Abstract
Mucormycosis is a difficult condition to diagnose and treat. In developed nations, haematological malignancies are the most prevalent underlying disease, uncontrolled diabetes is the most common condition. Pulmonary mucormycosis is reportedly associated radiologically with multiple (?10) nodules and pleural effusion. An additional observation from a computerized tomography (CT) scan appears to be the reverse halo sign, which suggests the presence of mucormycosis. Diagnosis relies heavily on culture and direct microscopy on histopathology. Mucormycosis is a rare disease with a high morbidity and death rate that is challenging to diagnose. Disease tends to advance quickly, and diagnosis is frequently postponed. Medical and surgical intervention performed quickly can save lives. The prognosis may be improved with guidance on complex multidisciplinary management, although methods vary depending on the healthcare setting. There are not many regional differences in diagnostic management worldwide. If mucormycosis is suspected, appropriate imaging is strongly advised to determine the extent of the disease, and surgery is also strongly advised after that. It is challenging to diagnose mucormycosis. Mucorales frequently do not grow in microbiologic cultures when samples from suspected cases are used. According to the recently released guidelines by ECIL-6, antifungals is slightly enriched by the addition of two newer azoles (posaconazole and isavuconazole) to liposomal amphotericin B, which remains the drug of choice for the initial antifungal treatment. Mucormycosis (MCR) is an opportunistic fungal infection that can be fulminant and potentially fatal. Diabetes, immunocompromised states and increased serum iron levels are the most major risk factors for getting MCR infection. Due to a complex interaction between corticosteroid medication and metabolic variables, MCR co-infections have recently been observed in individuals with COVID-19 disease. The most prevalent clinical type
of metastatic cerebral reinfection (MCR) is rhino-orbito-cerebral mucormycosis (ROCM), which is defined as an infection of the nasal canals, paranasal sinuses, neck spaces, orbits, and intracranial structures
Keywords
Mucormycosis, Posaconazole, Isavuconazole, ROCM
Introduction
Mucormycosis is a new, rare fungal infection that causes significant morbidity and mortality. Mucormycetes are classified in the Mucorales order and the Mucoromycotina subphylum. Due to the disease's rarity, large, randomized clinical trials are nearly impossible to conduct and the majority of the available data on epidemiology, diagnosis and treatment come from case reports and series[1]. Mucormycosis need immediate management due to the infection's frequently rapid progression and destruction. Delayed starting of therapy correlates with greater mortality. Readily available guidance is necessary for achieving efficient diagnosis and treatment, as well as to optimize patient prognosis [2]. In a recent study of invasive fungal illness in hematopoietic stem cell transplant (HSCT) recipients, mucormycosis was the third most prevalent infection follow candidiasis and aspergillosis. Despite its rising incidence, mucormycosis is difficult to diagnose. Mucormycosis can be difficult to distinguish from other frequent invasive mold infections, such as aspergillosis, both radiographically and clinically. Histopathology is considered the "gold standard" for diagnosis. However, histopathologic identification of Mucorales in tissue specimens takes extensive pathological skill and does not provide species identification [3]. In a French research , mucormycosis incidence increased by 7.3% year, notably in individuals with neutropenia. These infections are challenging to treat for a variety of reasons. First, diagnosis is problematic due to clinico radiological parallels with invasive aspergillosis and a historical absence of diagnostic instruments. However, new methods in serum and tissue, as well as the detection of highly suggestive radiological indications, have updated diagnostic options[4]. Secondary, treatment is an emergency that combines surgery, which is frequently required due to the angio-invasive and necrotic nature of the illness, with antifungal therapy. Primary in vitro resistance to several antifungal medications restricts therapeutic options[5]. The current guidelines in haematology are limited to specific patient categories or geographical regions, and they need to be updated. Recent key developments have radically altered the management of this illness[2]
Epidemilogy
The most frequent causes of mucormycosis are Lichtheimia spp, Rhizopus spp, and Mucor spp. Other Mucorales genera that are less prevalent are Rhizomucor , Saksenaea, Cunninghamella, and Apophysomyces. The etiology of mucormycosis differs greatly throughout nations. For example, in patients with mucormycosis in Europe, Rhizopus spp. (34%), Mucor spp. (19%), and Lichtheimia spp. (19%) were commonly observed[6]. A large percentage of cases of mucormycosis arise by direct organism inoculation into damaged skin or gastrointestinal tract mucosa, or by inhaling fungal sporangiospores that have been released into the air. Mucormycosis incidence varies seasonally; most infections happen between August and November. Trauma patients were more frequently infected with uncommon, non-Rhizopus spp.; patients infected with Apophysomyces spp. or Saksenaea spp. were all immunocompetent, had mostly acquired infection through trauma, and had infection frequently localized to the skin, soft tissues, and bones, according to a recent study that presented the epidemiology of mucormycosis in Australia[7]. The condition is still rare in rich countries, where it primarily affects people with hematological malignancies (HM). However, mucormycosis is more frequent in underdeveloped nations, particularly India, where cases are primarily associated with trauma or uncontrolled diabetes mellitus (DM). As a result, mucormycosis prevalence ranges from 0.01 to 0.02 per 100,000 people in Europe and the US, but it is significantly higher in India (14,000 per 100,000 people)[8].
