Abstract
The prevalence of liver cancer has risen in several nations in recent decades, and it is an important contributing factor to the global cancer burden. As the primary histological type of liver cancer, Hepatocellular Carcinoma (HCC) is responsible for the majority of liver cancer diagnoses and deaths. HCC constitutes approximately 80-90% of all primary liver cancers and a high mortality rate while many detected in later stages of development and therapeutic options are limited. Worldwide, HCC is now the sixth most common cancer and fourth leading cancer related-death. Hepatitis B virus (HBV) and Hepatitis C virus (HCV) remain, at present, the most important global risk factors for HCC, with very poor prognosis. Unfortunately, the prevalence of metabolic syndrome, Obesity, type II diabetes and NON- alcoholic fatty liver diseases (NAFLD) are increasing and may jointly become the major cause of HCC globally. Over indulgence in drinking is still an uncontrollable risk factor. In united states, there has been only minimal improvement in the prognosis for HCC patients over 15 years. It's still unknown exactly how HCC progresses in terms of its molecular causes and other mechanisms. Consequently, there is urgent need for better understsanding of these mechanisms. This review provides summary of the known mechanisms that either cause HCC or contribute to its progression.
Keywords
Liver, Cancer, Hepatitis, Hepatocellular carcinoma, PI3K, mTOR, ? -catenin/Wnt pathway, VEGF signalling pathway, RAS/RAF/MAPK pathway, Fibroblast Growth Factor Pathway.
Introduction
The liver, which makes up 2% to 3% of the normal body weight, is the largest solid organ in the human body with two lobes, it is situated beneath the right hemidiaphragm in the right upper quadrant of the abdominal cavity. It is shielded by the rib cage and is held in place by peritoneal reflections, also known as ligamentous attachments. The liver performs around 500 different functions [1].

Figure 1: Anatomy of the liver from Johns Hopkins medicine of liver anatomy and functions [2]
FUNCTIONS OF LIVER
Hepatocytes, or liver cells, have a unique ability to proliferate in response to any type of liver injury [3]. This metabolically active liver performs a variety of essential functions, including the following

Flow chart 1: It represents different functions of liver.
DISEASE RELATED TO LIVER
- Hepatitis
Hepatitis is defined as inflammation of the liver that can results from a variety of causes such as heavy alcohol consumption, autoimmune conditions, drugs, or toxins. However, the most frequent cause of a hepatitis is due to viral infection and referred to as viral hepatitis. Hepatitis A, B, and C are the three most prevalent forms of viral hepatitis. The other types of viral hepatitis are hepatitis D and E are less frequently encountered [4].
- Fatty liver disease
Fatty liver disease can result from a build-up of fat in the liver.
There are two kinds of liver disease that are:
- Alcoholic fatty liver disease, a condition brought on by excessive alcohol intake.
- Non-alcoholic fatty liver disease (NAFLD) is a common cause of chronic liver disease worldwide. NAFLD is a spectrum of the characterized by hepatic steatosis when no other causes for secondary hepatic fat accumulation (e.g., excessive alcohol consumption) [5].
Both forms of fatty liver disease have the potential to damage the liver and result in cirrhosis and liver failure if left untreated. Changes in diet and lifestyle can typically alleviate symptoms and reduce the likelihood of problems.
- Autoimmune conditions
The exact etiology of autoimmune hepatitis is unknown. Various factors like drugs, environmental agents, or viral infections with hepatitis viruses or Epstein-Barr virus may trigger an autoimmune response. Patients develop autoantibodies, and they more commonly present in those who have chronic hepatitis C virus infection. In these cases, hepatitis improves when the patient stops the offending drug. [4]
- Primary biliary cirrhosis (PBC):
This is an autoimmune and progressive disease of the liver, which is the destruction of intrahepatic biliary channels and portal inflammation and scarring. It leads to cholestatic jaundice and fibrosis of liver parenchyma. PBC is more common in middle-aged women. Alkaline phosphatase levels increase in PBC.
- Primary Sclerosing Cholangitis (PSC):
Commonly associated with ulcerative colitis. This condition is characterized by a decrease in the size of intrahepatic and extrahepatic bile ducts due to inflammation and fibrosis.
- Autoimmune hepatitis (AIH):
This is a form of chronic inflammatory hepatitis, more common in women than men, and is characterized by elevated autoantibodies such as antinuclear antibodies, anti-smooth muscle antibodies, and hypergammaglobulinemia.[6]
- Genetic conditions
The liver can also be impacted by a number of hereditary disorders.
- Alpha-1 antitrypsin deficiency:
This is the most common genetic cause of CLD among children.
- Hereditary hemochromatosis:
It is an autosomal recessive disorder of iron absorption. Here due to a mutation involving the HFE gene that regulates the iron absorption from the intestine, excessive iron is absorbed from the gastrointestinal tract. As a result, there is a pathological increase in total body iron (such a ferritin and hemosiderin). This process leads to the generation of hydroxyl free radicles, which in turn cause organ fibrosis.
Autosomal recessive disorder leading to copper accumulation.[7]
- Drug-induced liver disease
According to a 2019 study, over exposure to several medications and supplements may harm the liver. Drug induced liver injury (DILI) is an adverse toxic drug reaction resulting in liver injury. It is an uncommon occurrence with estimated incidence of 14-19 cases per 100,000 population, accounting for less than 1% of acute liver injury (ALI). Between 1998 and 2007, the United States Acute Liver Failure Study Group (ALFSG) network group identified that paracetamol was the commonest cause of ALI (46%), followed by determinate DILI (15%) and idiosyncratic DILI (12%). Idiosyncratic DILI was attributed to anti-tuberculous medications, sulpha compounds, phenytoin and herbal and dietary supplements. [8]
- Liver failure
Acute liver failure (ALF) is defined as the acute development of jaundice, synthetic failure and hepatic encephalopathy in patients without a previous history of liver disease. It is a severe and complex condition that results from acute and massive hepatocellular destruction. ALF is an infrequent condition, with an incidence of 1-8 cases per million inhabitants, and it is responsible for 6% of deaths due to liver disease and up to 7-8% of liver transplants. ALF mainly affects young adults, with a peak age between 35 and 45 years. Women account for approximately 60% of cases. The development of cerebral oedema, sepsis and multiple organ failure are the main causes of mortality. [9]
Main aetiology of Acute Liver failure:
- Acetaminophen intoxication, Hepatitis B virus, Hepatitis C virus, Hepatitis E virus, Cytomegalovirus, Epstein Barr virus, Herpes simplex virus, Varicella Zoster virus, Autoimmune hepatitis, Wilsons disease, Budd- Chiari syndrome, Drug-induced liver injury (idiosyncratic reaction), Amanita phalloides intoxication, Acute fatty of pregnancy, HELLP syndrome, Ischaemic hepatitis.
Chronic liver failure usually occurs when the liver is severely damaged and unable to function correctly. Liver failure associated with cirrhosis and liver disease typically progresses slowly. Some symptoms are:
- Rash,
- Diarrhoea,
- Bewilderment,
- Exhaustion and weakness,
- Queasy
Acute- on- chronic liver failure (ACLF) is a frequent complication in liver cirrhosis that has high short-term mortality. It is characterized by acute decompensation (AD) of liver cirrhosis, intra – and extrahepatic organ failure, and severe systemic inflammation (SI). AD in patients with liver cirrhosis-related complications such as cute gastrointestinal bleeding, development of ascites, HE (Hepatic Encephalopathy), and bacterial infection. The Chronic Liver Failure Acute- on- Chronic Liver in cirrhosis (CANNONIC) study was able to determine major risk factors for mortality in patients presenting with AD, and defined ACLF as a distinct syndrome patient with decompensated cirrhosis.[10]
- Cirrhosis
The term "cirrhosis" describes the scarring caused by liver disorders and other liver damage
Sources, like alcohol use disorders. Cirrhosis is a severe form of liver disease wherein normal liver tissue is replaced by scar tissue (fibrous tissue).
Cirrhosis is a condition in which the liver slowly deteriorates and malfunctions due to chronic injury. Scar tissue replaces healthy liver tissue, partially blocking the flow of blood through the liver.

