Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disorder resulting from the loss of dopaminergic neurons in the midbrain substantia nigra pars compacta, characterized by both motor and non-motor symptoms. Its etiology involves a complex interplay of genetic predispositions, environmental factors, and neuronal degeneration. PD affects approximately 1% of the global population over 60, with prevalence and incidence rates varying by region and age group. The annual incidence is estimated at 10–20 new cases per 100,000 individuals worldwide. Symptomatically, PD patients experience tremors, bradykinesia, rigidity, and postural instability, along with non-motor symptoms such as cognitive impairment and autonomic dysfunction. The primary goal of PD pharmacotherapy is symptom alleviation and quality of life improvement. Current medications include dopamine agonists, levodopa, monoamine oxidase-B inhibitors, and catechol-O-methyl transferase inhibitors. However, these treatments are limited by side effects, motor fluctuations, dyskinesias, and non-motor complications, posing significant challenges in long-term management. Emerging therapies targeting novel pathways, such as alpha-synuclein aggregation inhibitors, neuroprotective agents, and gene therapies, show promise in addressing these limitations and advancing PD treatment paradigms. Alpha-synuclein aggregation inhibitors aim to prevent the formation of toxic protein aggregates that contribute to neuronal death, while gene therapies are being developed to modify disease progression at the genetic level, offering potential for more durable and profound disease modification. Understanding the multifaceted nature of PD and exploring these innovative therapeutic avenues are crucial for optimizing patient care and enhancing disease management strategies.
Keywords
Parkinson’s Disease, ?-Synuclein Aggregation, Dopaminergic Neuron Degeneration, Motor and Non-Motor Symptoms, Gene Therapy in PD.
Introduction
Parkinson’s disease (PD) is the most prevalent form of parkinsonism, a group of neurological disorders that cause movement issues such as rigidity, slowness, and tremors. Affecting over 6 million people globally, PD is recognized as the second most common progressive neurodegenerative disorder1. The disease is primarily associated with the loss of dopaminergic neurons in the substantia nigra pars compacta (SNc), leading to a deficiency of dopamine (DA) in the striatum, which causes motor deficits. This dopamine shortage creates an imbalance between the inhibitory dopaminergic and excitatory cholinergic systems in the striatum, resulting in motor dysfunction2. PD is characterized by a combination of motor symptoms, such as bradykinesia and tremors, and non-motor symptoms, including depression, constipation, and sleep disturbances. Interestingly, these non-motor symptoms can sometimes appear before the motor symptoms become evident. The neuronal degeneration seen in PD is driven by several cellular and molecular processes, including the accumulation of misfolded proteins, failure of protein clearance mechanisms, mitochondrial damage, oxidative stress, neuroinflammation, immune system dysregulation, apoptosis, excitotoxicity, calcium (Ca++) dysregulation, autophagy, and dysbiosis. A key feature of PD pathogenesis is the presence of abnormal intra-neuronal aggregates of a protein called ?-synuclein, which form Lewy bodies and Lewy neurites. Under normal conditions, ?-synuclein is a presynaptic neuronal protein, but in PD, it misfolds and aggregates into Lewy bodies, which are considered hallmarks of the disease. Genetic mutations, duplications, or triplications in the SNCA gene, which encodes ?-synuclein, can cause autosomal dominant familial PD, underscoring the critical role of ?-synuclein in the development of the disease3. PD affects approximately 1% of people over the age of 50 and around 2.5% of those over 70. The lifetime risk of developing PD is estimated at 2.0% for men and 1.3% for women. Although the disease is most common in individuals over 60 years old4, most PD cases in this age group arise sporadically, influenced by factors such as neuroinflammation, oxidative stress, dysfunction of the innate and/or adaptive immune systems, disruptions in mitochondrial activity, genetic mutations, protein denaturation and aggregation, and environmental influences5. To manage PD, disease-modifying and neuroprotective strategies are crucial as they aim to improve motor symptoms by inhibiting or slowing the death of dopaminergic neurons in the brain. However, currently available medications like levodopa offer only temporary relief, as their effects are often short-lived6. The presence of abnormal intra-neuronal aggregates of ?-synuclein remains a defining characteristic of PD. Although the precise mechanistic role of Lewy bodies is not fully understood, the centrality of ?-synuclein in PD pathogenesis is emphasized by the fact that mutations, duplications, or triplications in the SNCA gene lead to autosomal dominant familial PD7. This review will explore emerging treatment approaches and discuss how PD management might evolve in the coming years.