Symptoms
62 patients out of 400 reported symptoms other than those typical to rhino-orbito-cerebral mucormycosis. Along with the typical symptoms of rhino-orbito-cerebral mucormycosis, 34 patients complained of facial palsy, 19 of gum ulcers, 6 of cheek infections, and 2 of nose maggots. The palate or nasal mucosa may occasionally display necrotic lesions. Intraocular hyphae cause thrombosis and gradual necrosis of the tissues, which may affect the palate, orbital or sinusoidal bones, nasal septum, and sinus lining. Symptoms that may be present include mucosal necrosis, fever, proptosis, ophthalmoplegia, orbital cellulitis, discomfort, and purulent nasal discharge. Phonotypic or hemiplegic symptoms, seizures, cavernous sinus thrombosis, and progressive expansion of necrosis to the brain are possible outcomes [17]. Where the fungus is growing in the body determines the symptoms of mucormycosis.
Symptoms includes –
- One sided facial swelling
- Fever
- Nasal or sinus congestion
- Headache
- Black lesions on nasal bridge or upper inside of mouth that quickly become more severe
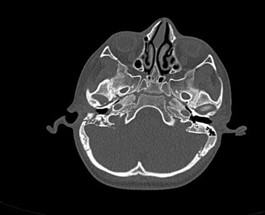
Fig no 1 Facial Palsy

Fig no 2 Nose Maggots
Clinical Manifestation Of Mucormycosis
Sporangiospore inhalation appears to be the primary route of infection in immunocompromised patients, leading to pulmonary illness. individuals with graft-versus-host disease and significant neutropenia are more likely to develop pulmonary mucormycosis, whereas individuals with diabetes usually present with rhino-orbital illness. Due to histocompatibility discrepancies, immunocompetent T cells in the graft attack immunodeficient recipient tissue within 100 days, resulting in tissue damage and graft-versus-host disease (GVHD).
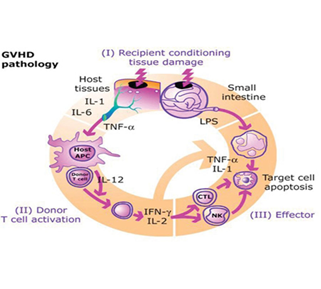
Figure no 3 GVHD pathology
Aspiration of hyphae from an upper respiratory tract infection focus, or fertilization of inhaled sporangiospores, leads to pulmonary mucormycosis, which is caused by the fungus growing within the airways. Because the fungus is so invasive, it can cause thrombosis and lung damage by penetrating the bronchial wall and entering the nearby blood vessels, especially the arteries [9]. The most prevalent types of mucormycosis in immune-compliant people are cutaneous and soft-tissue types, which usually develop due to skin disturbance from burns or traumatic injury surgery. Typical appearances include eschars, dry ulcers, necrosis, skin swelling, and abscesses [10]
Rhino – orbital -cerebral mucormycosis (ROCM)
An infectious disease known as mucormycosis (MCR) is brought on by fungus of the Mucorales group. Species from the genera Rhizopus, Lichtemia, and Mucor are frequently recorded. Saprophytic in nature, these fungi are frequently found in soil, decomposing food, and animal waste. Spore inhalation is the primary form of transmission for human infection; Cutaneous and rhino- orbito – Cerebral mucormycosis contaminated food consumption and traumatic injections are less prevalent routes of transmission [18]. Diabetes patients rarely get lung infections, but they frequently get rhino-orbito-cerebral mucormycosis. Patients with haematology have also reported experiencing it.Typically, paranasal sinuses are the source of rhino-orbital-cerebral infections, which cause bone loss and encroach on the orbit, eye, and brain. ROCM denotes a fulminant infection brought on by Mucorales species that affects the nasal canals, paranasal sinuses, neck spaces, orbits, and intracranial structures. Acute settings are similar to sinusitis in how ROCM manifests itself [10].
