The study aims to develop and validate a UV-visible spectrophotometric method for analyzing Calcium Pantothenate. The method uses a solvent system of methanol and water (1:1), chosen for its solubility and cost-effectiveness. A stock solution (1000 µg/ml) and subsequent dilutions were prepared for analysis. The absorption maxima (?max) of Calcium Pantothenate was determined to be 212 nm. Validation parameters included linearity , precision , accuracy , specificity , robustness , and sensitivity. Linearity was confirmed over the concentration range of 5 to 25 µg/ml, with an R² value of 0.999. Precision studies both intra-day and inter-day demonstrated consistency with %RSD values below 2%. Accuracy was verified using the standard addition method with recoveries within 100±2%. The method’s specificity was confirmed by comparing the UV spectra of the tablet and standard solutions with no interference from excipients. The developed method is thus validated as reliable and accurate for the quantitative analysis of Calcium Pantothenate.
UV Visible Spectrophotometer, Calcium Pantothenate, Water soluble vitamin, Method Validation.
Vitamin B5 (Calcium Pantothenate) is chemically, calcium; 3-[[(2R)-2,4 dihydroxy3,3dimethylbutanoyl]amino]propanoate
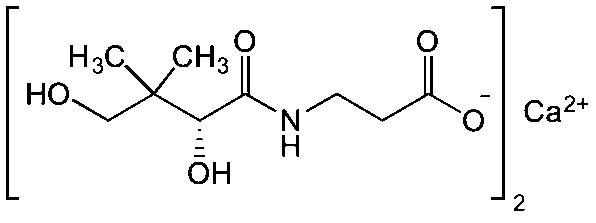
Figure 1. Vitamin B5 (Calcium Pantothenate)
It is a White or slightly yellowish or crystalline or powder form. It acts as a precursor to Coenzyme A (CoA), an essential coenzyme involved in various biochemical reactions that are crucial for energy production. It participates in the metabolism of Carbohydrates, fats, proteins. Characteristic lesions of vitamin B5 deficiency are numbness and burning of the hands and feet, headache, extreme, restlessness, stomach pain, heartburn, diarrhoea and loss of appetite.
Survey of the literature review revealed that few analytical, methods are available for the estimation of water-soluble vitamin (calcium Pantothenate) by using HPLC, Fluorimetry, Colorimetry. Yet there is no method reported in the literature for the estimation of Calcium Pantothenate by using UV Spectroscopy.
The aim of this work is to develop a method and validate for the estimation of calcium Pantothenate by using UV Spectrophotometer by using aqueous solvent system.
2. MATERIAL AND METHOD
API of calcium Pantothenate by I Dreamz Health Care, No.274C, Road No.5A, Harohalli Industrial area, 2nd Phase, Harohalli, Karnataka 62112 as gift sample. Calcium Pantothenate dosage form was purchased from Ipca Laboratories Ltd. AR grade Methanol and Water were obtained from Vasa Scientifics. Shimadzu (U-19000i) UV/Visible spectrophotometer coupled with UV- probe data acquisition software.
2.1 Method Development
2.1.1 Selection of Solvent
The study investigated the solubility of Calcium pantothenate in different solvents including water, methanol, 0.1N NaOH and acetonitrile. The results showed that the Calcium pantothenate was highly soluble in methanol and water. Water and methanol
(1 : 1) were chosen as the preferred solvents for dissolving the drug.
2.1.2 Preparation of Stock Solution (1000µg/ml)
Accurately weighed quantity of pure Calcium Pantothenate (100mg) was transferred into 100 ml volumetric flasks dissolved with the solvent system of methanol and water (1:1) and made up to 100 ml with the same solvent to give a solution containing 1000µg/ml. The solution was sonicated for 5mins.