Figure 2: Cirrhosis effected liver. [12]
Cirrhosis is widely prevalent worldwide and can be a consequence of different causes, such as obesity, non- alcoholic fatty liver disease, high alcohol consumption, hepatitis B or C infection, autoimmune diseases, cholestatic disease and iron or copper overload. [1]
Differences between Cirrhosis and Liver Cancer

Table 1: This table contains differences between liver cancer and cirrhosis.
- Other liver disease
Alcohol abuse, regular heavy drinking may lead to liver disease. There is a higher chance of developing hepatitis and cirrhosis, an irreversible and progressive condition. [19] Drug overdoses, large doses of many medicines can damage a normal liver. Drug-induced liver injury (DILI) is adverse toxic drug reaction resulting in liver injury. It is an uncommon occurrence with an estimated incidence of 14-19 cases per 100,000 population, accounting for less than 1% of acute liver injury (ALI). Nevertheless, DILI is the most common cause of acute liver failure (ALF) in the West, with a case fatality rate of 10-50%, although no definite causative agent has been attributed several cases. Such potentially fatal events have a critical impact on policymaking to both tighten pharmaceutical restriction of and, at times, complete market withdrawals of drugs. Between 1998 and 2007, the United States Acute Liver Failure Study Group (ALFSG) network group identified that paracetamol was the commonest cause of ALF (46%), followed by determinate DILI (15%) and idiosyncratic DILI was attributed to Anti-tuberculous medications, sulpha compounds, Phenytoin and herbal and dietary supplements. [ 20,21,22]

Flowchart 2: Classification of Drug induced liver injury, Medication Known to cause hepatocellular, Cholestatic or mixed pattern of liver injury. [21]
- NASH- associated HCC
Numerous organ cancers are associated with an increased risk of obesity. Obesity can lessen systemic alterations, which are characteristic of many cancer types and include alterations in immune function and systemic endocrine changes. According to available data, fatty liver disease is quickly overtaking other causes of HCC in the western world. Research has shown that oxidative stress, pathological inflammatory reactions, changed endocrine or adipokine signalling, altered immunological function, and metabolic and oxidative stress are fatty liver-specific pathways by which NAFLD or NASH induce HCC. [22]
- Cancer
In liver cancer is a cancer that originates in the liver, and is an aggressive tumor that frequently occurs in the setting of chronic liver disease and cirrhosis. Primary liver carcinoma (HCC), is the fifth most common cancer in males and the seventh most common cancer in females, and is the third leading cause of cancer of cancer-related death worldwide. [23]
LIVER CANCER
According to WHO Liver cancer is malignant tumor that starts in the cells of the liver. It arise from various causes, including hepatitis, infections, excessive alcohol consumption, or other factors leading to damage and abnormal cell growth. [24]
Types of liver cancer
Although the most common liver cancer types and primary and secondary liver cancer, this condition is categorized based on the characteristics and types of abnormal cells found in the liver. Primary liver cancer Originates in the liver where abnormal cells begin dividing and multiply rapidly.
- Hepatocellular carcinoma (HCC):
Also called hematoma, this is the most common type of liver cancer. It starts in the hepatocytes, the main cell type in the liver. HCC accounts for about 75% of all liver cancers.
Also known as bile duct cancer. It occurs in the ducts that drain bile from the liver to the small intestine and it is accounts for 10-20%.
Rare type, Idiopathic type of carcinoma.
Blood vessels in the liver, 1%.
Secondary metastatic liver cancer: It occurs when cancer spreads to the liver
From other parts of the body. [25]
The most common types of liver metastasis are:
- Colorectal cancers.
- Gastrointestinal cancers.
- Melanoma.
CLINICAL FEATURES /SYMPTOMS OF LIVER CANCER
Fatigue or weakness, Jaundice, Loss of appetite, Hard lump on the right side, below the rib cage, Pain or swelling in the upper abdomen, Pain around the right shoulder blade, Unexplained weight loss, Fever, Jaundice, which causes dark urine and yellow skin and eyes, feeling of fullness after a small meal, Itching, Swollen veins on the belly, becoming sicker if have hepatitis or cirrhosis.
Certain types of liver cancer also produce hormones that may cause:
- High blood calcium levels may cause constipation, nausea, or confusion.
- Low blood sugar levels may cause tiredness or a faint feeling.
- Enlarged breasts or shrinking of testicles in men.
- A high red blood cell count may cause redness in the face.[26]
STAGES OF LIVER CANCER
The American Cancer Society states that there are four stages of liver cancer: I (1) to IV (4). The lower the number, generally speaking, the less the cancer spread. A higher number, such stage 4, indicates more metastases of the malignancy.
Typical cancer stages include:
Stage 0: Very early
Stage 1: Preliminary
Stage 2: Immediate
Stage 3: Progression
Stage 4: Final stage
Stage 1 liver cancer
No blood vessels have formed into the solitary main tumor, regardless of size. Neither distant sites nor neighbouring lymph nodes have seen the cancer's spread. We have two subcategories for this stage.
- Stage 1A: A primary tumour that is two centimetres or less.
- Stage 1B: More than two centimeters surround the main tumor.
Stage 2 liver cancer
Either a single primary tumor (of any size) has invaded blood vessels, or there are several tumors (none larger than 5 cm). Neither distant sites nor neighbouring lymph nodes have seen the cancer's spread.
Stage 3 liver cancer
We have two subcategories for this stage.
Multiple tumors have been detected, with at least one of them measuring more than 5 cm. Neither distant sites nor neighbouring lymph nodes have seen the cancer's spread.
Multiple tumors have been identified, and at least one of them is expanding into a hepatic vein or portal vein branch. Neither distant nor nearby lymph nodes have been affected by the liver malignancy.
Stage 4 liver cancer
Stage 4 liver cancer can have distant internal locations or adjacent lymph nodes that have been affected by the malignancy. Although metastasis from advanced liver cancer is uncommon, it usually occurs to the lungs and bones. There are two subcategories in this step:
One or more tumors, irrespective of size, have been detected, and the cancer has progressed to neighbouring lymph nodes but not to remote locations.
Any number of tumors, regardless of size, have been discovered. The cancer has migrated to distant organs like the lungs or bones, although it may or may not have reached neighbouring lymph nodes.

