Abstract
Microspheres overcome the challenges of using powders and granules in pharmaceutical delivery. They consistently release medication at a steady pace, ensuring a dust-free environment without abrasion due to their free-flowing nature. These spheres, typically composed of proteins or synthetic polymers, range in size from 1 to 1000 micrometers.Various techniques can be employed to create microspheres, offering flexibility in controlling drug release and enhancing therapeutic effects. Nanospheres, a subset of microspheres, refer to those sized between 10 and 500 nanometers. Beyond drug delivery, microspheres find applications in cell sorting, diagnostics, gene and genetic studies, and targeted in vivo delivery. This review article delves into the details of microspheres, covering their types, formulation, evaluation, and diverse uses.
Keywords
Microspheres, Types, Method of preparation, Evaluations, Applications.
Introduction
Oral medication is often preferred, but limitations like short half-life and restricted intestinal absorption can reduce its effectiveness. This necessitates more frequent dosing to maintain therapeutic levels. To address this, controlled and sustained release methods have been developed, ensuring bioavailability and improving the drug's pharmacokinetic and pharmacodynamic profile.Microcapsules and micromatrices offer convenient ways to deliver drugs with minimized side effects and maximized therapeutic efficacy. Microcapsules encapsulate the drug within a distinct wall, while micrometrics disperse the drug throughout. Microspheres, also known as microparticles, can encapsulate various drug types and are easily administered via injection. Introduced in 1997, microspheres have become a viable alternative to traditional formulations for prolonged and controlled drug release. 1 They are particularly useful for delivering poorly water-soluble drugs, allowing for improved therapeutic concentrations, Cmax, Tmax, and area under curve. Microsphere-based formulations can deliver consistent doses and even target specific sites in the body.2
History and Development
Chemical microencapsulation emerged as an alternative drug delivery method between the 1940s and 1960s, with polymer/membrane technology becoming a prominent concept in the 1980s.3
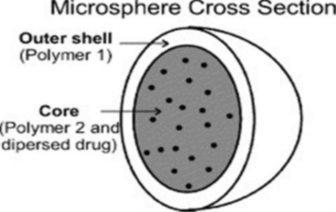
Structure and Composition
Microspheres are tiny particles mainly made up of polymers, which are large molecules formed by repeating units. These polymers can be categorized as either synthetic, created artificially, or natural, derived from living organisms.Synthetic polymers used in microspheres come in two varieties: non-biodegradable, meaning they resist breakdown by natural processes, and biodegradable, which can be broken down over time. Examples of non-biodegradable polymers include acrolein, glycidyl methacrylate, and epoxy polymers. On the other hand, biodegradable options include polyanhydrides, polyalkylcyanoacrylates, and various polymers based on lactides and glycolides.Natural polymers, in contrast, are sourced from biological materials. These can be derived from proteins like albumin, gelatin, or collagen, carbohydrates like starch, agarose, or carrageenan, or chemically modified versions of carbohydrates, such as poly(acryl dextran) and poly(acryl starch).4
Ideal Microspheres:
The perfect microsphere would be stable with a long shelf life, suitable for therapeutic use. Their size and ability to disperse evenly in injectable solutions should be adjustable. They should release the active drug in a controlled manner over time, be compatible with the body and break down naturally, adaptable to chemical changes, and able to hold high drug concentrations.5
Benefits of Microspheres:
Microspheres offer numerous benefits in drug delivery. They enhance the solubility of poorly soluble drugs, provide consistent and long-lasting therapeutic effects, and maintain stable drug levels in the blood, improving patient adherence to treatment. They can reduce both drug toxicity and the required dosage, while extending the duration of the drug's action. Their production is simple, and they are generally well-tolerated by patients.