2.1.3 Preparation of Standard solution (100µg/ml)
10ml of stock solution was taken and transferred to another separate 100ml volumetric flask and the same solvent system was added up to 100ml for additional dilution to give a solution containing 100µg/ml. From the above solution 1ml was further diluted to 10ml to obtain 10 µg/ml of Calcium pantothenate.
2.1.4 Preparation of sample solution (10µg/ml)
The tablet powder equivalent to 10mg of Calcium pantothenate was transferred into 100ml volumetric flask diluted to 100ml with diluent. The resultant solution was further diluted with the same diluent to obtain a solution containing 10µg/ml concentration of Calcium pantothenate. The sample solution was filtered through the 0.25µm Nylon filter.
2.1.5 Determination of Absorption Maxima (?max)
25µg/ml of Calcium Pantothenate solution was prepared by diluting 2.5ml of working standard solution again diluted to 10 ml with the same solvent system. The produced solutions of Calcium Pantothenate was scanned in the UV spectrophotometer from 200-400nm to determine the ?max of given compounds. The ?max of Calcium Pantothenate was observed to be 212nm.
2.2 Method Validation
Analytical method validation is the process of proving that an analytical method is suitable for its intended purpose and capable of producing reliable and consistent results. In pharmaceuticals, chemical manufacturing, and food safety industries, method validation is essential for ensuring product quality, safety, and efficacy.
The main parameters evaluated during the validation process such as Accuracy, Precision, Specificity, Linearity, Sensitivity, Robustness
2.2.1 Accuracy
The recommended method's accuracy was verified through the standard addition method at 50%, 100%, and 150% concentrations. This involved adding stated concentrations of pure drug solutions to a predetermined amount of the Calcium Pantothenate tablet sample (10µg/mL) and measuring the absorbance at the respective wavelength. The analysis of the percentage recovery at each level was conducted.
2.2.2 Precision
Precision studies were conducted to evaluate intraday and inter-day variations of Calcium Pantothenate at 10µg/mL concentrations. The stated concentration was subjected to analysis for 6 times. The % RSD can be evaluated for obtained absorbance values.
2.2.3 Specificity
Specificity refers to the capability of the method to accurately detect the analyte in the presence of other potentially interfering components. The specificity of the developed method for determining calcium Pantothenate in tablet dosage form was assessed by comparing the spectral characteristics of the tablet solution to those of the standard solution. The sample spectrum was thoroughly examined to identify any potential interferences arising from the presence of excipients
2.2.4 Linearity
The method's linearity means concentration immediately affects test findings. The linearity of the current approach was tested by measuring absorbance 212nm for calcium Pantothenate concentrations from 5 to 25 ?g/mL. Finally, the concentration-absorbance linearity graph was plotted, and the regression coefficient (R2) was calculated.
2.2.5 Sensitivity
Standard deviation equations were utilized to calculate LOD and LOQ.
LOD=3×?/S
LOQ = 10×?/S
Where,
? is the standard (SD) of the intercept
S -slope of the linear plot
2.2.6 Robustness
The maximum absorption wave length were purposely changed to test the method's resilience. After changing the wavelength maximum (± 2nm), % RSD can be evaluated for obtained absorbance values.
2.3 Assay of Dosage Form
The marketed 20 tablets were weighed and the powder equivalent to 10mg of Calcium Pantothenate was transferred into 100ml volumetric flask diluted to 100ml with diluent. The resultant solution was further diluted with the same diluent to obtain a solution containing 10µg/ml of Calcium Pantothenate. The sample solution was
filtered through the 0.25µm Nylon filter. Both sample and standard solutions of Calcium Pantothenate were analyzed. Assay of the compound was analyzed by following formula:
% Assay=ATASxWSDSxDTWTxP100xAVG WtLabel Claimx100
Where:
AT - Absorbance of sample (tablet) solution.
AS - Absorbance of standard solution.
