Abstract
Diabetes Mellitus (DM) is a type of metabolic disease caused by high blood sugar levels. Diabetes Mellitus is usually caused by hyperglycemia caused by poor insulin or secretory function, or both. In general, type 2 diabetes mostly affects the elderly and is caused by inadequate production or resistance of insulin in the body. Diabetes type 1 is a chronic illness in which the pancreas generates little or no insulin on its own. It was formerly referred to as juvenile diabetes or insulin-dependent diabetes. Dapagliflozin (DAPA) belongs to the group of drugs known as sodium-glucose co-transporter 2 (SGLT2) inhibitors. Through increasing the amount of glucose excreted in urine by the kidneys, it decreases blood sugar. Dapagliflozin causes glycosuria, which is the result of blocking glucose reabsorption in the proximal tubule of the nephron, hence enhancing glycemic management. Dapagliflozin has been studied as a stand-alone medication or in combination with other oral hypoglycemic medications, such as insulin. In this we reviewed various analytical methods like as UV spectroscopy, High Performance Liquid Chromatography, all these methods for determination of Dapagliflozin as single or by using other additional medications has been documented. An attempt has been made to cover all of the most recent analytical techniques utilized to analyse dapagliflozin in this review.
Keywords
Diabetes mellitus, Dapagliflozin, SGLT2, Analytical methods
Introduction
Diabetic mellitus, sometimes known as diabetes, is a collection of metabolic disorders characterized by elevated blood sugar levels [1]. This condition is caused by insufficient insulin production by the body or by impaired insulin uptake by the cells [2]. The most prevalent endocrine condition, affects about 100 million people globally (6% of the population). It is brought on by the pancreas' inability or lack of ability to produce enough insulin, which causes variations in blood glucose levels [3]. Numerous body systems, including blood vessels, the eyes, kidneys, hearts, and nerves, have been proven to be harmed by it [4].
Insulin-dependent diabetes (IDDM, type I) and non-insulin-dependent diabetes (NIDDM, type II) are two types of diabetes [5]. Type I diabetes is an autoimmune disease caused by a local response that occurs by selective destruction of insulin-secreting cells in and around the pancreas, but type II Diabetes is characterized by peripheral insulin resistance and impaired insulin secretion [6]. Many complications such as peripheral vascular disease, stroke, neuropathy, kidney failure, retinopathy, blindness and amputation are increased in people with diabetes [7].
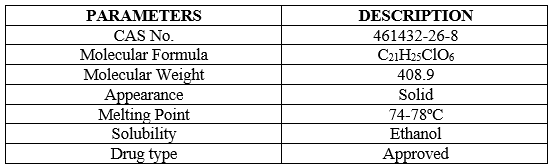
There are two main subtypes of endocrine cells in the islets of Langerhans in the pancreas: insulin-producing beta cells and glucagon-secreting alpha cells [8]. Beta and alpha cells constantly change their hormone secretion in response to the sugar environment. If there is no balance between insulin and glucagon, blood sugar will be inappropriate [9]. In Diabetes Mellitus, insulin is not lost or does not work well (insulin resistance), causing blood sugar to rise [10]. Approximately 366 million people had diabetes in 2011; in 2030, this number will increase to 552 million. The number of people with type 2 diabetes is increasing in all countries; 80% of people with diabetes live in low- and middle-income countries. Diabetes killed 4.6 million people in 2011 [11]. According to projections, the number of people with diabetes will increase in the next two years; Type 2 diabetes is most common in developing countries, and most patients are between the ages of 45 and 64 [12,13]. Dapagliflozin is an anti-diabetic medication that affects the kidneys' ability to reabsorb glucose through the sodium-glucose co-transporter [14]. Globally, dapagliflozin, also known as Farxiga, is a sodium-glucose cotransporter-2 inhibitor that is extremely effective, reversible, and selective, and is prescribed to treat type2diabetes [15]. Dapagliflozin as monotherapy and combination therapy with other antihyperglycemic drugs achieved good glycaemic control and lowered bodyweight and blood pressure (BP) across a broad range of patients in multiple well-designed clinical studies and their extensions [16]. Empagliflozin has a positive impact on other non-glycemic outcomes in addition to decreasing glucose, such as slight decreases in bodyweight. The best other SGLT2 inhibitor that has established benefits for the kidneys and cardiovascular system [17]. Its critical and important physic-chemical properties are showed in Table 1.
Mechanism Of Action: Dapagliflozin inhibits isoform 2 of the sodium glucose transporter (SGLT2), which is responsible for at least 90% of glucose reabsorption in the kidneys. Blocking this transport causes sugar to be released into the urine [18, 19]. In patients with poorly controlled type 2 diabetes and renal dysfunction, a combined treatment regimen containing dapagliflozin and metformin 10 mg daily reduced HbA1c by 0.54–0.84% compared to metformin monotherapy [20].