The extended-release capabilities of microspheres have attracted attention, especially in delivering anticancer drugs directly to tumors. Studies exploring their uptake by macrophages suggest potential in targeting infections within cells. These versatile particles offer reliable ways to maintain drug concentrations at specific sites without side effects, and modifications can enhance their targeting ability. Solid biodegradable microspheres offer controlled drug release within the particle itself, with their size, surface charge, and water affinity influencing their behavior in the body.Beyond drug delivery, microspheres can be used to assess regional blood flow using larger particles (10-15 ?m) and to shield the gastrointestinal tract from drug irritation. Typically injected into the bloodstream at specific locations, they gradually settle into capillaries. By extracting tissue samples and measuring fluorescence, blood flow can be determined, with fluorescent microspheres proving advantageous over radiolabelled ones in long-term blood flow studies.7
Disadvantages
However, microspheres have certain drawbacks. Once injected, their removal is challenging. Variations in drug content may occur during production, and the toxicity of beads remains uncertain. Large-scale manufacturing presents challenges, and maintaining drug stability and adequately controlling drug release rates can be difficult. Additionally, microspheres exhibit limited reproducibility.11 Parenteral microspheres, in particular, may have low drug loading, difficulty in complete carrier removal from the body, potential interactions or complex formation with blood components, and the possibility of altered formulation release, with any deviation potentially being harmful. It is important to note that controlled release dosage forms should not be crushed or chewed.8
Types of Microspheres 5
Microspheres can be categorized into various types based on their properties and applications:
Bioadhesive Microspheres: These microspheres utilize the adhesive properties of water-soluble polymers to attach themselves to mucosal membranes (nasal, rectal, ophthalmic, buccal), prolonging their residence time at the application site for improved drug absorption and therapeutic effects. 3
Magnetic Microspheres: By incorporating materials like chitosan and dextran, these microspheres respond to magnetic fields, allowing targeted drug delivery to specific disease sites. They find applications in both therapeutic delivery (e.g., chemotherapy to liver tumors) and diagnostic imaging (e.g., visualizing liver metastases). 7
Floating Microspheres: Designed with a lower density than gastric fluid, these microspheres remain buoyant in the stomach, delaying gastric emptying and facilitating controlled, prolonged drug release. This leads to steadier drug levels in the blood and less frequent dosing.3
Radioactive Microspheres: Used in radioembolization therapy, these microspheres lodge in tumor-supplying arteries, delivering localized radiation directly to the tumor site. Different types emit alpha, beta, or gamma radiation. 5
Mucoadhesive Microspheres: Composed of mucoadhesive polymers, these microspheres adhere to various mucosal tissues (e.g., gastrointestinal, nasal, ocular, urinary), providing localized or systemic drug delivery. Their high surface-to-volume ratio and close contact with the mucus layer enhance drug absorption and bioavailability.5
Polymeric Microspheres: This broad category encompasses both biodegradable and synthetic microspheres. Biodegradable ones are derived from polymers that break down into non-toxic products, while synthetic ones have proven safe and versatile for various clinical uses, including drug delivery and tissue fillers.5
Criteria for Microsphere Preparation:
The creation of microspheres involves encapsulating solids, liquids, or gases within polymeric coatings. Different methods are employed depending on factors such as particle size, intended route of administration, and desired drug release duration. Considerations like rpm, cross-linking method, drug type, evaporation time, and coprecipitation are taken into account. Microspheres should ideally exhibit high drug loading capacity, stability, controlled particle size and dispersibility, sustained drug release, biocompatibility, biodegradability, and the ability to be chemically modified.7
METHODS OF PREPARATION:
Various standard methods exist for microsphere preparation:
Single Emulsion Evaporation Technique:
This technique is primarily used for proteins and carbohydrates. It involves dispersing natural polymers in an oil phase after dissolving them in an aqueous medium. The dispersed globules are then cross-linked, either through heat denaturation or chemical cross-linkers such as glutaraldehyde, formaldehyde, or acid chloride. Heat denaturation is not suitable for heat-sensitive compounds, and chemical cross-linking may expose the active ingredient to chemicals for extended periods.3