WS - Weight of standard substance (mg)
WT - Weight of sample (tablet) powder (mg)
DS - Dilution factor of standard solution
DT - Dilution factor of sample solution
P - % purity of standard substance
AVG WT - Tablet weight in average (mg)
3. RESULTS AND DISCUSSION
3.1 Determination of Absorption Maxima (?max)
Fig 2, shows that scanned spectra of Calcium Pantothenate bulk and pharmaceutical dosage form shows absorption between 200 to 250nm. The maximum absorption (?max) scanned at 212nm.
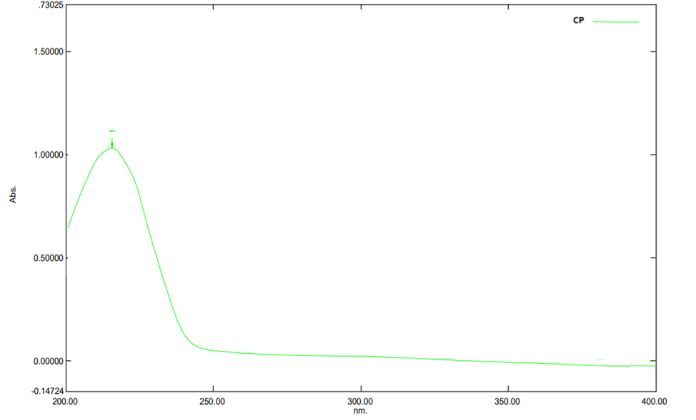
Figure 2. Absorption Maxima of Calcium Pantothenate
3.2 Method Validation
3.2.1 Linearity
The Calibration curve of Calcium Pantothenate shows good Linearity at concentration ranging from 5-25µg/mL.The graph were plotted against Absorbance vs Concentration and fit a linear Regression line: y=mx+b
The three trials was conducted as Set1, Set2, Set3 with same concentration.
The Correlation co-efficient (R?2;) is calculated.
r=n(?xy)-?x?y?n?x2-?x2n?y2-?y2
The correlation coefficient (R?2;) was
was within the working concentration.
The intercept and slope of Calcium Pantothenate were respectively.
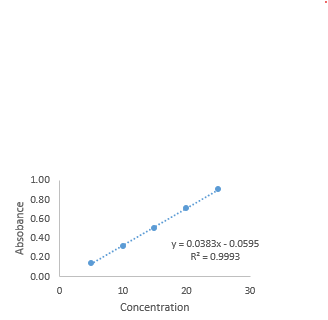
Figure-3: Linearity curve of Calcium Pantothenate at 212nm
The statistical analysis confirmed that the method is linear over a 5 to 25 µg/ml concentration range, with a strong correlation coefficient (R?2; = 0.999), ensuring consistent performance across different concentrations. Precision studies, both intra-day and inter-day, revealed %RSD values below 2%, indicating the method's repeatability and reliability. Accuracy was verified through the standard addition method, with recoveries within the acceptable range of 100±2%, proving the method’s ability to measure Calcium Pantothenate without interference from excipients accurately.
Moreover, the technique is highly sensitive, with a limit of detection (LOD) of 0.09 µg/ml and a limit of quantification (LOQ) of 0.3 µg/ml, making it suitable for detecting low concentrations. The method's specificity was confirmed by the absence of any spectral interference from excipients in the tablet formulations. Overall, this newly developed UV spectrophotometric method is a reliable, accurate, and sensitive approach for estimating calcium pantothenate making it ideal for pharmaceutical quality control.
Authors are thankful to Dr. Kalyani Peluri of the Department of Pharmaceutical Chemistry/Analysis, Faculty of Vydehi Institute of Pharmacy, for her assistance in the laboratory during this research.


 Kalyani Peluri*
Kalyani Peluri*
 Ambika S.
Ambika S.
 Pallavi K. C.
Pallavi K. C.
 Ruchitha M.
Ruchitha M.
 Tejaswini V.
Tejaswini V.








 10.5281/zenodo.14028818
10.5281/zenodo.14028818