Their protection against heart failure is mainly due to the hemodynamic effects of SGLT2 inhibitors, osmotic diuretics and natriuretic drugs, which effectively reduce vascular volume [21]. This will therefore lead to a reduction in preload and afterload, thus reducing heart rate and improving left ventricular function [22].
Drug Disposition: Dapagliflozin is well absorbed after oral administration. The maximum plasma concentration of dapagliflozin occurs within two hours after administration [23]. Bioavailability is 78% at a dose of 10 mg once daily. It can be taken with meals or on an empty stomach. It is 91% protein bound and liver or kidney diseases do not affect it [24].
Bioactivity: Greater diuresis and dose-related glycosuria are associated with dapagliflozin. There is a transient increase in urinary sodium excretion but this does not appear to affect blood sodium. Uric acid excretion increases temporarily, but the level of uric acid in the blood continues to decrease [25, 26].
Indications: For adults with type 2 diabetes, Farxiga is recommended as an adjunct to diet and exercise to improve blood sugar levels [27].
Dapagliflozin, a medication primarily used to treat type 2 diabetes by lowering blood glucose levels, has several additional benefits beyond its glucose-lowering effects:
- Cardiovascular Benefits: Dapagliflozin has been shown to reduce the risk of hospitalization for heart failure and cardiovascular death in patients with heart failure and reduced ejection fraction, regardless of diabetes status [28].
- Renal Protection: It helps slow the progression of chronic kidney disease (CKD) in patients with and without diabetes, reducing the risk of worsening kidney function, end-stage kidney disease, and cardiovascular death [29].
- Weight Loss: By promoting the excretion of glucose in the urine, dapagliflozin can contribute to modest weight loss, which is beneficial for many patients with type 2 diabetes who are often overweight or obese [30].
- Blood Pressure Reduction: It can lead to a modest reduction in blood pressure due to its diuretic effect, which is beneficial for patients with hypertension [31].
- Reduction in Albuminuria: Dapagliflozin helps reduce albuminuria (protein in the urine), which is an important marker of kidney damage and cardiovascular risk [32].
These additional benefits make dapagliflozin a valuable therapeutic option for managing not only blood glucose levels but also improving overall cardiovascular and renal health in patients with type 2 diabetes.
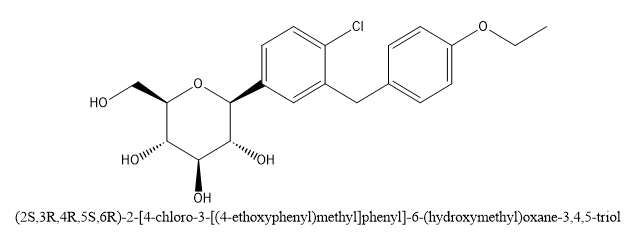
Fig 1 Structure of Dapagliflozin
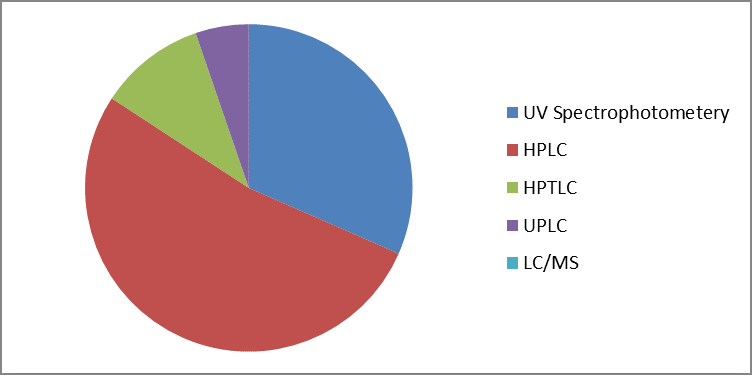
Fig 2 Overview of analytical methods for estimation of Dapagliflozin in biological and pharmaceutical samples.