Double Emulsion Technique:
The creation of a double emulsion involves the formation of either an oil-in-water-in-oil (o/w/o) or water-in-oil-in-water (w/o/w) system. This method commences with the dispersion of an aqueous drug solution within a continuous lipophilic organic phase. Subsequently, a polymer solution encapsulates the dispersed aqueous phase containing the drug, thereby forming the primary emulsion. This pre-formed emulsion is then subjected to homogenization or sonication and introduced into an aqueous polyvinyl alcohol (PVA) solution, leading to the development of the double emulsion. This particular technique has been effectively utilized in the production of controlled-release microspheres, exhibiting drug release profiles that are governed by diffusion.3
Quasi-Emulsion Solvent Diffusion:
A distinct quasi-emulsion solvent diffusion technique has been reported for producing acrylic polymer-based controlled-release microspheres. This method utilizes a quasi-emulsion with an external phase composed of polyvinyl alcohol and distilled water. The internal phase consists of the drug, ethanol, and a portion (20%) of the polymer to enhance plasticity. The internal phase, after synthesis at 60ºC, is added to the external phase at room temperature and continuously stirred for two hours. Filtration is then employed to separate the microsponges, followed by washing and drying in a vacuum oven at 40ºC for 24 hours.7
Supercritical Fluid (SCF) Technique:
A novel supercritical fluid (SCF) technique has been developed for preparing microspheres intended for pulmonary drug delivery. Based on the anti-solvent process, this method incorporates advanced technical design elements, enabling enhanced control over particle generation. SCF techniques have found application in the production of fine particles of peptides and proteins. However, a primary challenge associated with protein processing using these methods is the exposure of the protein to organic solvents, often resulting in a significant reduction in bioactivity and re-agglomeration of the protein upon storage.6
Phase Separation Coacervation Technique:
This technique is a simple method involving the separation of a micromolecular solution into two immiscible liquid phases. The principle of coacervation involves decreasing the solubility of the polymer in the organic phase to induce the formation of polymer-rich phases known as coacervates. In this approach, drug particles are dispersed in a polymer solution, and an incompatible polymer is added to the mixture. This addition causes the first polymer to undergo phase separation and envelop the drug particles.3
Polymerization:
The microspheres commonly available are typically made of lactide and glycolide homo and copolymers (PLGA), known for their long history of regulatory approval and established safety profiles in humans. The two main categories of polymerization methods used in microsphere production are normal polymerization and interfacial polymerization, both occurring in the liquid phase.
Normal Polymerization:
In bulk polymerization, the process is usually initiated by heating a monomer or a mixture of monomers along with an initiator or catalyst. The resulting polymer is then molded into microspheres, and the drug can be incorporated during the polymerization process. While this method produces pure polymer, the heat generated during the reaction is difficult to dissipate, which can be harmful to heat-sensitive active compounds. Suspension polymerization, also known as pearl polymerization, is a low-temperature process involving heating a monomer mixture containing an active drug, with droplets dispersed continuously in an aqueous phase. The resulting microspheres are typically smaller than 100 ?m. Emulsion polymerization differs from suspension polymerization by occurring at a lower temperature due to the presence of water in the aqueous phase, facilitating efficient heat dissipation. This allows for the formation of higher molecular weight polymers at a faster rate, but it also carries the risk of forming associations between the polymer, unreacted monomer, and other additives.5
Interfacial Polymerization:
This process involves the reaction of different monomers at the interface between two immiscible liquid phases, resulting in a polymer film that encapsulates the dispersed phase. In this method, two reactive monomers are used: one is dissolved in the continuous phase, while the other is dispersed in the continuous (aqueous) phase, emulsifying the second monomer. The solubility of the resulting polymer in the emulsion droplet leads to two possible outcomes: a monolithic carrier if the polymer is soluble within the droplets, or a capsule-like structure if the polymer becomes insoluble.5
Hot Melt Microencapsulation:
