharmaceutical Research and Development, 2013; 1(3): 115-122.
- Sudhamani T, Priyadarisini N, Radhakrishnan M. Proniosomes -a promising drug carriers. International Journal of PharmTech Research, 2010; 2(2): 1446-1454.
- Almira I, Blazek-Welsh, Rhodes DG. Maltodextrin-Based Proniosomes. AAPS Pharm SciTec., 2001; 3(1): 1-8.
- Blazek-Walsh AI and Rhodes DG. SEM imaging predicts quality of niosomes from maltodextrin-based proniosomes. Pharmceutical Research, 2001; 18: 656-661.
- Gandhi M, Paralkar S, Sonule M, Dabhade D and Pagar S. Niosomes: novel drug delivery system. International Journal of Pure and Applied Bioscience, 2014; 2(2): 267-274.
- Akhilesh D, Kamath JV. Review on span-60 based non-ionic surfactant vesicles as novel drug delivery. International journal of research in pharmaceutical and biomedical sciences, 2012; 3(1): 6-12.
- Nagalakshmi, Aruljothy M, Shanmuganathan S. An overview on niosome as carrier in dermal drug delivery. Journal of Pharmaceutical science and Research, 2015; 7(11): 923-927.
- Sharma P, Jain AP, Pandey P, Gupta R, Roshan S. Niosome a novel approach for drug delivery system: an overview. Asian Journal of Pharmaceutical science and Research, 2013; 3(5):18-30.
- Cummings J, Staurt JF, Calman KC. Determination of adriamycin, adriamycinol and their 7- deoxyaglycones inhuman serum by high performance liquid chromatography. Journal of Chromatography, 1984; 311: 125-33.
- Vadlamudi CH, Sevukarajan M. Niosomal drug delivery system- a review. Indo American Journal of Pharmceutical Research, 2012; 2(9).
- Nasseri B. Effect of cholesterol and temperature on the elastic properties of niosomal membranes. International Journal of Pharmaceutics, 2005; 300: 95-101.
- Mahale NB, Thakkar PD, Mali RG, Walunj DR, Chaudhari SR. Niosomes: novel sustained release nonionic stable vesicular systems - an overview. Advances in Colloid and Interface Science, 2012; 183-184: 46–54.
- Sarker A, Shimu IJ, Alam SAA. Niosome: as dermal drug delivery tool. IOSR Journal of Pharmacy and Biological Science, 2015; 10(2): 73-79.
- Sakthivel M, Kannan K, Manavalan, Senthamarai. Non-ionic surfactant vesicles review. Research Journal of Pharmaceutical, Biological and Chemical, 2012; 3(1):604-614.
- Jindal K. Niosomes as a potential carrier system: a review. International Journal of Pharmaceutical, Chemical and Biological Sciences, 2015; 5(4): 947-959.
- Carafa M, Marianecci C, Marzio LD, Rinaldi F, Celia C, Donatella Paolino D, Alhaique F, Esposito S. Niosomes from 80s to present: the state of the art. Advances in Colloid and Interface Science, 2014; 205: 187-206.
- Singh SK, Rajera R, Nagpal K, and Mishra D. Niosomes: a Controlled and novel drug delivery system. Biological and Pharmaceutical Bulletin, 2011; 34(7): 945-953.
- Gandhi A, Sen SO, Paul A. Current trends in niosome as vesicular drug delivery system. Asian Journal of Pharmacy and Life Science, 2012; 2 (2): 339-353.
- Stahl F, Seleci DA, Seleci M, Walter JG, and Scheper T. Niosomes as nanoparticular drug carriers: fundamentals and recent applications. Hindawi Publishing Corporation Journal of Nanomaterials, 2016; 2016: 1-13.
- Mujoriya R, Bodla RB, Dhamande K, Singh D and Patel L. Niosomal drug delivery system: the magic bullet. Journal of Applied Pharmaceutical Science, 2011; 1(9): 20-23.
- Kalra N, Jeyabalan G, Singh G, Choudhary S. Non-ionic surfactant vesicles and their therapeutic potentials. Journal of Innovations in Pharmaceuticals and Biological Sciences, 2016; 3 (2): 193-201.
- Azeem A, Anwer MK and Talegaonkar S. Niosomes in sustained and targeted drug delivery: some recent advances. Journal of Drug Targeting, 2009; 17(9): 671-689.
- Koh RY, Yeo PL, Chye SM, and Ling APK. Niosome: a mini-review on its structure, properties, methods of preparation, and medical applications. Journal of Chemical and Pharmaceutical Research, 2016; 8(10): 231-239.
- Pawar P, Sankhyan A. Recent trends in niosome as vesicular drug delivery system. Journal of Applied Pharmaceutical Science, 2012; 2(6): 20-32.
- Lalani R, Misra A, Amrutiya J, Patel H, Bhatt P, Patel V. Challenges in Dermal Delivery of Therapeutic Antimicrobial Protein and Peptides. Current Drug Metabolism. 2017; 18(5):426-36.
- Abdelkader H, Alani AWG and Alany RG. Recent advances in non-ionic surfactant vesicles (niosomes): self-assembly, fabrication, characterization, drug delivery applications, and limitations. Drug Delivery, 2013; 21(2): 87-100


 Aman Londhe*
Aman Londhe*
 Rohan Jadhav
Rohan Jadhav


 10.5281/zenodo.14739890
10.5281/zenodo.14739890