ANALYTICAL METHODS:
UV VISIBLE SPECTRIPHOTOMETERIC METHOD:
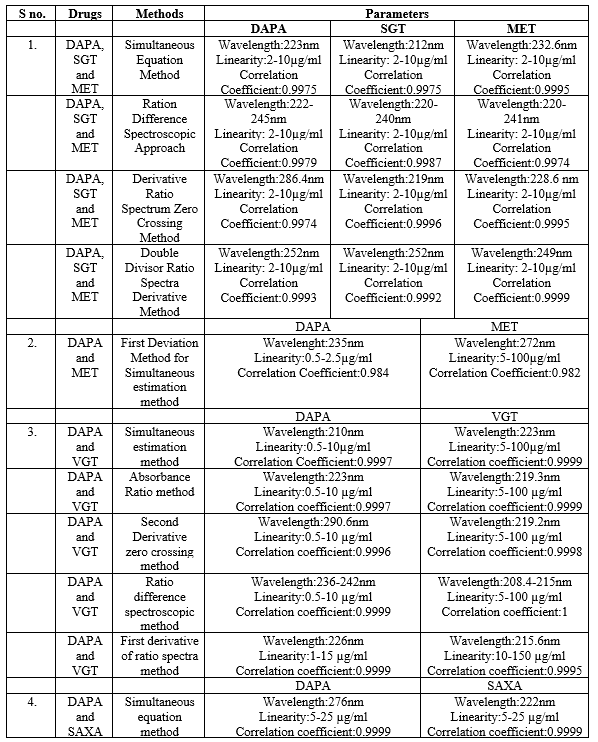
When analyzing drug compounds, UV spectroscopy is a basic method that is both easy to use and efficient. It assists with the identification and measurement of the drug material by providing a concise overview of the solubility, lambda max of the entity, and UV absorbance pattern of the substance. This section reviews the limited number of reported techniques for DAPA analysis. For the simultaneous evaluation of the ternary combination, the proposed study offers four spectrophotometric techniques that are rapid, simple, accurate, repeatable, and environmentally benign. The first method relies on the idea of simultaneously solving pre-existing equations by measuring absorbance for DAPA, SAXA, and MET respectively, at 223, 212, and 232.6 nm. The second technique, ratio difference spectroscopy, measures the amplitude variation in the ratio spectra at two different wavelengths. On the other hand, the third strategy, which uses the derivative ratio spectrum zero-crossing approach, depends on using the derivative ratio signals at zero-crossing sites. The fourth strategy is the double divisor-ratio spectrum derivative strategy, which quantifies the concentrations of all three medications when combined after obtaining the ratio spectrum's first derivative. Using the simultaneous equation approach, all three medications showed strong linear correlation in the concentration series of 2–10 µg/mL and an extraordinary correlation coefficient value for all other methods, 0.5–10 µg/mL [33]. In the second paper, A new, simple, sensitive, fast, accurate, cost-effective and reliable first derivative spectroscopy method was developed for the combination of dapagliflozin (DAPA) and metformin hydrochloride (MET). The method includes the solution of the first-order differentiation method based on absorbance measurements at two wavelengths (235 nm and 272 nm) using a UV-visible spectrophotometer equipped with a conformal quartz cell with a diameter of 1 cm and deuterated methanol [34].
In the third paper, the study that is being proposed offers five spectrophotometric approaches that are simple, quick, easy, accurate, and repeatable for the simultaneous evaluation of the combined tablet. These approaches are the simultaneous equation, absorbance ratio, second derivative zero crossing, ratio difference, and first derivative of ratio spectra. For the first, second, third, and fourth approaches, as well as for the fifth method, there was a strong linear association between the concentration series for DAPA and values of between 1 and 15 µg/ml. Nonetheless, VILG demonstrated remarkable linear correlation in the range of 5–100 µg/ml for the initial, subsequent, and fourth techniques; 10–150 µg/ml for the fifth approach [35].
In the fourth paper, A simple, precise, and accurate UV-spectrophotometric simultaneous equation approach for estimating Dapagliflozin (DAPA) and Saxagliptin (SAXA) was developed and verified in accordance with ICH recommendations. With this method, absorbance at two wavelengths (?max of SAXA and DAPA) in phosphate buffer pH 6.8 (222 nm and 276 nm) is measured and simultaneous equations are solved. In the 5–25µg/ml concentration range, both medications abide by Beer's law. % The range of recovery for both medications was 98.44–99.05%, which indicates exceptional accuracy. With a relative standard deviation for both medications of less than 2%, the procedures were accurate. All methods mention above shown in Table 2 [36].
HPLC METHOD:
One sensitive method for quantifying chemicals, medications, and biological products is high-performance liquid chromatography. The partition and adsorption processes in HPLC are mostly in charge of achieving separation. Medications and other substances that have a greater affinity for the mobile phase will elute faster than those that have a higher affinity for the stationary phase. Depending on the type of column, there are two types of separation: reverse phase and normal phase. In the normal phase, the mobile phase is nonpolar and the column is polar; in the reverse phase, the mobile phase is polar and the column is nonpolar. Nowadays, the HPLC's main advantages are its excellent and sensitive reproducibility, minimal solvent consumption, and large separation capacity.
Pharmaceutical Samples: HPLC-based analytical techniques for determining dapagliflozin levels in pharmaceutical dose forms.
