Abstract
Probiotics are bacteria that help to maintain natural balance of microorganisms in gut. The main aim associated with probiotic delivery is maintenance of bacterial viability. The microencapsulation of probiotics and use of biocompatible materials is gaining popularity. Encapsulation is proved to be the best way for the protection of probiotics to ensure their stability without any change in native strain properties. Encapsulation of probiotics provides the protection made up of encapsulating materials that stabilize the probiotics during processing, storage, and at the site of action by enhancing stress resistance
Keywords
Probiotic, microencapsulation, extrusion process, electrospinning, microencapsulating agents
Introduction
Probiotics have multifactorial benefits to human health and are a part of daily diet for centuries in the form of traditional fermented foods or beverages. For health or food applications, probiotic microorganisms should be well characterized and must have proven efficacy. Among all probiotic microorganisms, several Lactobacillus spp. and Bifidobacteria spp. have been extensively used for their health benefits (1). Probiotics are live microorganisms, which are defined by their ability to confer health benefits to the host, if administered adequately. Probiotics are not only used as health supplements but have also been applied in various attempts to prevent and treat gastrointestinal (GI) and non-gastrointestinal diseases such as diarrhea, colon cancer, obesity, diabetes, and inflammation. One of the challenges in the use of probiotics is putative loss of viability by the time of administration. (2). In this review, we focus on various formulation technologies available for probiotics
Microencapsulation of Probiotics:
The oral administration of most bacteria results in a large loss of viability associated with passage through the stomach, which is attributed to the high acid and bile salt concentrations present. This loss of viability effectively lowers the efficacy of the administered supplement. Encapsulation is proved to be the best way for the protection of probiotics to ensure their stability without any change in native strain properties. Encapsulation of probiotics provides the protection made up of encapsulating materials that stabilize the probiotics during processing, storage, and at the site of action by enhancing stress resistance. (3) The technology is based on the immobilization of bacteria into a polymer matrix. Encapsulation technologies are required to maintain the viability of probiotics during storage and within the human gut so as to increase their ability to colonize the colon. (4)
Purpose of Microencapsulation
The purpose of microencapsulation of probiotic is to protects certain compound or biological cells against surrounding environment which destruct the core. It protects the bacteria from low pH, bile salts and other constituents that it encounters during GI transit. It also improves the flow properties during formulation development. (5-7). An immobilized environment confers additional protection to probiotic cells during rehydration.
Structure of Microcapsule
Microcapsules, formed by using natural materials like sugar, gums, protein, lipid, and synthetic or modified polymers, can be formulated as gel beads or in dried powder form. The coating material used can be sodium alginate, or a mixture material such as xanthan, gellan gum, alginate, and Chitosan. Microcapsule consists of semipermeable, spherical, thin and strong polymer membrane surrounding a liquid core, with a diameter varying from a few microns to 1mm.Coating material also affects the structure of microcapsule. Generally, sodium alginate produces microcapsules with smooth surface [8], while slow gelling property of milk results in formation of irregular shaped capsule [9, 10]
Formulation Technologies for Microencapsulation of Probiotics
The presence of diverse condition in human digestive system makes designing of the probiotic release system difficult. Hence, highly tailored system like specific target location system is required. Probiotic cell is commonly encapsulated by extrusion, emulsion, adhesion to starch and spray drying. In these methods, probiotic bacteria are entrapped in the gel matrix using different gel forming mechanisms [11, 12].

Figure 1 Different encapsulation techniques
Microencapsulation techniques are divided into two parts: Encapsulation process And Drying process.
Encapsulation Process
There are two basic techniques of microencapsulation that are used for encapsulation of probiotic bacteria.
Extrusion Technique:
It is the oldest common technique for probiotic formulation [13] Extrusion method in the case of alginate capsule consists of the following stages: preparation of hydrocolloid solution and the addition of probiotic cell in hydrocolloid solution to form cell suspension. This material undergoes plastic deformation by the application of force causing that material to flow through an orifice or die and if the material has suitable properties that shape is retained in the final extrudate. Droplets of cell suspension are directly dripped into the hardening solution containing cations like calcium. When the droplets come in contact with hardening solution, alginate polymers surround the core to form a three-dimensional lattice structure by cross-linking calcium ions as shown in Figure 3 [14-18]

Figure 2 Extrusion of alginate and probiotic cells
Freeze Drying
Freeze drying or lyophilization or cryodesiccation is a low temperature dehydration process that involves freezing the product, lowering the pressure and removing the ice by sublimation [19]. Freezing causes damage to the cell membrane due to ice crystal formation and also imparts stress condition by high osmolarity. Recently, Lactobacillus and Bifidobacterium cells were first encapsulated into enzymatically gelled sodium caseinate [20]. Freeze-drying occurs in three steps: freezing, primary drying, and secondary drying. Probiotics along with the encapsulating material first undergo freezing in which the water is converted into ice at sub-zero temperatures. During the freezing stage, the product temperature is decreased, resulting in the formation of ice. In the primary drying step, frozen water is removed by sublimation under vacuum, and in the secondary drying step, unfrozen water is removed by the desorption process, resulting in the dried end-product.