In this method, a polymer is melted and combined with solid drug particles smaller than 50 ?m. The mixture is suspended in a non-miscible solvent, such as silicone oil, and continuously agitated while being heated to a temperature 5°C above the polymer's melting point. The emulsion is then cooled until the polymer particles solidify, and the resulting microspheres are separated by decantation using petroleum ether. This technique was developed to enable the microencapsulation of water-sensitive polymers, like polyanhydrides. It allows for the production of microspheres with diameters ranging from 1 to 1000 ?m, with size distribution easily controlled by adjusting the stirring rate. However, a notable drawback is the exposure of the drug to moderately elevated temperatures.5
Ionic Gelation Method:
This technique involves complexing a hydrophilic polymer with a multivalent cation (e.g., calcium chloride) or polyanion (e.g., sodium tripolyphosphate) to form highly viscous gel particles. The resulting opalescent suspension is centrifuged to isolate the microspheres, which are then lyophilized for 24 hours. The formation of microspheres is attributed to electrostatic interactions between positively and negatively charged ions. This method has been used to create chitosan-loaded acyclovir microspheres, demonstrating controlled drug release for treating ocular viral infections. Drug release from these microspheres follows a non-Fickian diffusion mechanism and first-order kinetics.7
Spray Drying Technique:
This method involves dissolving a polymer in a volatile organic solvent, such as acetone or dichloromethane, and then dispersing the drug in solid form within the polymer solution under rapid homogenization. The resulting dispersion is atomized into a hot air stream, forming tiny droplets from which the solvent rapidly evaporates, leaving behind microspheres ranging in size from 1 to 100 ?m. A cyclone separator is used to collect the prepared microparticles from the hot air, and any remaining solvent traces are removed by vacuum drying. This technique involves preparing a solution by dissolving both the drug and polymers in a suitable solvent, which is then sprayed through a nozzle in a spray dryer under various experimental conditions. Research has shown that spray drying different lactide-based polymers can yield Vitamin-D3 microspheres with varying release patterns depending on the polymer type.8
Spray Congealing:
In this process, the drug dissolved in molten lipophilic polymer to form a heated solution, which is then atomized through a pneumatic nozzle. The resulting microparticles are collected in a carbon dioxide-cooled ice bath container and subsequently air-dried under vacuum for several hours. This method has been employed to create lipid-polymer composite microspheres containing 10-hydroxy camptothecin for colon-specific drug delivery using an ultrasonic spray freeze-drying process. Drug release studies have shown that approximately 15% of the drug is released below pH 6.8, while over 30% is released below pH 7.4, suggesting the potential of these microparticles for targeted drug delivery to the colon.8
Wax Coating and Hot Melt:
This technique involves dissolving a polymer in a suitable dispersion medium and gradually cooling it to form microspheres, enabling the synthesis of low-melting point polymers into this format. Wax, particularly beeswax and carnauba wax, is commonly used for coating and coring particles. In this process, melted wax is used to disperse and encapsulate the medication. The wax suspension is then dispersed into a cold solution, such as liquid paraffin, using high-speed mixing. The mixture is continuously mixed for one hour, followed by the decantation of the external phase. The suspended microspheres are then collected from the solvent and allowed to air dry. This method is considered more cost-effective than other techniques and results in faster drug release. Various combinations of beeswax and carnauba wax can be utilized to achieve specific properties for the coating.6
Comprehensive Evaluation of Microspheres: 4
To ensure the effectiveness and safety of microspheres for drug delivery and other applications, a thorough evaluation process is undertaken, encompassing various aspects:
Physical Characterization:
- Size and Shape Analysis: The size and shape of microspheres are crucial factors influencing their behaviour in biological systems. Microscopic techniques like Scanning Electron Microscopy (SEM) and conventional Light Microscopy (LM) are employed to visualize and quantify these parameters. SEM provides high-resolution images of the microsphere surface and cross-section, revealing detailed morphological features. LM, while offering lower resolution, is particularly useful for assessing double-walled microspheres, allowing visualization of the core and coating layers. Microsphere size is often expressed as the volume mean diameter, calculated after suspending the particles in a solution containing Tween 80 to prevent clumping.