Figure -4 the different stages of liver cancer. [27]
For general health and wellbeing, a healthy liver is essential. On the other hand, hepatotoxicity brought on by some environmental pollutants, unhealthy eating patterns, alcohol use, and some prescription medications can result in serious illnesses such cirrhosis, hepatitis, and alcoholic liver disease, which can ultimately lead to hepatic cancer. Hepatocellular carcinoma is the most prevalent type of liver cancer, also known as hepatic cancer, which is a potentially fatal illness. Other etiological variables linked to hepatocellular carcinoma (HCC) include non-alcoholic fatty liver disease, alcoholic liver disease, and chronic viral hepatitis B and C.[25,28]
HEPATOCELLULAR CARCINOMA
Primary hepatic cancer (HCC) is the most prevalent type of liver cancer. With almost half a million new cases diagnosed each year, it is the fifth most prevalent cancer in the world among men and the seventh most common among women. It ranks as the second most common cause of cancer-related mortality worldwide. Since the majority of instances of hepatocellular carcinoma (HCC) are caused by chronic liver disease caused by hepatitis B virus (HBV) or hepatitis C virus (HCV), preventive interventions are strongly recommended. [22]
DIAGNOSIS/SCREENING
High risk individuals include those with hepatitis C and B, patients with alcohol-related cirrhosis and other alcohol abusers should have regular screening for living cancer.
PHYSICAL EXAMINATION
- Abdomen to check for lumps, swelling, ascites
- Changes in the liver, spleen.
- Signs of jaundice- yellowing of the skin and sclera.
- LFT – AFP (alpha fetoprotein), a type of protein, is produced by liver tumors.
IMAGING SCANS
- USG
- CT- to measure the tumour’s size.
- MRI- To identify the extent of the outbreak.
- PET scans- liver scans.
- Biopsy- a small sample of tissue is removed and analysed. The analysis can reveal whether the tumor is cancerous (malignant) or non-cancerous (benign).
- Arteriography.
- Leucocytosis, erythrocytosis, hypercalcemia, hypoglycaemia and hypocholesterolaemia.
- Molecular testing- To identify specific genes, proteins, to plan for targeted therapy.[25]
EPIDEMIOLOGY
Liver cancer is the third most common cause of cancer- related deaths in the world and has become an urgent problem for global public health. It is divided in to primary and secondary liver cancer. Primary liver cancer includes hepatocellular Carcinoma (HCC) [29]. As the principle histologic type of liver cancer, hepatocellular carcinoma (HCC) is responsible for the great majority of liver cancer diagnoses and deaths. Hepatitis B Virus (HBV) and hepatitis C Virus (HCV) remain, at present, the most important global risk factors for HCC, but it is likely their importance will decline in coming years. The effect of HBV vaccination of new-borns, already seen in young adults in some countries, will be more notable as vaccinated cohort age. IN addition, infections with both HBV and HCV should contribute to declines in the rate of viral-associated HCC. Primary liver cancer is the seventh most frequently occurring cancer in the world and second most common cause of cancer mortality. [30,31] The highest incidence rates in the world are found in Asia and Africa. Mongolia has the highest incidence at 93.7 per 100,000, but China has the greatest number of cases, due to both an elevated rate (18.3 per 100,000) and the world’s largest population (1.4 billion persons).[37] Liver cancer ranks sixth globally among all malignancies and is the fourth leading cause of cancer-related deaths globally, with 841,080 new cases reported in 2018. East Asia and Africa have the greatest rates of HCC incidence and mortality, however certain regions of Europe and the USA are seeing increases in these rates as well. In fact, since the early 2000s, HCC has been the leading cause of cancer-related deaths in the United States, according to surveillance epidemiology end results (SEER). If current trends continue, HCC is expected to overtake all other causes of cancer-related deaths by 2030. It is anticipated that between 2020 and 2040, there will be a 55% increase in the annual number of new instances of liver cancer worldwide, with 1.4 million new cases expected to be detected by that time. Furthermore, it is estimated that 1.3 million liver cancer deaths would occur globally in 2040—a 56.4% increase over 2060. According to NCR (National Cancer Institute) of Surveillance, Epidemiology, and End Results Program (SEER) Compared to other cancers, liver and intrahepatic bile duct cancer is relatively rare. [30]

Table 2: Compared to other cancers, liver and intrahepatic bile duct cancer is relatively rare.[30]


Figure 5: Cancer incidence and mortality rate worldwide. [31]
Data source:
GLOBOCON 2020.
Graph Production:
IARC,World Health Organization.