Emulsion Technique
Emulsification is a chemical process that involves the interaction between a continuous phase and a discontinuous phase with the addition of an emulsifier. In this technique, microcapsules are formed after the formation of a water in oil emulsion, usually stabilized by surfactants like Tween 80/Span 20/glycerol, and gelled by external gelation by the addition of calcium chloride solution [23,24]. Gelification is done by different mechanisms like ionic, enzymatic, and interfacial polymerization.
Emulsification and Ionic Gelification
Emulsification is a chemical technique to encapsulate probiotic using alginate, carrageenan and pectin as an encapsulating material. A small volume of the cell polymer suspension (i.e., the discontinuous phase) is added to a large volume of vegetable oil (i.e., the continuous phase). The mixture is then homogenized to form a water-in-oil emulsion. Once the water-in-oil emulsion is formed, the water-soluble polymer must be insolubilized to form tiny gel particles within the oil phase. Once W/O emulsion is formed, water soluble polymer becomes insoluble after addition of ions of calcium chloride, by means of cross-linking forming gel particles in the oil phase. Microbeads produced by this technique are recovered by membrane filtration technology [25].

Figure 3 Emulsification and ionic Gelification
Emulsification and Enzymatic Gelification
Milk proteins have excellent gelation properties and are a natural vehicle for probiotics [26]. This method is an example of encapsulation by means of rennet gelation as shown in Figure 5, which is based on the principle of using dairy proteins which have been put in contact with rennet at low temperature. Rennet is a complex set of enzymes produced in stomachs of ruminant animals. Rennet has been traditionally used to separate milk in to solid curds and liquid whey and also used in the production of cheese. The principle of the technique is based on using dairy proteins which have been put in contact with rennet at low temperature. This allows keeping a liquid system where ?-casein is cleaved by the enzyme. After that, dairy proteins have been emulsified in a cold oil to form water in oil emulsion.

Figure 4 Microencapsulation of probiotic cells by rennet gelation of milk proteins
Emulsification and Interfacial Polymerization
This technique is a single step. It requires formation of an emulsion in which discontinuous phase contains an aqueous suspension of the cell and continuous phase contains organic solvent. Interfacial polymerization is a type of step growth polymerization which occurs at the interface of two immiscible phases resulting in a polymer that is constrained to the interface. To initiate the polymerization reaction, biocompatible agent which is soluble in the continuous phase is added. The drops of probiotic cell are coated to form thin and strong membrane [27].
Electrospinning and Electro spraying:
Principally electrospinning and electro spraying are electrohydrodynamic processes where a polymer solution can be spun or sprayed by the application of high potential electric field to obtain fibers or particles respectively. Electrospinning is a process during which a liquid droplet is electrified to generate a jet, followed by stretching and elongation to generate fibers. It offers a simple and inexpensive method which enables production of homogenous, continuous and uniform nanofibers. Electro spraying is a liquid atomization-based technique which is used to produce and formulate micro or nanoparticular carriers for various biomedical applications. This uses high voltage electric field to break up a solution. The particle size is controlled by varying the solution properties such as concentration and conductivity, as well as parameters such as flow rate and applied voltage
Refractive window drying:
Refractive window (RW) drying and its variant, conductive hydro drying is an emerging drying technique for the encapsulation of probiotics. A typical RW dryer consists of a hot water reservoir above which a food-grade infrared transparent polyester film is placed. The homogenized mixture of probiotics and encapsulating material is spread over the film and is allowed to dry. Product temperatures are kept low and rapid drying occurs as all three modes of transfer of heat are involved. Raghavi et al. [27] have clearly explained the mechanism of the drying approach. Once the product gets dried, witha mismatch in the refractive index between dried product and hot water, the “window” is said to get closed at a Mylar film and deflecting the incident radiation back into the hot water [28]. The approach has been successfully used for the drying of several products such as slurries, purees [29], slices of fruits and vegetables [30], and meat powders [31]. The high-quality flakes or films can be effectively obtained using this low-cost (operational) technique [28].