Epidemiology and Etiology
PD is primarily a disease of older adults, typically affecting individuals over the age of 60. However, early-onset cases can occur, albeit less frequently. The prevalence of PD increases with age, making it a significant health concern in aging populations globally. According to recent data, approximately 1% of individuals over 60 and 2.5% of those over 70 are affected by PD, with the prevalence expected to double by 2040 due to the aging global population. However, early-onset cases can occur, albeit less frequently, affecting about 4% of people with PD under the age of 50th. The exact cause of PD remains elusive. However, research has identified contributing factors such as genetic mutations and environmental toxins. It is widely accepted that a complex interplay of genetic predisposition, environmental influences, and age-related changes in neuronal function contributes to the development of PD8,9. Genetic factors play a crucial role, with several genetic mutations linked to an increased risk of PD. Mutations in genes such as SNCA (encoding ?-synuclein), LRRK2 (Leucine-Rich Repeat Kinase 2), and Parkin (PRKN) have been identified in familial and sporadic cases of PD. These mutations can disrupt protein function, leading to neuronal dysfunction and the accumulation of ?-synuclein aggregates, a hallmark of PD pathology10. Pesticides, herbicides, and other environmental toxins have been implicated as potential risk factors, particularly in agricultural settings or occupations with high pesticide exposure. Studies have shown that individuals with long-term exposure to pesticides have a 70% higher risk of developing PD. Other environmental factors, such as heavy metals, solvents, and certain medications, have also been studied for their association with PD development11. Furthermore, age-related changes in neuronal function and neuroinflammatory processes contribute to the neurodegenerative cascade observed in PD. Oxidative stress, mitochondrial dysfunction, and impaired protein clearance mechanisms further exacerbate neuronal damage and cell death in affected brain regions. For instance, mitochondrial dysfunction and oxidative stress have been shown to be significantly higher in the brains of PD patients, leading to increased neuronal apoptosis and subsequent motor and non-motor symptoms12.
Pathophysiology
PD is characterized by complex neurodegenerative processes that involve both motor and non-motor pathways. Neuropathological features of PD (Figure 1) include the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the formation of intracellular aggregates known as Lewy bodies, primarily composed of ?-synuclein protein. The degeneration of dopaminergic neurons leads to a dopamine deficiency in the basal ganglia, particularly in the nigrostriatal pathway, resulting in the motor symptoms observed in PD, such as bradykinesia, rigidity, tremor, and postural instability13. Additionally, non-dopaminergic pathways, including cholinergic, serotonergic, and noradrenergic systems, are also affected, contributing to non-motor symptoms like cognitive impairment, mood disorders, and autonomic dysfunction. The pathophysiological cascade in PD neuroinflammation, impaired protein clearance mechanisms, and synaptic dysfunction. These processes ultimately lead to neuronal degeneration, synaptic loss, and neurochemical imbalances in various brain regions implicated in motor control, cognition, and behavior14.

Figure 1. Neuropathological features of Parkinson’s disease
The motor circuitry involved in PD encompasses various pathways such as the direct pathway responsible for motor excitation, the indirect pathway for motor inhibition, the hyper-direct pathway for baseline motor inhibition, and the nigrostriatal projection with modulatory functions. Neurochemically distinct pathways within the basal ganglia involve different dopamine receptors, leading to inhibitory or excitatory effects on motor control. Hypotonic-hyperkinetic symptoms like tremors are linked to disruptions in the indirect loop, causing a lack of motion inhibition, while hypertonic-hypokinetic symptoms such as bradykinesia result from issues in the direct pathway15,16. Environmental hazards contribute to an increase in ?-synuclein levels and the formation of Lewy bodies. Aging is associated with a decrease in mitochondrial complex-1 activity, which results in increased reactive oxygen species (ROS) and reduced ATP production, ultimately leading to the loss of dopaminergic neurons. Genetic factors also play a significant role: mutations in the DJ-1, PINK-1, and LRRK-2 genes lead to mitochondrial dysfunction, increased ROS, and decreased ATP, contributing to neuronal loss. Mutations in the SNCA gene result in elevated ?-synuclein levels and Lewy body formation, furthering neuronal degeneration. Additionally, mutations in the UCHL-1 gene impair the ubiquitin-proteasome system, causing dysfunction in the autophagy-lysosomal pathway and an increase in misfolded proteins. These combined effects from environmental, aging, and genetic factors converge to decrease the number of dopaminergic neurons17, leading to the development of PD shown in Figure 2.