Diagnosis –
The primary methods of the diagnosis of MCR are direct microscopy, histology, and fungal culture of surgical tissues. Mucorales hyphae have a wide, non-septate, branching (90? bifurcations) morphology and can be identified by direct microscopy or a wet mount preparation with potassium hydroxide (KOH). On histology, neutrophilic or granulomatous inflammation is the most common finding. Angioinvasion and prominent necrosis are frequent. For multiple carcinogenesis, histopathological tissue diagnosis remains one of the most reliable and efficient diagnostic methods. On Sabouraud dextrose agar, all Mucorales species grow quickly [3–7 days] at a temperature of 25–30 °C[19].
Treatment –
A high index of clinical suspicion, fast laboratory and radiographic diagnosis, and multidisciplinary management are necessary for the effective treatment of ROCM. Effective management of ROCM involves accurate surgical debridement, removal of the underlying risk factors, and antifungal therapy with 5–10 mg/kg liposomal amphotericin B. Extensive debridement, turbinectomy, palatal resection, lamina papyracea resection, and possibly orbital exenteration are all part of the surgical therapy. Amphotericin B injections in the retrobulbar region and/or sinus irrigation at doses of 3.5 mg/ml and 1 mg/ml, respectively, are possible[20].
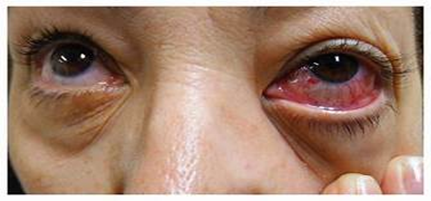
Figure no 4. Proptosis, palpebral erythema and cavernous sinus syndrome, 7 days after symptom onset in uncontrolled diabetes
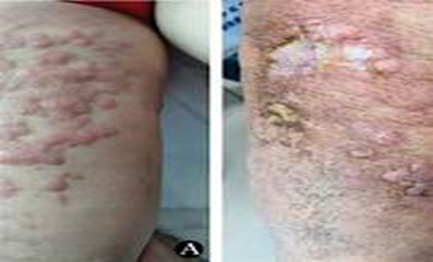
Figure no 5. Extensive primary cutaneous mucormycosis of the left leg due to Apophysomyces variabilis
Diagnosis –
The availability of imaging methods, skilled staff, and mycological and histological studies are necessary for the diagnosis of mucormycosis. Referrals to the highest level of treatment should be made right away for patients suspected of having mucormycosis. Should there be any delay, management ought to start by adhering to this guideline.
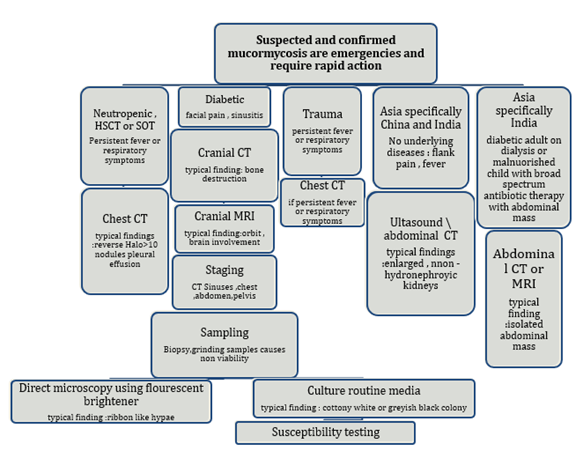
Figure no 6. Diagnosis pathway for mucormycosis
HSCT – haematopoietic stem cell transplantation, SOT – solid organ transplantation.
Recommendation –
A pulmonary CT scan is advised for patients with suspected pulmonary mucormycosis and a haematological malignancy in order to detect the reversed halo sign, vascular occlusion on CT pulmonary angiography, or a region of ground glass opacity surrounded by a ring of consolidation on thoracic CT. It is highly advised to perform a cranial CT or MRI in diabetic patients who have face pain, sinusitis, proptosis, ophthalmoplegia, newly diagnosed amaurosis, or both in order to ascertain whether sinusitis is present[11]. Due to its greater sensitivity than a CT scan, an MRI should be performed instead if disease of the eye or brain is suspected.