- Surface Chemistry and Composition: Electron Spectroscopy for Chemical Analysis (ESCA) is utilized to delve into the surface chemistry of microspheres, especially those made of biodegradable materials. This technique reveals the atomic composition and provides insights into how the surface might degrade over time. Additionally, SEM can be employed to analyze surface morphology by mounting and coating samples with a thin layer of gold for examination.
- Density Determination: The density of microspheres is a fundamental physical property that affects their behaviour in various environments. A multivolume pycnometer is used to accurately measure density. This instrument measures pressure changes within a helium-filled chamber containing the microsphere sample, allowing for precise calculation of volume and density.
- Surface Charge Assessment: The surface charge of microspheres plays a significant role in their interactions with biological systems. Microelectrophoresis is used to determine the isoelectric point, the pH at which the net surface charge is zero. This information sheds light on the microsphere's ionizable behaviour and its tendency to adsorb ions.
- Wettability Evaluation: The wetting properties of microspheres determine how they interact with liquids, particularly in biological fluids. The contact angle, measured at the interface between the microsphere, air, and water, is a key indicator of hydrophilicity (water-loving) or hydrophobicity (water-repelling). This measurement is typically done using a droplet placed in a circular cell on an inverted microscope, allowing for precise determination of advancing and receding contact angles.
Drug Loading and Stability:
- Drug Entrapment Efficiency: This parameter quantifies the amount of drug successfully loaded into the microspheres. It is determined by crushing and dissolving a known quantity of microspheres, followed by filtration and analysis using UV-visible spectroscopy. The ratio of the actual drug content to the theoretical drug content (based on the formulation) gives the entrapment efficiency.5
- Swelling Behavior: In the case of microspheres made from hydrophilic polymers like sodium alginate, the swelling index is a crucial parameter. It assesses how much the microspheres expand when placed in different solutions, such as distilled water or buffer solutions with varying pH. This is done by measuring weight changes over time, providing insights into the polymer's water uptake capacity.8
- Stability Assessment: To ensure the shelf-life and efficacy of microsphere formulations, stability studies are conducted. Microspheres are stored under different conditions (e.g., varying temperature and humidity) for a set period, typically 60 days. The drug content is then analyzed to evaluate any degradation or changes in the formulation over time.9
Drug Release Profiling:
- In Vitro Release Studies: These studies are essential for understanding how drugs are released from microspheres under controlled laboratory conditions. Various methods, including the beaker method, interface diffusion systems, modified Keshary Chien cells, and dissolution apparatus, are employed. These techniques provide valuable information about drug release kinetics, mechanisms, and potential for sustained or controlled delivery.10
- In Vivo Studies: To evaluate drug absorption and distribution in living organisms, in vivo studies are conducted. Animal models, often using rats with ligated esophagi to isolate buccal absorption, are commonly used. The dosage form is administered to the animals, and blood samples are collected at intervals to analyze drug concentration. Additionally, buccal absorption tests can be performed to directly assess drug permeability through mucosal tissues.
- In Vitro-In Vivo Correlations: These studies aim to establish relationships between in vitro drug release profiles and in vivo drug availability, typically measured by blood concentration or urinary excretion. Different types of correlations can be used to predict in vivo performance based on in vitro data, aiding in the development and optimization of microsphere formulations.10
Applications of Microspheres:5
Diverse Applications of Microspheres in Drug Delivery and Therapy:
Microspheres have revolutionized various fields, particularly in drug delivery and therapeutic interventions, due to their unique properties and versatility:
- Revolutionizing Vaccination: Microspheres offer a promising alternative to traditional vaccines, addressing their limitations and enhancing safety, efficacy, and ease of administration. Biodegradable microspheres can act as parenteral carriers, boosting immune response, modulating antigen release, and improving vaccine stability.