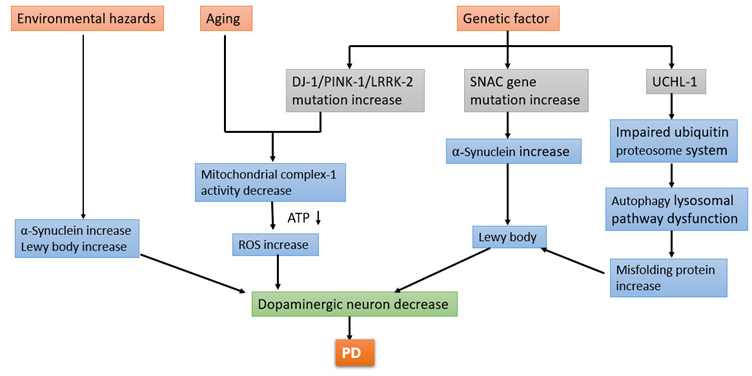
Figure 2: The diagram illustrates the multifactorial causes and mechanisms leading to Parkinson's disease
Clinical Manifestations
PD presents with a heterogeneous array of motor and non-motor symptoms. Motor symptoms (Table 1), including bradykinesia, rigidity, resting tremor, and postural instability, are the most recognizable features. As much as 80% of dopaminergic cells in the nigro-striatal system can be lost before these cardinal motor features appear. The disease typically begins typically begins unilaterally and progresses contralaterally over years, leading to stooped posture, shuffling gait, reduced arm swing, micrographia, and resting tremors, notably a pill-rolling type18. Non-motor symptoms (Table 2) significantly contribute to disability and often precede motor signs. Cognitive impairment, mood disorders, sleep disturbances, autonomic dysfunction, and sensory abnormalities are common. Speech difficulties, swallowing problems, and drooling occur in a substantial portion of patients. Dystonia, characterized by sustained muscular contractions, can manifest before PD diagnosis with varied presentations like foot position abnormalities, writer's cramp, oromandibular dystonia, and torticollis19. The neuroanatomical and neurochemical bases for non-motor symptoms remain incompletely understood, but non-dopaminergic cellular dysfunction is implicated. Sleep disturbances are prevalent, with disruptions in the sleep-wake cycle and excessive daytime sleepiness affecting up to 50% of patients20. Constipation, depression, anxiety, hallucinations, and psychosis are also common non-motor manifestations, with dopamine agonists potentially exacerbating hallucinosis. In addition to these symptoms, PD patients often experience fluctuations in their response to medication, such as "on-off" phenomena and dyskinesias, which further complicate management and quality of life. Additionally, emerging research is focusing on the role of gut-brain axis dysfunction, inflammation, and oxidative stress in PD pathogenesis, opening avenues for novel therapeutic targets21.