Treatment –
A multimodal approach is required for the successful management of mucormycosis. This approach involves the use of various adjunctive therapies, early administration of active antifungal agents at the optimal dose, complete removal of all infected tissues and if possible reversal or absence of underlying predisposing factors. The lowest dose of immunosuppressive medications, such as corticosteroids should be gradually reduced. Timely diagnosis is essential to start therapeutic measures that minimize the impact of deformity corrective surgery improve result and survival and stop progressive tissue invasion and its terrible after effects[12]. Likewise, mucormycosis is identified by a large-scale angioinvasion that results in tissue necrosis and vascular thrombosis. Hematogenous dispersion of the organism is the outcome of angioinvasion, while immune cells and antifungal agents cannot reach the infection focus due to necrosis of the afflicted tissues. Compared to other fungi like Aspergillus or Candida, some Mucorales like R. oryzae are less susceptible to innate host defense, which makes them harder to treat and consequently more likely to cause mortality[1]. A further salvage treatment option that ECIL-6 suggests is a lipid amphotericin B combination with either caspofungin or posaconazole. The use of two antifungals as first line therapy is not supported by any data. A propensity score analysis was used in a recent study to compare the effects of combination therapy versus monotherapy in a group of 106 patients with hematologic malignancies. The group receiving combination treatment did not show any improved outcome. Salvage treatment is one of the type of the treatment which is given after the cancer has not responded to other treatments. This treatment is depending on the the type of the cancer[13]. When a condition does not improve with traditional therapy, a type of therapies known as salvage therapy, or rescue therapy, is used. HIV and other malignancies are the most common diseases that need salvage therapy. The phrase is used to refer to both a second and a final attempt, but its definition is unclear. The negative effects of medications used in salvage therapy are typically far more severe than those of the conventional course of treatment. This frequently holds true for a last-resort medicine. It is unknown how long active antifungal medication treatments will last. Oral formulations of active medicines, like isavuconazole and posaconazole, are favored since they can be taken for several months, if necessary. When surgery is necessary, it must be done with extreme vigor. Because the Mucorales hyphae spread the infection so quickly, it is important to remove both necrotic tissues and the surrounding healthy-looking tissues that are infected. When it comes to soft tissue infections and rhino-orbito-cerebral infections, surgery is especially helpful[14].
Multicenter Epidemiologic Study of Mucormycosis Associated with Coronavirus Disease
The coronavirus disease (COVID-19) clinical course is known to be complicated by secondary infections. Although systemic fungal infections are becoming more frequently reported, bacterial infections remain the most common secondary infection. Less than 1% of COVID-19 patients' secondary infections during the early stages of the pandemic were fungi. The emergence of systemic fungal infections in severe COVID-19 cases may be caused by pre-existing conditions, careless use of antimicrobial and glucocorticoid medications, and gaps in infection control procedures[16]. To assess the epidemiology and outcomes of cases of coronavirus disease (COVID-19)-associated mucormycosis (CAM), we carried out a multicenter retrospective study across India from September to December 2020. Patients with uncontrolled diabetes mellitus have a high rate of mucormycosis in India, and a large number of patients with severe COVID-19 also have diabetes. India is among the nations most seriously affected by the COVID-19 pandemic. India should have a large number of CAM cases. In the patients under study's management, the prevalence of complementary and alternative medicine was 1.6% in ICUs and 0.27% in hospital wards. Comparing September through December 2020 to the same months in 2019, the researchers found 2.1-fold increase in mucormycosis cases; attribute the increase to COVID-19. The majority of CAM cases were identified more than eight days after COVID-19 cases. The development of late CAM was found to be independently correlated with hypoxemia brought on by COVID-19 and improper use of glucocorticoids. CAM patients had a high mortality rate of 44%, which was similar to non-CAM patient rates of 49%[15].
India is the country with the second-highest case and death burden in the world, with 30.3 million cases and 398,000 deaths reported. 5 million low-income and low-middle-income people who live in the Pune region of Maharashtra state, western India, are served by this tertiary care teaching hospital, which is run by the state. When resting oxygen saturation (SpO2) was greater than 94% and a positive SARS-CoV-2 infection was present, it was considered mild COVID-19. The presence of breathlessness, respiratory rate (RR) greater than 24 breaths per minute, or SpO2 lower than 93% on room air was classified as moderate COVID-19. Breathlessness, RR >30 breaths/min, or SpO2 <90>
CONCLUSION –
Mucormycosis is a potentially fatal fungal infection that mostly affects immunocompromised patients. Despite the availability of treatment, the disease's incidence and mortality are on the rise. It is characterized by host tissue infarction and necrosis. About half of the total inpatient costs were related to the medications used for antifungal treatments. Mucormycosis raises mortality, utilization of healthcare resources and expenses. To better optimize induction and consolidation treatment, comparative studies are required.