Figure 6: Global age-adjusted incidence rates of liver cancer, estimated for 2020.[31]
Data Source:
GLOBOCAN 2020.
Graph production:
IARC, World Health Organization.
MOLECULAR PATHOGENESIS
HCC pathophysiology is a multifaceted, intricate process. The early stages of hepatocyte malignant transformation and the development of HCC are caused by the interaction of several variables. These variables include the degree of the underlying chronic liver disease, the cellular milieu and different immune cells, the reciprocal interactions between viral and non-viral risk factors, and a genetic predisposition. One of the main factors that makes cancer possible is a changed microenvironment, which is known to have a role in the entire malignant development process, starting with the early stages of transformation and continuing through invasion and metastasis. The primary oncogenic triggers and signalling pathways connected to the start, growth, and spread of HCC were covered in our earlier primer.
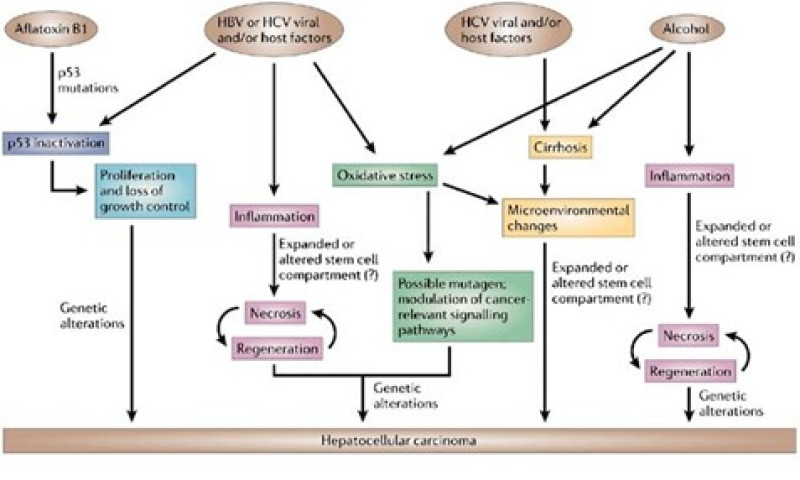
Figure 7: Mechanisms of hepatocarcinogenesis.
(The suspected mechanisms of hepatocarcinogenesis for the various risk factors are show.) Commonalities are indicated using the same colour. In addition to these mechanism, hepatitis B virus (HBV) and aflatoxin B1 share the characteristic of affecting the genome-HBV can integrate in to the host genome and aflatoxin B1 is a mutagen. HCV, hepatitis C virus.[32] In spite of liver cancer's variability both inside and between various tumors, the cell of origin of HCC is still unknown. Previous research has indicated that liver stem cells may be the cause of HCC, as they have been in many other cancer forms. However, mature hepatocytes or a transit-amplifying population may also be to blame. The possibility that matures hepatocytes, rather than progenitor cells, are the cellular source of HCC is supported by certain preclinical murine models of the disease. Hepatocytes may undergo this transformation through a series of genetic changes, dedifferentiation into hepatocyte precursor cells, which subsequently develop into HCC cells that express progenitor cell markers, or transdifferentiation into biliary-like cells, which results in intrahepatic cholangiocarcinoma. The latter two scenarios suggest extraordinary hepatocyte cell plasticity. A combination of the amplification of areas that include FGF19 (5% of tumors) and mutations in RPS6KA3 and RSK2 (5–9% of instances), the receptor tyrosine kinase (RAS-RAF-MAPK), phosphatidylinositol-3-kinase, protein kinase B, and mammalian target of rapamycin (PI3K-AKT-mTOR) pathways are typically activated in HCC. The inactivation of Kelch like ECH-associated protein 1 (KEAP1) or activating mutations in nuclear factor erythroid 2-related factor 2 (NFE2L2 or NFR2) also trigger the oxidative stress signaling system. Chromosome regions 11q13 and 6p21 are the sites of DNA amplifications that impact the oncogenes cyclin D1 (CCND1) and neoangiogenic vascular endothelial growth factor A (VEGFA), respectively. The latter promotes tumor proliferation by secreting hepatocyte growth factor (HGF) that is mediated by macrophages. Regretfully, the majority of HCC mutations arise in non-druggable pathways such the TERT promoter, p53, or WNT-?-catenin, and the mutations found in more readily treatable targets are found in only a small fraction of patients, making it challenging to administer certain medicines. A molecular classification of HCC has been established by the use of genomic, transcriptomic, and epigenomic profiling investigations. This classification correlates with clinical features even though it hasn't been applied in clinical practice yet. The proliferation class and the non-proliferation class are the two recognized molecular subtypes. The proliferation class is characterized by cell proliferation and survival pathways like PI3K-AKT-mTOR, RAS-MAPK and MET, chromosomal instability, TP53 inactivation, amplifications of FGF19 and CCND1, and overexpression of ?-fetoprotein. This proliferation class has a poor clinical outcome and is linked to HBV infection. Conversely, tumors in the non-proliferation class frequently have increased TERT promoter mutations and CTNNB1 activation.[33