Table 1 : Motor symptoms of Parkinson’s disease22

Table 2 : Non-motor symptoms of Parkinson’s disease23
Treatment Approaches
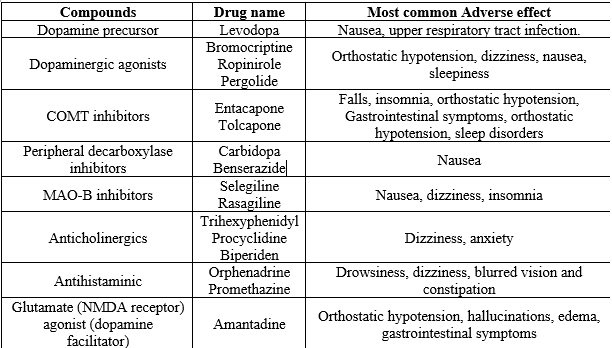
Medications for PD are designed to address both motor and non-motor symptoms associated with the condition. In treating motor symptoms, the primary approach is dopamine replacement therapy, which involves medications that either supply dopamine directly or mimic its effects in the brain. The term "early disease" refers to individuals newly diagnosed with PDor those experiencing functional disability necessitating symptomatic treatment24. Another term used is "de novo" PD. Conversely, "later disease" refers to patients already receiving levodopa therapy who have developed motor complications. Adjuvant therapy in later-stage disease aims to minimize "off" time, reduce levodopa dosage, and enhance control over Parkinsonian motor impairments25. About 10% of patients may develop motor complications within a year of starting levodopa therapy, prompting the need for adjunctive treatment soon after initiating levodopa. Levodopa-carbidopa (Sinemet) is a common medication used for dopamine replacement therapy. Levodopa converts to dopamine in the brain, replenishing dopamine levels in the striatum to compensate for deficiency in Parkinson’s disease26. Carbidopa, by inhibiting peripheral levodopa-to-dopamine conversion, reduces side effects like nausea and vomiting. Other medications used in PD management include dopamine agonists like pramipexole and ropinirole, which stimulate dopamine receptors directly, aiding in compensating for dopamine deficiency27. MAO-B inhibitors such as rasagiline and selegiline increase dopamine levels by blocking dopamine metabolism in the brain. COMT inhibitors like entacapone and tolcapone prolong levodopa’s action, reducing motor fluctuations28. Anticholinergics like trihexyphenidyl and benztropine alleviate tremors and rigidity by counteracting overactive acetylcholine due to dopamine deficiency29. Additionally, antidepressants such as SSRIs, TCAs, and SNRIs help manage depression and anxiety, while antipsychotics target psychosis30. Cholinesterase inhibitors like donepezil improve cognitive function, and wakefulness-promoting agents like modafinil enhance alertness31 shown in Table 3.
Table 3 :- Medication for motor symptoms of PD32

Table 4:- Medication for non-motor symptoms of PD33

Emerging Therapies
Emerging therapies for PD are targeting alpha-synuclein aggregation, a key process in the pathology of the condition. Alpha-synuclein is a protein that, when misfolded, forms toxic aggregates known as Lewy bodies, contributing to neuronal damage. One approach involves developing therapeutics that inhibit the formation of these aggregates or promote their clearance by the brain's immune system. These treatments may include small molecules, antibodies, or compounds that bind to alpha-synuclein, preventing its aggregation. Another promising avenue is the use of alpha-synuclein misfolding inhibitors. In its normal state, alpha-synuclein regulates neurotransmitter release without causing harm34. However, in Parkinson's disease, it undergoes misfolding and aggregation. Misfolding inhibitors interfere with this process, stabilizing alpha-synuclein's normal conformation and preventing toxic aggregate formation35. Antisense oligonucleotides (ASOs) are synthetic nucleic acid strands that target specific RNA molecules. In Parkinson's, ASOs can be designed to reduce the production of alpha-synuclein protein by targeting its RNA transcripts, potentially slowing disease progression. Efficient delivery of ASOs to the brain is crucial, and strategies to enhance brain penetration are being explored36,37. Beta-2 adrenergic agonists, primarily used for respiratory conditions, are being investigated for their neuroprotective effects in Parkinson's. They activate receptors that may protect neurons from degeneration and promote the release of neurotrophic factors supporting neuron function38,39. Clinical trials are needed to assess their safety and efficacy in Parkinson's. Lymphocyte activation gene 3 (LAG3), involved in immune regulation, has garnered interest due to its potential role in Parkinson's neuroinflammation. Studies suggest increased LAG3 expression in Parkinson's brains, implicating it in immune responses to disease-related changes. Modulating LAG3 activity or expression is being explored as a therapeutic strategy to mitigate neuroinflammation and neurodegeneration40.