REFERENCE –
- Skiada A, Lass-Floerl C, Klimko N, Ibrahim A, Roilides E, Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018 Apr 1;56(suppl_1):93-101.
- Cornely, O. A., Alastruey?Izquierdo, A., Arenz, D., Chen, W., Dannaoui, É., Hochhegger, B., Hoenigl, M., Jensen, H. E., Lagrou, K., Lewis, R. E., Mellinghoff, S. C., Mer, M., Pana, Z. D., Seidel, D., Sheppard, D. C., Wahba, R., Akova, M., Alanio, A., Al?Hatmi, A. M. S., . . . Chakrabarti, A. (2019). Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. The Lancet Infectious Diseases, 19(12), e405–e421.
- Roden M. M., et al. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41:634–653
- Ribes JA, Vanover-Sams CL, Baker DJ: Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236–301.
- Pilmis B, Alanio A, Lortholary O, Lanternier F. Recent advances in the understanding and management of mucormycosis. F1000Res. 2018 Sep 7;7:F1000 Faculty Rev-1429.
- Skiada A, Pagano L, Groll A et al.. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011; 17: 1859–1867.
- Kennedy KJ, Daveson K, Slavin MA et al.. Mucormycosis in Australia: contemporary epidemiology and outcomes. Clin Microbiol Infect. 2016; 22: 775–781.
- Bitar D, Van Cauteren D, Lanternier F et al.. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg Infect Dis 2009;15: 1395–1401.
- Xhaard A, Lanternier F, Porcher R, et al. Mucormycosis after allogeneic haematopoietic stem cell transplantation: a French multicentre cohort study (2003–2008). Clin Microbiol Infect 2012; 18: e396–400.
- Pai, V., Sansi, R., Kharche, R., Bandili, S. C., & Pai, S. B. (2021). Rhino-orbito-cerebral mucormycosis: Pictorial review. Insights Into Imaging, 12(1).
- Chermetz M, Gobbo M, Rupel K, et al. Combined orofacial aspergillosis and mucormycosis: fatal complication of a recurrent paediatric glioma-case report and review of literature. Mycopathologia 2016; 181: 723–33.
- Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008; 47: 503–509.
- Planegger, Andrea MD*; Uyulmaz, Semra MD*; Poskevicius, Audrius MD†; Zbinden, Andrea MD‡; Müller, Nicolas J. MD§; Calcagni, Maurizio MD*. Cutaneous Invasive Fungal Infections with Saksenaea Species in Immunocompetent Patients in Europe: A Systematic Review and Case Report. Plastic and Reconstructive Surgery - Global Open 10(4):p e4230, April 2022. |
- Kyvernitakis A, Torres HA, Chamilos G et al.. Initial use of combination treatment does not impact survival of 106 patients with hematologic malignancies and mucormycosis: a propensity score analysis. Clin Microbiol Infect. 2016; 22:e1–811.e8.
- Roilides E, Antachopoulos C, Simitsopoulou M. Pathogenesis and host defence against Mucorales: the role of cytokines and interaction with antifungal drugs. Mycoses. 2014; 57: 40–47.
- Patel, A., Agarwal, R., Rudramurthy, S. M., Shevkani, M., Xess, I., Sharma, R....Chakrabarti, A. (2021). Multicenter Epidemiologic Study of Coronavirus Disease–Associated Mucormycosis, India. Emerging Infectious Diseases, 27(9), 2349-2359. .
- Ministry of Health and Family Welfare, Government of India. Clinical management protocol for COVID-19. 2021.
- Kataria, T., Sharma, S., Jat, P. S., Singh, S., Grover, M., Sharma, S., Agarwal, Y., & Agarwal, S. (2022). Coronavirus-associated mucormycosis: different from sinonasal mucormycosis. The Journal of Laryngology & Otology, 136(12), 1296–1303.
- Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi. 2019;5(1):26.
- Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi. 2020;6(4):265.
- Skiada A, Lass-Floerl C, Klimko N, Ibrahim A, Roilides E, Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56(1):S93–S101


 Pratik Borawake*
Pratik Borawake*
 Akash Doke
Akash Doke






 10.5281/zenodo.11063473
10.5281/zenodo.11063473