Table 3: Signalling in HCC. [34]
Different Cellular Signalling Pathways Linked to HCC
Growing research on tumor signal transduction pathways demonstrate that aberrant activation of several molecules in various signalling pathways controlling cell cycle, proliferation, differentiation, cell survival, and apoptosis causes HCC progression.
- Wnt/?-catenin signalling pathway in the liver physiological process
Wnt/?-catenin pathway in liver disease
The liver is a “metabolic factory” in humans. The Wnt/?-catenin pathway is associated with several common liver diseases, including cholestasis, liver fibrosis, fatty liver, and polycystic liver disease. Differentiated hepatocytes multiply to rebuild the liver during acute liver injury, which is one of the liver's unique physical characteristics. An essential regulator that governs liver growth, metabolic liver zonation, and liver regeneration following liver damage is the Wnt/?-catenin pathway. Mutations of several components of the system, especially the silence or mutation of the Wnt tumour suppressor, which is common in many cancer types, are commonly the source of dysregulation of Wnt/?-catenin signalling.[35] Cell surface ligands known as Wnt ligands are important for maintaining healthy liver function. They assemble into complexes with LRP-5/LRP-6 coreceptors and Frizzled receptors. ?-catenin binds to a variety of tumor suppressor proteins, including APC, axin, and Ser/Thr kinase GSK3?. APC and axin proteins phosphorylate ?-catenin via altering the structure of GSK3?. This results in the cytosolic degradation of ?-catenin. Axin is recruited to the film of LPR-5 upon Wnt interaction with a ligand, which results in the inactivation of the ?-catenin destruction complex. [36, 37] This makes it easier for unphosphorylated ?-catenin to build up and migrate into the nucleus. To begin the TCF-LEF target gene, ?-catenin then forms a complex with a TCF-LEF group of DNA constraining record variables. A considerable portion of the target gene, cyclin D1, is involved in cell division.

Figure 8: Wnt/?-catenin signaling pathway in Hepatocellular carcinoma
A crucial step in the development of tumor metastasis is the process of cell-to-cell adhesion, which is carried out by phosphorylated ?-catenin binding with E-cadherin. Strong evidence of Wnt/?-catenin's involvement in liver cancer was discovered by the researcher. The majority of HCC cases had overexpressed levels of ?-catenin, which accumulates, promotes cell proliferation, and prevents differentiation. Additionally, partner ?-catenin transformations or start are thought to be associated with compounded HCC outcomes, such as larger tumor growth, increased vascular incursion, and point mutation or deletion. [37,38]
- VEGF signaling pathway:
A vital growth factor for angiogenesis during the development of hypervascular HCC malignancy is VEGF. Tumor cells that are buried deep must travel 100–200 ?m to obtain nutrients and oxygen necessary for their survival. A tumor larger than 2 to 3mm needed to undergo angiogenesis. VEGF-A, VEGF-B, VEGF-C, VEGF-D, and PIGF are the family's principal members; VEGF-A121 and VEGF-A165 are among their putative variants. Three primary subtypes of VEGF exist: VEGFR-1, VEGFR-2, and VEGFR-3. Each of them is implanted in the cell membrane; the intracellular region contains a split tyrosine kinase domain, while the extracellular region has a single TM seven immunoglobulin-like domain. After ligand binding, they get phosphorylated, activating PLC-?, PKC, and the MAPK signaling cascade. Additionally, activating endothelial NO stimulates cell proliferation and vascular permeability. Moreover, it makes Rho GTPase active. [45, 46] Above all, VEGFR-2 appears to play a major role in blocking almost all of the documented cell responses to VEGFs. VEGFR-3 is important for lymphangiogenesis, while VEGFR-2 initiates endothelial cells' proliferation, migration, increased endurance, and advancement of vascular penetrability. [38,40] The majority of HCC patients had VEGF mRNA articulation in their liver tumors. The liver's entrance vein is the primary route for HCC dissemination and metastasis, and the formation of portal vein tumor thrombus (PVTT) in HCC is closely correlated with VEGF mRNA expression. High VEGF articulation, as identified by immunohistochemistry, is significantly isolated from HCC and from the areas around the HCC tissues. [45, 48] The earliest evidence of the VEGF pathway's function in HCC came from therapy advances that hampered this pathway later in the process.

Figure 9: The Vascular endothelium growth Factor (VEGF) Hepatocarcinoma Pathogenesis.
- Ras/Raf/MAPK Corridor
Pathway of Mitogen-Activated Protein Kinase Signaling. Three members of the mammalian mitogen-activated protein kinase (MAPK) family are engaged in a range of cellular activities: p38, c-Jun NH2-terminal a kinase (JNK), and extracellular signal-regulated kinase (ERK). [33] The promotion of cell proliferation, migration, survival, and tumor growth is one of these functions of the ERK pathway. Tyrosine kinase domain activation in the ERK pathway is caused by ligand binding to receptor tyrosine kinases (RTKs). [38,43] that serve as GRB2 and SOS protein docking sites. As a result, MEK, Ser/Thr kinase RAF, and small GTPase RAS activate in a cascade. The different actions of transcription factors and the level of gene expression can be changed by ERK activation, which changes how the cell cycle progresses. Cell growth and proliferation are regulated by c-myc, which can be activated by phosphorylated ERK (ERK-P) [38,45].

Figure 10: The RAS/MAPK pathway Hepatocarcinoma Pathogenesis
- PI3/AKT/mTOR Corridor
The control of cell survival, metabolism, and apoptosis is mediated by this route. 30 to 50 percent of HCC cases have activation of this pathway. The signaling pathway for phosphatidylinositol-3 kinase (PI3K). The AKT/mammalian target of rapamycin (mTOR)/phosphatidylinositol-3 kinase (PI3K) signaling pathway belongs to a broad class of similar kinases with two subunits—catalytic and regulatory. It is an enzyme that transduces signals inside cells and has the ability to phosphorylate phosphatidyl-inositol's -OH group. A PI3K regulatory subunit called p85 interacts with phosphor-tyrosines on active receptor-type calcium channels (RTKs) to draw ligands to the plasma membrane and start the enzymes' processes. In response to PI3K activation, lipid second messenger phosphatidyl-inositol-triphosphate (PIT) is activated. The pleckstrin homology (PH) domain on the C terminus of AKT kinase, which binds to PIT and phosphoinositide-dependent kinase 1 (PKDK1), is the downstream ligand of PI3K. [38, 46] By attaching to and activating serine threonine kinase Akt, PI3 kinase (PI3K) phosphorylates the membrane lipid phosphatidylinositol 4, 5-biphosphate (PIP2). The tumor suppressor gene PTEN targets the lipid products of PI3K for dephosphorylation, acting as a negative regulator of this system in normal cells. By decreasing PIP3 levels and excessively activating the PI3/AKT/mTOR pathway, a PTEN mutation prevents apoptosis and encourages the development of tumors. Tumor grade, upregulation of p-AKT and p-mTOR, vascular invasion, intrahepatic metastasis, and matrixmetalloprotease-9 are all linked to PTEN loss.