Clinical Trials of Immunotherapeutic Interventions in PD Management
Current clinical trials of immunotherapeutic interventions for PD involve various approaches, including immunomodulatory therapies, active immunization, and passive immunotherapy. Among the immunomodulatory therapies, GM-CSF is under investigation (NCT03790670, Phase Ib, Recruiting) for its ability to enhance regulatory T cell (Treg) quantity and functionality without affecting effector T (Teff) cell levels41. Active immunization approaches include PD01A and PD03A, both C-terminal alpha-synuclein (?-Syn) mimicking peptide vaccinations. PD01A has completed its Phase I trial (NCT02618941), as has PD03A (NCT02267434)42. Additionally, a nucleic acid vaccine, pVAX1-IL-4/SYN-B, is designed to increase antibody titers against ?-Syn, but further trial information is not provided43. For passive immunotherapy, several monoclonal antibodies are being explored. Prasinezumab, a humanized IgG1 monoclonal antibody targeting C-terminal epitopes on ?-Syn, is in a Phase IIb trial (NCT04777331, Recruiting)44. BIIB054, targeting N-terminal ?-Syn aggregate species, had its Phase II trial terminated (NCT03318523)45. Lu AF82422, another humanized IgG1 monoclonal antibody focusing on the C-terminal of ?-Syn, has completed Phase I (NCT03611569)46. Finally, ABBV-0805, aimed at ?-Syn oligomeric and protofibrillar species, had its Phase I trial withdrawn (NCT04127695)47. These trials represent a diverse array of strategies aimed at modifying the immune response in PD to slow disease progression and improve patient outcomes.
CONCLUSION
PD presents as a multifaceted and progressive neurodegenerative disorder, marked by an interplay of genetic, environmental, and age-related factors. This review underscores the complexity of PD pathophysiology, which involves both motor and non-motor symptoms arising from dopaminergic neuronal loss and alpha-synuclein aggregation. Despite advancements in pharmacotherapy, including dopamine replacement and symptom management, long-term treatment remains challenging due to side effects and the progression of both motor and non-motor symptoms. Emerging therapies, such as alpha-synuclein aggregation inhibitors, neuroprotective agents, and gene therapies, offer promising avenues for more effective management of PD. The exploration of novel biomarkers for early diagnosis and the development of personalized medicine approaches are crucial steps toward improving patient outcomes. Understanding the intricate mechanisms underlying PD and continuing to innovate in therapeutic strategies are essential for enhancing disease management and the quality of life for patients with Parkinson's disease. Future research must focus on these promising areas to create a comprehensive and effective treatment paradigm for PD.
REFERENCE
- Bloem BR, Okun MS, Klein C. Parkinson's disease. The Lancet. 2021 Jun 12;397(10291):2284-303.
- Iarkov A, Barreto GE, Grizzell JA, Echeverria V. Strategies for the treatment of Parkinson’s disease: beyond dopamine. Frontiers in aging neuroscience. 2020 Jan 31;12:4.
- LeWitt PA, Chaudhuri KR. Unmet needs in Parkinson disease: Motor and non-motor. Parkinsonism & Related Disorders. 2020 Nov 1;80:S7-12.
- Canonico M, Artaud F, Degaey I, Moisan F, Kabore R, Portugal B, Nguyen TT, Pesce G, Boutron-Ruault MC, Roze E, Elbaz A. Incidence of Parkinson’s disease in French women from the E3N cohort study over 27 years of follow-up. European journal of epidemiology. 2022 May;37(5):513-23.
- Caggiu E, Arru G, Hosseini S, Niegowska M, Sechi G, Zarbo IR, Sechi LA. Inflammation, infectious triggers, and Parkinson's disease. Frontiers in neurology. 2019 Feb 19;10:122.
- Krishnamurthy PT, Kumari M, Byran G, Gangadharappa HV, Garikapati KK. Neuroprotective approaches to halt Parkinson's disease progression. Neurochemistry International. 2022 Sep 1;158:105380.
- Niu Y, Zhang J, Dong M. Nrf2 as a potential target for Parkinson’s disease therapy. Journal of Molecular Medicine. 2021 Jul;99(7):917-31.
- Chesnokova AY, Ekimova IV, Pastukhov YF. Parkinson’s disease and aging. Advances in Gerontology. 2019 Apr;9:164-73.
- Kolicheski A, Turcano P, Tamvaka N, McLean PJ, Springer W, Savica R, Ross OA. Early-onset Parkinson’s disease: creating the right environment for a genetic disorder. Journal of Parkinson's disease. 2022 Jan 1;12(8):2353-67.
- Bardien S, Lesage S, Brice A, Carr J. Genetic characteristics of leucine-rich repeat kinase 2 (LRRK2) associated Parkinson’s disease. Parkinsonism & related disorders. 2011 Aug 1;17(7):501-8.
- Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Frontiers in cellular neuroscience. 2015 Apr 10;9:124.
- Calabrese V, Santoro A, Monti D, Crupi R, Di Paola R, Latteri S, Cuzzocrea S, Zappia M, Giordano J, Calabrese EJ, Franceschi C. Aging and Parkinson's Disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radical Biology and Medicine. 2018 Feb 1;115:80-91.
- Jellinger KA. Parkinson's disease. Neurodegeneration: the molecular pathology of dementia and movement disorders. 2011 Sep 2:194-223.
- Murueta-Goyena A, Andikoetxea A, Gómez-Esteban JC, Gabilondo I. Contribution of the GABAergic system to non-motor manifestations in premotor and early stages of Parkinson’s disease. Frontiers in pharmacology. 2019 Oct 30;10:485371.
- Pierucci M, Galati S, Valentino M, Di Matteo V, Benigno A, Pitruzzella A, Muscat R, Di Giovanni G. Nitric oxide modulation of the basal ganglia circuitry: therapeutic implication for Parkinson's disease and other motor disorders. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders). 2011 Nov 1;10(7):777-91.
- Boonstra JT, McGurran H, Temel Y, Jahanshahi A. Nigral neuropathology of Parkinson’s motor subtypes coincide with circuitopathies: a scoping review. Brain Structure and Function. 2022 Sep;227(7):2231-42.
- Larsen SB, Hanss Z, Krüger R. The genetic architecture of mitochondrial dysfunction in Parkinson’s disease. Cell and tissue research. 2018 Jul;373:21-37.
- Abusrair AH, Elsekaily W, Bohlega S. Tremor in Parkinson’s Disease: From Pathophysiology to Advanced Therapies. Tremor and Other Hyperkinetic Movements. 2022;12.
- Silva AB, de Oliveira RW, Diógenes GP, de Castro Aguiar MF, Sallem CC, Lima MP, de Albuquerque Filho LB, de Medeiros SD, de Mendonça LL, de Santiago Filho PC, Nones DP. Premotor, nonmotor and motor symptoms of Parkinson's disease: a new clinical state of the art. Ageing Research Reviews. 2023 Feb 1;84:101834.
- Yousaf T, Pagano G, Wilson H, Politis M. Neuroimaging of sleep disturbances in movement disorders. Frontiers in Neurology. 2018 Sep 11;9:335709.
- Sprenger F, Poewe W. Management of motor and non-motor symptoms in Parkinson’s disease. CNS drugs. 2013 Apr;27:259-72.
- Shahed J, Jankovic J. Motor symptoms in Parkinson's disease. Handbook of clinical neurology. 2007 Jan 1;83:329-42.
- Factor SA. The clinical spectrum of freezing of gait in atypical parkinsonism. Movement disorders: official journal of the Movement Disorder Society. 2008;23(S2):S431-8.
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE. Parkinson disease. Nature reviews Disease primers. 2017 Mar 23;3(1):1-21.
- Borghammer P, Just MK, Horsager J, Skjærbæk C, Raunio A, Kok EH, Savola S, Murayama S, Saito Y, Myllykangas L, Van Den Berge N. A postmortem study suggests a revision of the dual-hit hypothesis of Parkinson’s disease. npj Parkinson's Disease. 2022 Nov 30;8(1):166.
- Aradi SD, Hauser RA. Medical management and prevention of motor complications in Parkinson's disease. Neurotherapeutics. 2020 Oct 1;17(4):1339-65.
- Cabreira V, Soares-da-Silva P, Massano J. Contemporary options for the management of motor complications in Parkinson’s disease: updated clinical review. Drugs. 2019 Apr 1;79(6):593-608.
- Fabbri M, Barbosa R, Rascol O. Off-time treatment options for Parkinson’s disease. Neurology and therapy. 2023 Apr;12(2):391-424.
- Fox SH. Non-dopaminergic treatments for motor control in Parkinson’s disease. Drugs. 2013 Sep;73:1405-15.
- Connolly B, Fox SH. Treatment of cognitive, psychiatric, and affective disorders associated with Parkinson's disease. Neurotherapeutics. 2014 Jan 1;11(1):78-91.