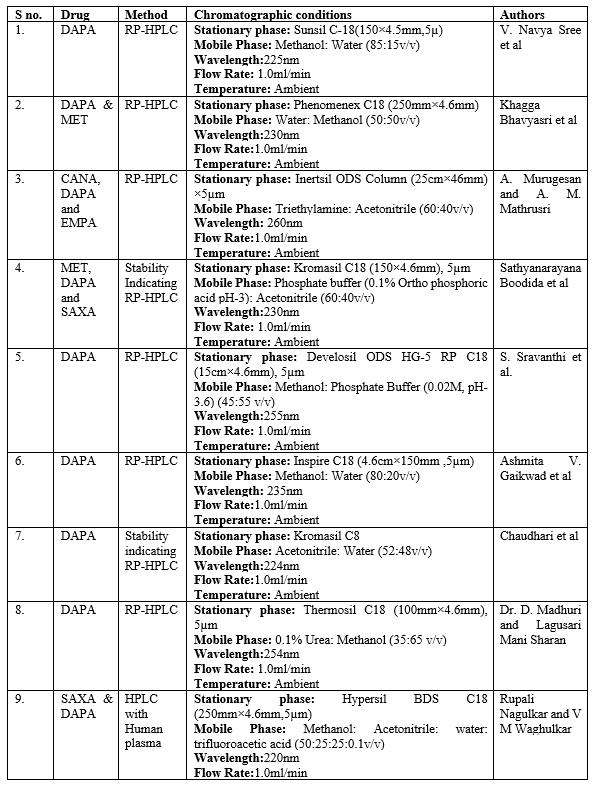
V. Navya sree et al. devised a speedy, precise, and accurate RP-HPLC method to estimate dapagliflozin in bulk and formulation in accordance with ICH requirements. Using a mixture of methanol and water at various ratios, the chromatography was performed on a Sunsil C18 (150 x 4.5 mm, 5µ) column HPLC (Waters). The mobile phase was eventually optimised to be 85:15 v/v with a flow rate of 1.0 ml/min at 225 nm [37]. Khagga Bhavyasri et al. devised an easy-to-use, reliable, and cost-effective RP-HPLC technique for estimating Dapagliflozin and Metformin HCl in bulk and tablet dose form. As chromatographic conditions, stationary phase Phenomenex C18 250mm x 4.6 mm were used. The fluid phase was a 50:50 mixture of water and methanol. The diluent was water, the flow rate was kept at 1.0 ml/min, and the wave length of detection was kept at 230 nm [38]. Reverse phase HPLC was created by A. Murugesan and A. M. Mathrusri to investigate the simultaneous estimation of gliflozin derivatives such as canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. This approach is dependable, straightforward, and stable. The chromatographic separation was achieved using an isocratic flow Inertsil ODS column (25 cm x 46 mm) with a 5 µm internal diameter. 1 ml/min of ACN pH (50:50) is the flow rate of the mobile phase TEA. At 260 nm, a UV detector was used to find the analytes. Gliflozin derivatives were subjected to forced degradation investigations in accordance with ICH criteria; the resulting degradations were described by their peak area at their retention time in accordance with the suggested methodology [39].
In order to estimate the combination of metformin, dapagliflozin, and saxagliptin in bulk and tablet dose forms, Sathyanarayana Boodida et al. devised a straightforward, accurate, and quick stability-indicating reversed-phase-HPLC approach. This method has been verified. A Kromasil C18 column (150 4.6 mm, 5 ?m) with a column oven temperature of 30 C and a mobile phase made up of 40?etonitrile and 60% phosphate buffer (pH = 3) is used in the suggested procedure. The injection volume was 10 ?L, and the flow rate was fixed at 1.0 mL/min. A PDA detector was used for the 230 nm detection, and the entire run time was 4 minutes [40]. A new, basic, quick, accurate, repeatable, and exact HPLC method for estimating dapagliflozin in bulk and commercial formulations was created by S. Sravanthi et al. Dapagliflozin was successfully separated using an isocratic mode of separation on a Develosil ODS HG-5 RP C18, 5µm, 15cmx4.6mm i.d. column. Methanol: Phosphate buffer (0.02M, pH-3.6) was used in a 45:55% v/v ratio at a flow rate of 1mL/min, and the detection was done at 255 nm [41].
A straightforward, accurate HPLC method for the simultaneous measurement Of DAPA in pharmaceutical dosage forms was developed by Ashmita V. Gaikwad et al. On an Inspire (4.6 x 150mm, 5 ?m) 5 micro column with isocratic flow, the separation was accomplished. The mobile phase consisted of water and methanol in a ratio of 80:20 and a flow rate of 1mL/min. UV detection at 235 nm. According to the rule, the retention time of dapagliflozin is 4.422 minutes [42].