Figure 11 : PI3K/AKT/mTOR signaling pathway in Hepatocellular Carcinoma.
Reduced PTEN expression has been connected to high tumor stage, low survival rate, and high recurrence rate in the pathophysiology of HCC. The PI3K/AKT/mTOR signaling pathway is elevated in the treatment plan, and inhibitors may be crucial.[47]
- Fibroblast Growth Factor Pathway.
The 20 ligands that make up the fibroblast growth factor (FGF) ligand family are linked to carcinogenesis and have an intracellular tyrosine kinase domain and an external transmembrane domain. [45,55] Numerous research findings indicated that FGF plays a crucial part in the development of chronic hepatitis. Following dimerization, the tyrosine kinase domains can initiate several intracellular signalling cascades. [38,49] Importantly, FGF-substrate2 (FRS-2) is an adapter of the FGF receptor that may bind diverse proteins, including as SOS and GRB2, and phosphorylate them to activate RAS-GTPase, which in turn activates different downstream signalling pathways, including PI3K/AKT, Wnt, and MAPK.

Figure 12: Fibroblast Growth Factor Signalling Pathway
Through angiogenesis, FGF's downstream signalling causes cancer. In the mouse model, the growth of HCC is markedly accelerated by FGFR1 overexpression. FGF8, 17, and 18 boost the survival of HCC cells, indicating a role in the disease's progression. FGF15, on the other hand, also encourages hepatocellular growth in mice, which may also be involved in the formation of HCC. Furthermore, activation of FGFR4 via the GSK3? signalling pathway by FGFR19 promotes the epithelial-to-mesenchymal transition. [38,50]
- Serine/Threonine Kinase (AKT) Pathway.
Another name for it is protein kinase B (PKB), which through several Ser/Thr kinase family members, including liver kinase B1 (LKB1), calcium/calmodulin-dependent protein kinase IV (CAMKIV), and sulfatase (SULF2), plays a significant role in angiogenesis in pathological conditions and tumor formation. LKB1 regulates apoptosis, proliferation, metabolism, and cell polarity through a variety of phenotypic manifestations. Pseudo kinase Ste20-related adaptor (STRAD?) and the scaffolding protein MO25 are activated and localized as a result of LKB1 phosphorylating at Ser428 in HCC, which phosphorylates AMP-activated protein kinase (AMPK) microtubule affinity-regulating kinase (MARK). A complex consisting of MO25 and STRAD? binds to LKB1, causing it to relocalize from the nucleus to the cytoplasm, where it promotes angiogenesis and cell proliferation. [51] Through a multitude of signaling pathways, calcium (Ca+2) functions as a second messenger to regulate a range of biological activities. It increases the affinity toward calmodulin-kinase, such as Ca+2± /CAM-protein kinase-IV (CAMKIV), via binding to downstream effector calmodulin (CAM). In HCC, CAMKIV expression is elevated and exhibits control of the cell cycle and proliferation of cells. [52]