- Targeted Drug Delivery: By engineering microspheres for specific interactions with target receptors, drug delivery can be precisely localized to specific body sites. This targeted approach maximizes therapeutic benefits while minimizing adverse effects through controlled and focused drug release.
- Monoclonal Antibody-Guided Targeting: The high specificity of monoclonal antibodies (Mabs) allows them to guide microspheres carrying bioactive compounds to desired locations. This immunological targeting can be achieved through various mechanisms, enhancing the precision of drug delivery.
- Chemoembolization for Tumor Treatment: Microspheres are instrumental in chemoembolization, a technique used to treat tumors. This involves selectively blocking tumor blood vessels and simultaneously delivering chemotherapy directly to the tumor, achieving sustained drug levels and vascular occlusion.
- Imaging with Radiolabelled Microspheres: Radiolabelled microspheres enable visualization of various cells, tissues, and organs. The size of the microspheres determines the specific sites that can be imaged, offering valuable diagnostic information.
- Topical Delivery through Microsponges: Porous microspheres, known as microsponges, are incorporated into topical formulations like creams and lotions. These microsponges can hold and release active ingredients such as emollients and fragrances in a controlled manner.
- Surface-Modified Microspheres for Enhanced Delivery: Various techniques can modify the surface of microspheres, altering their distribution in the body and protecting them from immune clearance. For example, poloxamer adsorption can increase hydrophilicity and reduce uptake by the immune system.
- Improved Oral Drug Delivery: Microspheres are valuable for oral delivery of drugs with low bioavailability and poor water solubility. Micronization through encapsulation in microspheres can significantly enhance their absorption and bioavailability.
- Intranasal Delivery of Peptides and Proteins: Microspheres offer a solution for the intranasal delivery of peptides and proteins, overcoming rapid clearance issues. Bioadhesive microspheres provide improved control over release and surface properties compared to gels.
- Sublingual and Buccal Drug Delivery: The buccal mucosa is ideal for certain peptide drugs. Mucoadhesive microspheres can prolong drug residence time in the buccal cavity, bypassing the liver's first-pass metabolism.
- Gene Delivery with Non-Viral Vectors: Microspheres are being investigated as non-viral vectors for gene therapy, offering advantages over viral vectors like reduced immunogenicity, ease of preparation, and targeted delivery.
- Colon-Specific Drug Delivery: Microspheres can be designed to release drugs specifically in the colon, protecting them from absorption in the stomach and small intestine. This is valuable for drugs targeting colon-related conditions.
- Breast Cancer Treatment: Cytotoxin-loaded microspheres can be directly injected into arteries supplying breast tumors, embolising the tumor vasculature and delivering the drug to the site.
- Brain Tumor Treatment: Microspheres are used to deliver diagnostic and therapeutic agents to brain tumor cells. Controlled release microspheres have shown promise in sustained drug delivery for improved outcomes.
- Lung Cancer Treatment: Microspheres have also found applications in lung cancer treatment. Studies have demonstrated enhanced anti-cancer activity and prolonged drug release using PEGylated microparticles.
Future Challenges:11
Microspheres, with their diverse applications in molecular biology, tumor prevention, vaccine and protein delivery, hold great promise for the future of medicine. Continued research and development are necessary to address challenges such as optimizing microsphere properties for specific applications, ensuring their safety and efficacy in various therapeutic contexts, and developing scalable and cost-effective manufacturing processes.