A stability-indicating reverse phase HPLC method was created by Chaudhari et al. to find DAPA in its API. The Kromasil 100-5-C8 (100 mm ×151; 4.6 mm) column, UV detector (224 nm), mobile phase composition (acetonitrile: water; 52:48), and flow rate (1.0 mL/min) were all used in the stability-indicating RP-HPLC technique for the experiment. The validation process for the approach adhered to ICH criteria [43].
Dr. D. Madhuri and Lagusari Mani Sharan developed a new, simple, accurate, fast, accurate, reproducible and cost-effective RP-HPLC method for the quantitative determination of dapagliflozin in bulk materials and pharmaceuticals. The RP-HPLC method for quantifying dapagliflozin is based on absorbance measurement at 254 nm using 0.1% urea and methanol (35:65% v/v) as solvent. After preparing the dapagliflozin solution, the mobile phase was diluted as necessary to obtain a standard curve. The standard drug, Dapagliflozin, exhibits an absorbance maximum at 254 nm [44].
BIO-ANALYTICAL METHODS: There are various methods for determining DAPA in biological samples such as plasma, blood and urine.
Rupali Nagulkar and V M Waghulkar created an efficient and accurate isocratic RP-HPLC technique that complies with ICH criteria to measure DAPA and SAXA in human plasma simultaneously while using linagliptin as an internal standard. The Youglin-HPLC system SP930D was used for the analysis, and a Hypersil BDS C18 column (250 mm x 4.6 mm, particle size: 5 um) was used to separate the analytes. A mobile phase made up of acetonitrile, methanol, and A 50:25:25:0.1 ratio of water to trifluoroacetic acid was used, with a flow rate of 1.0 ml/min [45]. All methods mention above shown in Table 3.
DAPAGLIFLOZIN'S ULTRA PERFORMANCE LIQUID CHROMATOGRAPHY METHOD:
Madhavi et al. described an ultra-performance liquid chromatography technique utilising a C-18 column with an acetonitrile (60) and 0.1% orthophosphoric acid (40) mobile phase at a wavelength of 254 nm for the combination of dapagliflozin and saxagliptin [46].
Livia Maronesi Bueno et al. devised a UPLC method to simultaneously detect dapagliflozin and three synthesis impurities. Method development and validation utilised a Waters® Acquity UPLC H-Class model. Separation was achieved on a Zorbax phenyl column (50 x 3.0 mm, 1.8 ?m), with a constant mixture of acetonitrile and water (70:30, v/v) as the mobile phase. The detection of all peaks was accomplished using a photodiode array detector (PDA) set at 230 nm [47].
Liquid Chromatography-Tandem Mass Spectrometry Method For Dapagliflozin:
Sahil Kalyan and Amrita Parle devised a meticulous and exceptionally responsive LC-MS/MS technique, which was thoroughly tested and confirmed for the concurrent determination of DAPA and MET in pharmaceutical formulations. The chromatographic division was executed using the Agilent Infinity Lab Poros hell 120 EC-C18 column with dimensions of 2.1×100 mm and a particle size of 2.7 µm [48]. Annemarie B. van der Aart-van der Beek presented a straightforward and robust LC-MS/MS method, enabling the concurrent measurement of three SGLT2 inhibitors (canagliflozin, dapagliflozin, and empagliflozin) in human plasma, serum, and urine, with a rapid runtime of 1 minute. Chromatographic separation was achieved using a Waters ACQUITY UPLC HSS T3 column (1.8 ?m particle size, 2.1 × 50 mm) coupled with a Waters ACQUITY UPLC HSS T3 VanGuard Pre-column (1.8 ?m particle size, 2.1 × 5 mm). The separation utilized gradient elution with a mobile phase comprising 20 mm ammonium acetate (pH 5) and acetonitrile, running at a flow rate of 0.8 ml/min [49].
High Performance Thin Layer Chromatography For Dapagliflozin:
Saloni A. Desai and colleagues employed a straightforward, accurate, and stability-assuring HPTLC technique for the concurrent assessment of MET, DAPA, and SAXA in both bulk and tablet formulations. The optimized chromatographic parameters included pre-coated silica gel 60 F254 HPTLC aluminium plates, a mobile phase consisting of methanol and 0.5% aqueous ammonium sulphate in a ratio of 8:2 (v/v) with a pH of 5.5, saturation duration of 20 minutes, and a solvent front advancement of 95 mm [50]. In order to simultaneously determine DAPA and SAXA in both their pure form and dose form, Hytham M. Ahmad et al. devised a novel validated high-performance thin-layer chromatography method. This method was then employed as a stability-indicating assay for both medications. The suggested technique combined dual-wavelength detection at 210 nm for saxagliptin and 225 nm for dapagliflozin with high-performance thin-layer chromatography. Chromatographic separations were carried out using a mobile phase of hexane/methanol/ethyl acetate (4:2:4, v/v/v) on silica gel 60-F254 aluminium plates. For dapagliflozin and saxagliptin, the retention factor values were 0.66 and 0.18, and the correlation coefficients were 0.9994 and 0.9995 [51].