Figure 13: Serine/Threonine Kinase (AKT) Hepatocarcinoma Pathogenesis.
When calcium is overexpressed in HCC, it binds to calmodulin, which then forms a complex with upregulated Ca+2/CAM dependent protein kinase-2 (CAMKK2). Additionally, this combination activates AMPK and CAMKIV, which promotes angiogenesis. Elevated sulfatase 2 (SULF2) in HCC is associated with a higher rate of tumor recurrence, hepatoblast phenotype, and enhanced tumor development. When the SULF2 enzyme is dephosphorylated, 6-O-desulfurase is produced.[53]
Current Treatments of HCC:
Therapies Surgical resection, trans-arterial chemoembolization (TACE), radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), and liver transplantation are still among the options for treating early to intermediate stages of HCC. Nevertheless, these operations are not appropriate for many patients, mostly those without cirrhosis, and frequently result in problems following surgery. The 2007 approval of the multi-kinase inhibitor sorafenib dramatically changed the way that intermediate and advanced stage HCC patients were treated. TACE plus pharmacological medications are a common combination used to advance intermediate HCCs since it is more effective than monotherapy. Significantly, the current therapy strategy for advanced HCC now includes regorafenib, cabozantinib, ramucirumab, nivolumab, and pembrolizumab as second-line add-on medications and sorafenib and lenivatinib as first-line options. This is because more drugs have been approved. Tyrosine kinase inhibitors (TKI) with several targets, including angiogenesis pathways and tumor cell proliferation, include sorafenib, regorafenib, lenvatinib, and cabozantinib. Since regorafenib is a sorafenib derivative, sorafenib and regorafenib have similar targeting profiles. Regorafenib also blocks v-raf murine sarcoma viral oncogene homolog B1 (B-RAF), proto-oncogene c-KIT (KIT), ret proto-oncogene (RET), angiotensin 1 receptor (TIE2), PDGFR?, and fibroblast growth receptors (FGFRs) 1 and 2. Sorafenib targets tyrosine kinase signaling, including rapidly accelerated fibrosarcoma (RAF), vascular endothelial growth factor (VEGF) receptors 1–3, and platelet-derived growth factor (PDGF) receptor ?. Tyrosine kinase inhibitor lenvatinib reduces angiogenesis by targeting tumor cell-expressed RET and KIT, fibroblast growth factor receptor (FGFR)1-4, and VEGFR1-3. According to the previously listed proteins, cabozantinib also targets AXL receptor tyrosine kinase (AXL), MET (hepatocyte growth factor receptor), neurotrophic receptor kinase 2 (NTRK2), and Fms Related Receptor Tyrosine Kinase 3 (FLT3). Ramucirumab is a direct binding antagonist of VEGFR2 that prevents VEGF ligands -A, -C, and -D from binding. [54] As a first-line treatment for advanced HCC, sorafenib barely extends the median survival time by three months and benefits fewer than one-third of patients. Additionally, using chemotherapeutic medications after six months of starting treatment can result in drug resistance. Toxic side effects and drug inefficacy requiring greater dosages are some other side consequences of therapy. This is troubling since the first-line treatments now on the market are TKIs that target the PI3K/Akt/mTOR pathway. Consequently, in this review, we will discuss about the role of the PI3K/Akt/mTOR pathway in HCC, the efficacy of TKI drugs that are already available, and how they might be used in creative therapeutic approaches. [54,55]
CONCLUSION:
The prevalence of NAFLD/NASH is increasing and may soon over take viral factors as the major cause of HCC globally. Excessive alcoholic consumption also remains important risk factors. These has been minimal improvement in the progenesis for HCC patients over the past two decades. Uncertainty surrounds the precise molecular pathways of HCC growth, and a deeper comprehension of these mechanisms is important in order to design novel and efficacious therapeutic approaches as well as trustworthy prognostic markers. Additionally, a deeper comprehension of the mechanisms behind the development of HCC can support efforts to create potent prevention measures. This review provides a summary of HCC etiology, epidemiology and some of the well-established as well as a few recently proposed mechanisms of HCC progression.
REFERENCES
- Shrief R.Z. Abdel-Misih. MD and MarkBloomston.MD*: Liver Anatomy Surg clin North Am. 2010; 90 (4):643-653.
- Johns Hopkins medicine of liver anatomy and functions.
- Rola Ajjawi, Department of health research, International medicinal university, Kuala Lumpur, Malaysia study of liver anatomy and its functions (2022); JCMEDU-22-52444
- Parth Mehta; Anil Kumar Reddy Reddivari, oct24, Hepatitis stat pearl. 2022. PMID: 32119436.
- Sjaak Pouwels, Nasser Sakran2, Yitka Groham8.4, Angela Leal5, Tadeja pintar6 ,Wah Yang, Radwan Kassir, Rishisinghal, Kamal Mahawar,and Dharmanand Rammarain, non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss, BMC Endocr Disord. 2022:22:63. PMID: 35287643 (PubMed).
- Ashish Sharma: Shivaraj Nagalli: Chronic liver disease stat pearls (Internet) 2023 Jul3.
- Tom Hosack, Djamil Damry, and Sujata Biswas; drug induced liver injury: Mar 21, 2023.
- Maximiliano Rovegno, Magdalena Vera, Alex Ruiz, Carlos Benitez; current concept in acute liver failure: 10, 1016\j.aohep. 2019.04.08; July-Aug 2019:01.18. Issues 4. 543-552.
- Markus Kimmann. And Jonel Trebicka: acute-on-chronic liver failure: current Interventional treatment option and future challenges. pess Med 2023.13(7)1052.
- Pere Gines, Aleksander Krug, Juan and Abraldes, Elsa sola, Nuria Fabrellas, Patrick Kamath: liver cirrhosis; September 17, 2021.
- Liver cirrhosis: news-medical.net: eranicle\shutter stock.com.
- Mathias Pinter,1,2 Michael Trauncer, Markus Peck-Radosalvjevic1, 3and Wolfgang Sieghart1,4: cancer and liver cirrhosis: implications on prognosis and management: ESMO open 2016:1(2) Mar 17. PMID: 27843598.
- Dafina. Janevska. Viktorija Chaloska-Ivanova, and Vlado Janevski: hepatocellular carcinoma: risk factors, diagnosis and treatment open access Maced J. Med sci. 2015 Dec 15: 3(4):732-736.
- Gurtsevitch VE. Human Oncogenic Virus: Hepatitis B and Hepatitis C virus and their role in hepatocarcinogenesis. Biochemistry (Mosc) 2008; 73; 504-513. [PubMed] [Google scholar].
- Szabo E, Paska c, Kaposi Novak p, et al. similarities and differences in hepatitis B and C virus induced: hepatocarcinogenesis. Pathol oncol Res, 2004; 10: 5-11.
- Alan D, Herbst BA, Reddy KR. Risk factors for Hepatocellular carcinoma. Clinical liver disease. 2012:1 (6) (Google scholar).
- HSU Y-C, Yip TC-F, HO HJ, Wong VM-S, Huang Y-T, E1-Serag HB, et al. development of a scoring system to predict Hepatocellular carcinoma in Asians on antiviral for chronic Hepatitis B. Journal of Hepatology. 2018: 69 (2): 278-85.