CONCLUSION:
The uptake of medications within the gastrointestinal system is a multifaceted and inconsistent process, with the time that dosage forms spend in the stomach playing a significant role in how quickly drugs are absorbed..Microspheres are poised to make a significant contribution to the future of drug delivery innovation through the integration of various techniques. These tiny spheres offer potential applications in cell sorting, diagnostics, gene and genetic material delivery, as well as targeted and efficient drug delivery within living organisms. Additionally, they can serve as miniature models of damaged tissues and organs, providing a valuable platform for research and development of regenerative therapies.Due to their expanded surface area, precisely controlled microspheres enhance the accuracy of drug delivery to specific locations and expedite drug dissolution. This drug delivery strategy also holds the potential to improve the overall bioavailability of pharmaceuticals. Despite these challenges, microspheres offer a feasible alternative for systemic delivery of drugs with poor oral bioavailability and a desirable non-invasive method for delivering potent peptide and potentially protein-based therapeutics.
REFERENCE
- Varde NK, Pack DW. Microspheres for controlled release drug delivery. Expert opinion on biological therapy. 2004 Jan 1;4(1):35-51.
- Saini S, Kumar S, Choudhary M, Nitesh, Budhwar V. Microspheres as controlled drug delivery system: an updated review. International journal of pharmaceutical sciences and research. 2018 May 1;9(5):1760-8.
- Gavhane P, Deshmukh M, Khopade AN, Kunjir VV, Shete RV. A review on microsphere. Journal of Drug Delivery and Therapeutics. 2021 Jan 15;11(1):188-9
- Sarlesh rajput, Preeti agrawal, Ashish Pathak, Nikhil Shrivasatava, Satyendra Singh Baghel, Rajendra singh baghel . A Review On Microspheres: Methods Of Preparation And Evaluation. World Journal of Pharmacy and Pharmaceutical Sciences, volume 1 issue(1), 2012 pp.422–438.
- Patel S, Kumhal RP, Dinesh PA, Gorantli CC. A Review on Microspheres: Types, Methods of Preparation, Effects of Process Variables and Applications. American Journal of Pharm Tech Research. 2020;10(4):123-40.
- Dalbanjan Nikita S. Microsphere: A Complete Review. World Journal Of Pharmacy And Pharmaceutical Sciences, volume 7 no(11), 2018 pp.786–817.
- Prasad BS, Gupta VR, Devanna N, Jayasurya K. Microspheres as drug delivery system-a review. J Glob Trends Pharm Sci. 2014;5(3):1961-72.
- Sharma N, Purwar N, Gupta PC. Microspheres as drug carriers for controlled drug delivery: a review. International Journal of pharmaceutical sciences and research. 2015 Nov 1;6(11):4579.
- Soni LM, Kumar M and Namdeo PK: Sodium alginate microspheres for extending drug release: formulation and in vitro evaluation. International Journal of Drug Delivery 2010; 2:64-68.
- Mikhail AS, Negussie AH, Mauda-Havakuk M, Owen JW, Pritchard WF, Lewis AL, Wood BJ. Drug-eluting embolic microspheres: State-of-the-art and emerging clinical applications. Expert Opinion on Drug Delivery. 2021 Mar 4;18(3):383-98.
- Shanthi NC, Gupta R and Mahato KA: Traditional and emerging applications of microspheres: A review. International Journal of Pharm Tech Research 2010; 2(1): 675-81.
- Bansal H, Kaur SP and Gupta AK: Microspheres: Methods of Preparation and Applications, A Comparative Study. International Journal of Pharmaceutical Science Review and Research 2011; 12: 69-78.
- Parmar H, Bakliwal S, Gujarathi N, Rane B, Pawar S. Different methods of formulation and evaluation of mucoadhesive microsphere. International Journal of Applied Biology and Pharmaceutical Technology .2010;1:1160-11633


 FARSANA P P*
FARSANA P P*
 Dr.Preetha S Panicker
Dr.Preetha S Panicker
 NASRIN NASIR
NASRIN NASIR
 KEERTHI. R
KEERTHI. R

 10.5281/zenodo.12737198
10.5281/zenodo.12737198