CONCLUSION:
The analysis of available analytical methods for dapagliflozin indicates that various techniques such as spectrophotometry, LC-MS, HPTLC, and HPLC with UV-visible and photo diode array detectors have been documented. Only two studies have focused on its assay in human plasma thus far. HPLC-MS/MS is generally preferred as the method of choice for quantifying drugs in biological samples. Therefore, utilising HPLC-MS/MS for estimating dapagliflozin in biological samples seems highly advantageous. This review offers a comprehensive overview of current analytical methods employed for determining dapagliflozin in active pharmaceutical ingredients, tablets, and pharmaceutical dosage forms.
ABBREVATIONS:
- ACN: Acetonitrile
- BP: Blood pressure
- CANA: Canagliflozin
- CVD: Cardio vascular Disease
- DAPA: Dapagliflozin
- DM: Diabetes Mellitus
- HHF: Hospitalization for heart failure
- HPLC: High performance liquid chromatography
- HPTLC: High performance thin layer liquid chromatography
- ICH: International council for harmonization
- IDDM: Insulin dependent diabetes mellitus
- LC-MS: liquid chromatography- Tandem Mass spectrometry
- MET: Metformin
- NIDDM: Non-insulin dependent diabetes mellitus
- PDA: Photo diode array
- SAXA: Saxagliptin
- SGLT-2: Sodium glucose Co transporter-2
- UPLC: ultra-performance liquid chromatography
- UV: Ultra Visible
- VILG: Vildagliptin
REFERENCE
- American Diabetes Association, 2014. Diagnosis and classification of diabetes mellitus. Diabetes care, 37(Supplement_1), pp.S81-S90.
- Kolb, H., Kempf, K., Röhling, M. and Martin, S., 2020. Insulin: too much of a good thing is bad. BMC medicine, 18, pp.1-12.
- Sonksen, P. and Sonksen, J., 2000. Insulin: understanding its action in health and disease. British journal of anaesthesia, 85(1), pp.69-79.
- Balaji, R., Duraisamy, R. and Kumar, M.P., 2019. Complications of diabetes mellitus: A review. Drug Invention Today, 12(1).
- Olefsky, J.M., Revers, R.R., Prince, M., Henry, R.R., Garvey, W.T., Scarlett, J.A. and Kolterman, O.G., 1985. Insulin resistance in non-insulin dependent (type II) and insulin dependent (type I) diabetes mellitus. Comparison of Type I and Type II Diabetes: Similarities and Dissimilarities in Etiology, Pathogenesis, and Complications, pp.175-205.
- Clark, M., Kroger, C.J. and Tisch, R.M., 2017. Type 1 diabetes: a chronic anti-self-inflammatory response. Frontiers in immunology, 8, p.323070.
- Mezil, S.A. and Abed, B.A., 2021. Complication of diabetes mellitus. Annals of the Romanian Society for Cell Biology, pp.1546-1556.
- Gromada, J., Franklin, I. and Wollheim, C.B., 2007. ?-Cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocrine reviews, 28(1), pp.84-116.
- Hantzidiamantis, P.J. and Lappin, S.L., 2019. Physiology, glucose.
- Holt, R.I. and Hanley, N.A., 2021. Essential endocrinology and diabetes. John Wiley & Sons.
- Krishnaveni, M., Gowri, R.S., Sharmila, R., Krishnavani, J. and Haritha, P., 2022. Systematic Review On Type-2 Diabetes Mellitus And Its Prevalence In India. Journal of Pharmaceutical Negative Results, pp.5753-5758.
- Chen, L., Magliano, D.J. and Zimmet, P.Z., 2012. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nature reviews endocrinology, 8(4), pp.228-236.
- Misra, A., Gopalan, H., Jayawardena, R., Hills, A.P., Soares, M., Reza?Albarrán, A.A. and Ramaiya, K.L., 2019. Diabetes in developing countries. Journal of diabetes, 11(7), pp.522-539.
- Fioretto, P., Giaccari, A. and Sesti, G., 2015. Efficacy and safety of dapagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in diabetes mellitus. Cardiovascular diabetology, 14, pp.1-13.
- Dhillon, S., 2019. Dapagliflozin: a review in type 2 diabetes. Drugs, 79(10), pp.1135-1146.
- Tan, X. and Hu, J., 2016. Combination therapy for type 2 diabetes: dapagliflozin plus metformin. Expert opinion on pharmacotherapy, 17(1), pp.117-126.