- Hashem B. EL. Serag, Fashha Kanwal, Ziding Feng, Jorge A. Marrero. Saira khaderi, and Amit G. singal; risk factors for cirrhosis in contemporary Hepatology practices-findings from texas Hepatocellular carcinoma consortium cohort; Gastroenterology, 2020 Jul: 159 (1):376-377.
- What is alcohol use disorder and what is the treatment: Medically Reviewed by Timothy J. legg, Ph.D. PsyD-By Tim New man-updated on [November 28, Medical News today].
- Tom Hosack, Djamil Damry Sujala Biswas: Drug induced liver injury: a comprehensive review: therap Adv. Gastroenterol 2023:16:17562848231163410.
- Lee WM. Drug –induced acute liver failure. Clin liver Dis 2013; 17: 575-586, [ PMC free article] [ PubMed] [ Google scholar].
- Joseph M. LIOVET, Robin Kate Kelley, Augusto Villanueva, Amit G. singal, Eli Plkarsky, Sasan Roayaie, Riccardo Lencioh Kazuhiko Koike, Jessica Zucman-Russi, and Richards. Finn: (LIOVET, J.M.et al.) hepatocellular carcinoma, nature reviews disease, prim 7, Article number: 6 [2021].
- Chun-Yu Liu, Kuen-Feng Chen, and Pei-jer Chen: treatment of liver cancer: cold spring, Harb perspect Med, 2015 Sep: 5 (9).
- National cancer Institute.
- Slide share; liver cancer MS. Elizabeth.
- Mayo clinic: liver cancer symptoms and causes
- Image of liver cancer stages. \
- American cancer society; liver cancer stages.
- Song Gao, Xingyuge Jiang, Liang Wang, Shanshan Jiang, Hanyuan Luo, Yan Chen, and Cheng peng: the pathogenesis of liver cancer and the therapeutic potential of bioactive substance: front Pharmacol 2022: 13:1029601.
- Kartherine A. McGlynn. Jessica L. Petrick, and Hashem B. El – Serag: Epidemology of hepatocellular carcinoma: hepatology 2021 Jan: 73 (suppl 1): 4-13.
- Bray F, FerlayJ. Soerjomataram I, siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidensce and mortality worldwide for cancers in 185 countries, CA cancer J sclin 2018; 68; 394-424.
- Paraskevi A. Farazi & Ronald A. DePinho: Hepatocellular carcinoma pathogenesis: from genes to environment: Nature Reviews Cancer volume 6, pages674–687 (2006).
- Blanca Cucarull, Anna Tutusaus, Patricia Rider, Tania Hernaez, Carlos cuno, Pablo Garcia de Frutos, Anna Colell, and albert Morales: Hepatocellular Carcinoma: Molecular Pathogenesis and Therapeutic Advances: cancers 2022, 14, 621
- Jens U. Marquardt, Peter R. Galle?, Andreas Teufel: Molecular diagnosis and therapy of hepatocellular carcinoma (HCC): An emerging field for advanced technologies Journal of Hepatology 2012 vol. 56 j 267–275.
- Jiaqi Liu, Qing Xiao, Jiani Xiao, Chenxi Niu, Yuanyuan Li, Xiaojun Zhang, Zhengwei Zhou, Guang Shu & Gang Yin : Wnt/?-catenin signalling: function, biological mechanisms, and therapeutic opportunities: Signal Transduction and Targeted Therapy volume 7, Article number: 3 (2022).
- Finch P. W., He X., Kelley M. J., et al. Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proceedings of the National Academy of Sciences. 1997;94(13):6770–6775. doi: 10.1073/pnas.94.13.6770. [PMC free article] [PubMed] [CrossRef] [Google Scholar] [Ref list]
- Clevers H. Wnt/?-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [PubMed] [CrossRef] [Google Scholar] [Ref list]
- Divya Jain, 1 Yogesh Murti, 2 Wasi Ullah Khan, 3 Rajib Hossain, 4 Mohammad Nabil Hossain, 5 Krishn Kumar Agrawal, 6 Rana Azeem Ashraf, 7 Muhammad Torequl Islam, 4 Pracheta Janmeda, Yasaman Taheri, 8 Mohammed M. Alshehri, 9 Sevgi Durna Da?tan, 10 , 11 Balakyz Yeskaliyeva, 12 Aliya Kipchakbayeva, 12 Javad SharifiRad, and William C. Cho: Roles of Therapeutic Bioactive Compounds in Hepatocellular Carcinoma: Oxid Med Cell Longev. 2021; 2021: 9068850.
- R. Yamaguchi, H. Yano, A. Iemura, S. Ogasawara, M. Haramaki, and M. Kojiro, “Expression of vascular endothelial growth factor in human hepatocellular carcinoma,” Hepatology, vol. 28, no. 1, pp. 68–77, 1998.
- M. B. Thomas, R. Chadha, K. Glover et al., “Phase 2 study of erlotinib in patients with unresectable hepatocellular carcinoma,” Cancer, vol. 110, no. 5, pp. 1059–1067, 2007.
- M. J. Hawkins, “Clinical trials of antiangiogenic agents,” Current Opinion in Oncology, vol. 7, no. 1, pp. 90–93, 1995.
- S. M. Wilhelm, C. Carter, L. Y. Tang et al., “BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis,” Cancer Research, vol. 64, no. 19, pp. 7099–7109, 2004.
- E. K. Kim and E. J. Choi, “Pathological roles of MAPK signaling pathways in human diseases,” Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, vol. 1802, no. 4, pp. 396–405, 2010.
- Y. Keshet and R. Seger, “The MAP kinase signaling cascades: a system of hundreds of components regulates a diverse array of physiological functions,” in MAP Kinase Signaling Protocols, Springer, 2010.
- M. Soares-Silva, F. F. Diniz, G. N. Gomes, and D. Bahia, “The mitogen-activated protein kinase (MAPK) pathway: role in immune evasion by trypanosomatids,” Frontiers in Microbiology, vol. 7, p. 183, 2016.
- B. Vanhaesebroeck and M. D. Waterfield, “Signaling by distinct classes of phosphoinositide 3-kinases,” Experimental Cell Research, vol. 253, no. 1, pp. 239–254, 1999.
- S. Wullschleger, R. Loewith, and M. N. Hall, “TOR signaling in growth and metabolism,” Cell, vol. 124, no. 3, pp. 471–484, 2006.
- Beenken and M. Mohammadi, “The FGF family: biology, pathophysiology and therapy,” Nature Reviews Drug Discovery, vol. 8, no. 3, pp. 235–253, 2009.
- N. Turner and R. Grose, “Fibroblast growth factor signalling: from development to cancer,” Nature Reviews Cancer, vol. 10, no. 2, pp. 116–129, 2010.
- N. Zheng, W. Wei, and Z. Wang, “Emerging roles of FGF signaling in hepatocellular carcinoma,” Translational Cancer Research, vol. 5, no. 1, pp. 1–6, 2016.
- T. C. Delgado, F. L. Otsoa, and M. L. Martinez-Chantar, “Post-translational modifiers of liver kinase B1/Serine/threonine kinase II in hepatocellular carcinoma,” Journal of Hepatocellular Carcinoma, vol. 6, pp. 85–91, 2019.
- J. Li, C. Zhang, H. Jiang, and J. Cheng, “Andrographolide inhibits hypoxia-inducible factor-1 through phosphatidylinositol 3-kinase/AKT pathway and suppresses breast cancer growth,” OncoTargets and Therapy, vol. 8, pp. 427–435, 2015.
- R. M. Carr, P. A. R. Duran, E. J. Tolosa et al., “The extracellular sulfatase SULF2 promotes liver tumorigenesis by stimulating assembly of a promoter looping GLI1-STAT3 transcriptional complex,” Journal of Biological Chemistry: 2020 Feb 28;298(9)2698-2712.
- Sun, E.J.; Wankell, M; Palamuthusingam, P; McFarlane, C; Hebbard; Targeting the PI3K/Akt/mTOR Pathway in Hepatocellular Carcinoma: Biomedicines 2021,9(11),1639.
- El-Serag, H.B.; Marrero, J.A.; Rudolph, L.; Reddy, K.R. Diagnosis and Treatment of Hepatocellular Carcinoma. Gastroenterology 2008, 134, 1752–1763.


 Ch. Lakshmi Nandini*
Ch. Lakshmi Nandini*
 K.G. Rajya Lakshmi
K.G. Rajya Lakshmi


















 10.5281/zenodo.10839675
10.5281/zenodo.10839675