- Taximaimaiti R, Luo X, Wang XP. Pharmacological and non-pharmacological treatments of sleep disorders in Parkinson's disease. Current Neuropharmacology. 2021 Dec 12;19(12):2233.
- Shankar J, Geetha KM, Wilson B. Potential applications of nanomedicine for treating Parkinson's disease. Journal of Drug Delivery Science and Technology. 2021 Dec 1;66:102793.
- Müller T. Drug treatment of non-motor symptoms in Parkinson’s disease. Expert Opinion on Pharmacotherapy. 2002 Apr 1;3(4):381-8.
- Vekrellis K, Stefanis L. Targeting intracellular and extracellular alpha-synuclein as a therapeutic strategy in Parkinson's disease and other synucleinopathies. Expert opinion on therapeutic targets. 2012 Apr 1;16(4):421-32.
- Srinivasan E, Chandrasekhar G, Chandrasekar P, Anbarasu K, Vickram AS, Karunakaran R, Rajasekaran R, Srikumar PS. Alpha-synuclein aggregation in Parkinson's disease. Frontiers in medicine. 2021 Oct 18;8:736978.
- Nakamori M, Junn E, Mochizuki H, Mouradian MM. Nucleic acid–based therapeutics for Parkinson's disease. Neurotherapeutics. 2019 Apr 1;16(2):287-98.
- Testa CM. Antisense Oligonucleotide Therapeutics for Neurodegenerative Disorders. Current Geriatrics Reports. 2022:1-4.
- Magistrelli L, Comi C. Beta2-adrenoceptor agonists in Parkinson’s disease and other synucleinopathies. Journal of Neuroimmune Pharmacology. 2020 Mar;15:74-81.
- Paakinaho A, Tiihonen M, Koskela H, Koponen M, Tiihonen J, Hartikainen S, Tolppanen AM. ?2-Adrenoceptor Agonists in Asthma or Chronic Obstructive Pulmonary Disease and Risk of Parkinson’s Disease: Nested Case-Control Study. Clinical Epidemiology. 2023 Dec 31:695-705.
- Maruhashi T, Sugiura D, Okazaki IM, Okazaki T. LAG-3: from molecular functions to clinical applications. Journal for immunotherapy of cancer. 2020;8(2).
- Olson KE, Namminga KL, Lu Y, Schwab AD, Thurston MJ, Abdelmoaty MM, Kumar V, Wojtkiewicz M, Obaro H, Santamaria P, Mosley RL. Safety, tolerability, and immune-biomarker profiling for year-long sargramostim treatment of Parkinson's disease. EBioMedicine. 2021 May 1;67.
- Antonini A, Bravi D, Sandre M, Bubacco L. Immunization therapies for Parkinson’s disease: state of the art and considerations for future clinical trials. Expert opinion on investigational drugs. 2020 Jul 2;29(7):685-95.
- Gouda NA, Elkamhawy A, Cho J. Emerging therapeutic strategies for Parkinson’s disease and future prospects: a 2021 update. Biomedicines. 2022 Feb 3;10(2):371.
- Manoutcharian K, Gevorkian G. Recombinant Antibody Fragments for Immunotherapy of Parkinson’s Disease. BioDrugs. 2024 Jan 27:1-9.
- Weihofen A, Liu Y, Arndt JW, Huy C, Quan C, Smith BA, Baeriswyl JL, Cavegn N, Senn L, Su L, Marsh G. Development of an aggregate-selective, human-derived ?-synuclein antibody BIIB054 that ameliorates disease phenotypes in Parkinson's disease models. Neurobiology of disease. 2019 Apr 1;124:276-88.
- Henriquez G, Narayan M. Targeting ?-synuclein aggregation with immunotherapy: a promising therapeutic approach for Parkinson’s disease. Exploration of Neuroprotective Therapy. 2023 Aug 25;3(4):207-34.
- Manoutcharian K, Gevorkian G. Recombinant Antibody Fragments for Immunotherapy of Parkinson’s Disease. BioDrugs. 2024 Jan 27:1-9.


 Varsha Rawat* 2
Varsha Rawat* 2
 Renu Singh 1
Renu Singh 1





 10.5281/zenodo.13905162
10.5281/zenodo.13905162