- Fioretto, P. and Avogaro, A., 2017. Dapagliflozin: potential beneficial effects in the prevention and treatment of renal and cardiovascular complications in patients with type 2 diabetes. Expert Opinion On Pharmacotherapy, 18(5), pp.517-527.
- Kasichayanula, S., Liu, X., LaCreta, F., Griffen, S.C. and Boulton, D.W., 2014. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clinical pharmacokinetics, 53, pp.17-27.
- Wright, E.M., 2021. SGLT2 inhibitors: physiology and pharmacology. Kidney360, 2(12), pp.2027-2037.
- Kasichayanula, S., Liu, X., LaCreta, F., Griffen, S.C. and Boulton, D.W., 2014. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clinical pharmacokinetics, 53, pp.17-27.
- Delanaye, P. and Scheen, A.J., 2021. The diuretic effects of SGLT2 inhibitors: A comprehensive review of their specificities and their role in renal protection. Diabetes & metabolism, 47(6), p.101285.
- Nikolic, M., Zivkovic, V., Jovic, J.J., Sretenovic, J., Davidovic, G., Simovic, S., Djokovic, D., Muric, N., Bolevich, S. and Jakovljevic, V., 2021. SGLT2 inhibitors: a focus on cardiac benefits and potential mechanisms. Heart Failure Reviews, pp.1-15.
- Tirucherai, G.S., Lacreta, F., Ismat, F.A., Tang, W. and Boulton, D.W., 2016. Pharmacokinetics and pharmacodynamics of dapagliflozin in children and adolescents with type 2 diabetes mellitus. Diabetes, Obesity and Metabolism, 18(7), pp.678-684.
- Amin, M. and Suksomboon, N., 2014. Pharmacotherapy of type 2 diabetes mellitus: an update on drug–drug interactions. Drug safety, 37, pp.903-919.
- Kasichayanula, S., Liu, X., LaCreta, F., Griffen, S.C. and Boulton, D.W., 2014. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clinical pharmacokinetics, 53, pp.17-27.
- Scheen, A.J., 2015. Pharmacodynamics, efficacy and safety of sodium–glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs, 75, pp.33-59.
- Sachdeva, M., Dhingra, S. and Parle, M., 2014. Dapagliflozin; a new adjunct in the treatment of type-2 diabetes mellitus. International Journal of Basic & Clinical Pharmacology, 4, pp.741-74.
- McMurray, J.J., Solomon, S.D., Inzucchi, S.E., Køber, L., Kosiborod, M.N., Martinez, F.A., Ponikowski, P., Sabatine, M.S., Anand, I.S., B?lohlávek, J. and Böhm, M., 2019. Dapagliflozin in patients with heart failure and reduced ejection fraction. New England Journal of Medicine, 381(21), pp.1995-2008.
- Petrie, M.C., Verma, S., Docherty, K.F., Inzucchi, S.E., Anand, I., B?lohlávek, J., Böhm, M., Chiang, C.E., Chopra, V.K., de Boer, R.A. and Desai, A.S., 2020. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. Jama, 323(14), pp.1353-1368.
- Saleem, F., 2017. Dapagliflozin: cardiovascular safety and benefits in type 2 diabetes mellitus. Cureus, 9(10).
- Sjöström, C.D., Johansson, P., Ptaszynska, A., List, J. and Johnsson, E., 2015. Dapagliflozin lowers blood pressure in hypertensive and non-hypertensive patients with type 2 diabetes. Diabetes and Vascular Disease Research, 12(5), pp.352-358.
- Cherney, D.Z., Dekkers, C.C., Barbour, S.J., Cattran, D., Gafor, A.H.A., Greasley, P.J., Laverman, G.D., Lim, S.K., Di Tanna, G.L., Reich, H.N. and Vervloet, M.G., 2020. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. The lancet Diabetes & endocrinology, 8(7), pp.582-593.
- Sen, D.B., Jatu, S., Maheshwari, R.A., Zanwar, A.S., Velmurugan, R. and Sen, A.K., 2023. New eco-friendly UV-spectroscopic methods for simultaneous assessment of dapagliflozin, saxagliptin and metformin in ternary mixture. Indian J Pharm Educ Res, 57(2), pp.559-69.
- Jani, B.R., Shah, K.V. and Kapupara, P.P., 2015. Development and validation of UV spectroscopic first derivative method for simultaneous estimation of dapagliflozin and metformin hydrochloride in synthetic mixture. J Bioequiv, 1(1), p.102.
- Sen, A.K., Khatariya, S.B., Sen, D.B., Maheshwari, R.A., Zanwar, A.S. and Velmurugan, R., 2023. Various innovative UV spectroscopic methodologies for concurrent estimation of dapagliflozin and vildagliptin in combined tablet. Journal of Applied Pharmaceutical Science, 13(9), pp.213-223.
- Patel, A., Omray, D.L. and Soni, P., 2020. Method development for simultaneous estimation of Dapagliflozin and saxagliptin in fixed-dose combination and validation on UV spectroscopy. J. Pharm., 9(3), pp.2536-2543.
- Sree, V.N., Bhavyasri, D.K., Sumakanth, D.M. and Swethasri, R., Estimation of Dapagliflozin in Pure and Marketed Formulation by Validated Reverse Phase-High Performance Liquid Chromatographic Method.(2020). Int. J. Life Sci. Pharma Res, 10(4), pp.P70-84.
- Bhavyasri, K., Surekha, T., Begum, S. and Sumakanth, M., 2021. RP-HPLC Method for Dapagliflozin and Metformin HCL in Bulk and Combined Formulation. Archives of Pharmacy Practice, 12(4-2021), pp.106-110.
- Murugesan, A. and Mukthinuthalapati, A., 2022. Simultaneous Estimation of Gliflozin Derivatives Canagliflozin, Dapagliflozin, Empagliflozin and Ertugliflozin Using RP-HPLC Methods. Acta Scientific Pharmaceutical Sciences (ISSN: 2581-5423), 6(1).
- Vankalapati, K.R., Alegete, P. and Boodida, S., 2022. Stability?indicating HPLC method development and validation for simultaneous estimation of metformin, dapagliflozin, and saxagliptin in bulk drug and pharmaceutical dosage form. Biomedical Chromatography, 36(7), p.e5384.
- Sravanthi, S., Zarin, N., Shruthi, B., Krishna, D.R. and Manjeera, A., A New Analytical Method Development and Validation for the Estimation of Dapagliflozin by Using Reverse Phase-High Performance Liquid Chromatography.
- Gaikwad, A.V., Gawade, A.S., Hupparage Vrushabh, B., Mantry, S., Kale, A. and Kale, J., 2022. Method Development and Validation of Dapagliflozin by RP-HPLC. Journal of Pharmaceutical Negative Results, pp.4316-4335.
- Chaudhari, U., Sahu, J.K. and Dande, P.R., 2023. Analytical Method Development, Validation and Forced Degradation Study of Dapagliflozin by RP-HPLC. Drug Metabolism and Bioanalysis Letters Formerly: Drug Metabolism Letters, 16(2), pp.140-152.
- Sharan, L.M. and Madhuri, D., 2023. Doe Approach: A Validated Rp-Hplc Method For The Determination Of Dapagliflozin. Journal of Survey in Fisheries Sciences, pp.4019-4028.
- Nagulkar, R. and Waghulkar, V.M., SIMULTANEOUS ESTIMATION OF SAXAGLIPTIN AND DAPAGLIFLOZIN IN HUMAN PLASMA BY HPLC METHOD AND ITS VALIDATION AS PERICH GUIDELINES.
- Madhavi, S. and Rani, A.P., 2017. Development and validation of a method for simultaneous determination of dapagliflozin and saxagliptin in a formulation by RP-UPLC. World J Pharma Res, 6(12), pp.904-16.
- Bueno, L., Manoel, J.W., Koetz, M., Henriques, A.T., Steppe, M. and Schapoval, E.E.S., 2022. Simultaneous analysis of dapagliflozin and its three related impurities by stability-indicating UPLC method and in vitro toxicity evaluation. Drug Analytical Research, 6(2), pp.27-37.
- Kalyan, S. and Parle, A., A Validated LC-MS/MS Method for Simultaneous Estimation of Dapagliflozin and Metformin in Pharmaceutical Dosage Form.
- van der Aart-van, A.B., Wessels, A.M.A., Heerspink, H.J. and Touw, D.J., 2020. Simple, fast and robust LC-MS/MS method for the simultaneous quantification of canagliflozin, dapagliflozin and empagliflozin in human plasma and urine. Journal of Chromatography B, 1152, p.122257.
- Desai, S.A., Mardia, R.B., Suhagia, B.N. and Desai, H.T., 2022. Development and validation of stability-indicating HPTLC method for simultaneous estimation of Metformin, Saxagliptin, and Dapagliflozin in their combined matrix using AQbD. Bull. Env. Pharmacol. Life Sci, 12, pp.32-42.
- Ahmed, H.M., Omar, M.A., Batakoushy, H.A. and Hamid, M.A.A., 2020. HPTLC-densitometric analysis of selected antidiabetic drugs in presence of their degradation products. Microchemical Journal, 154, p.104560.


 Abhijeet Singh Rana*
Abhijeet Singh Rana*
 Kajol
Kajol
 Ankit Sharma
Ankit Sharma
 Arti Devi
Arti Devi





 10.5281/zenodo.13142608
10.5281/zenodo.13142608