Spray Drying
In the spray drying process, probiotics along with the encapsulating wall material (liquid feed) are atomized into the hot gas drying chamber, the wet droplets transit through high temperature. In a short span, crust formation occurs, leading to the formation of dried solid particles. The encapsulated powder can be separated from the drying air using a cyclone separator. This technique is known for its merits in terms of the flexibility to have high production volumes, high reproducibility, low production cost, and the convenience to scale-up [32,33].The solution is pressured and then atomized to form a mist into the drying chamber. The hot gas (air or nitrogen) is blown into the drying chamber too. This hot gas allows the evaporation of the solvent. The capsules are then transported to a cyclone separator for recovery. [34,35]
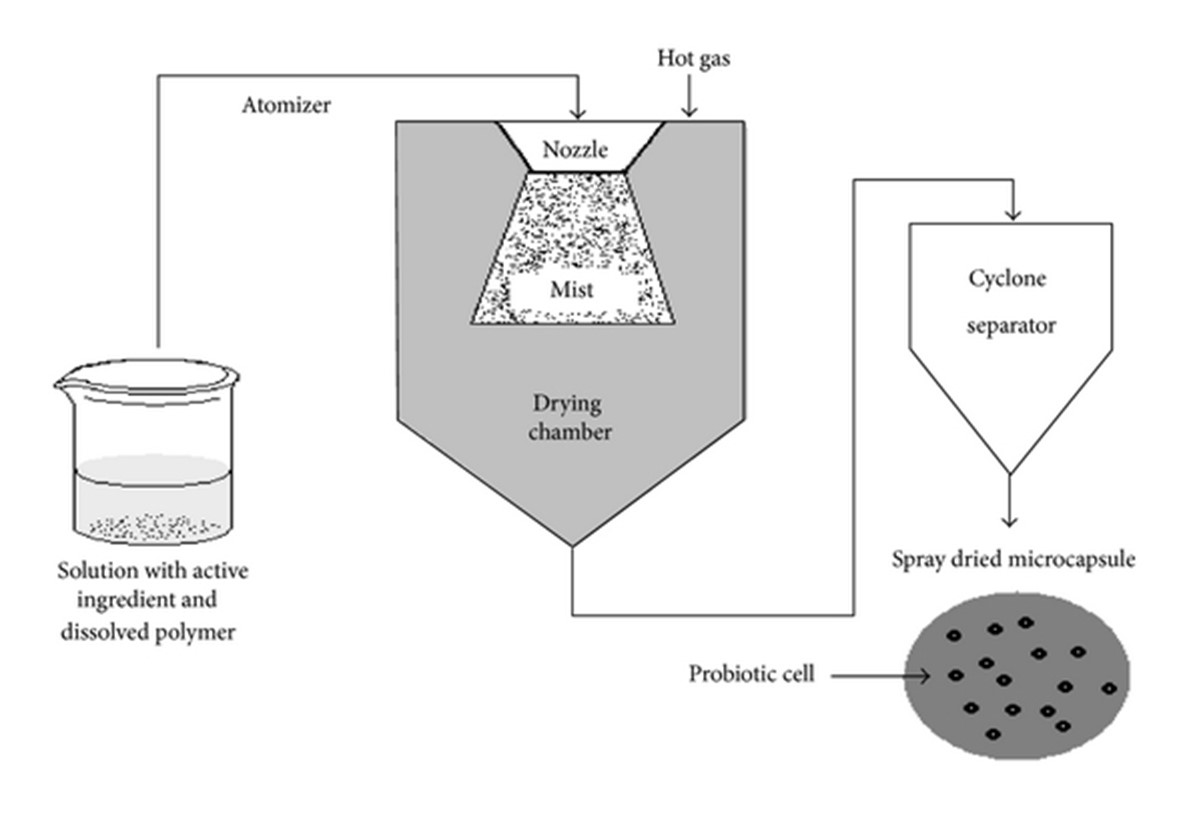
Figure 5 Spray drying procedure
Spray?freeze?drying
Spray-freeze-drying is carried out in three stages, namely atomization, freezing, and freeze-drying. In this process, probiotics along with the encapsulating wall material (liquid feed) are atomized, forming fine droplets with a high interfacial area. This is then allowed to get in contact with a cryogenic medium such as liquid nitrogen at very low temperatures. This freezes the probiotic cell in the wall matrix with the formation of frozen droplets. These frozen droplets are further subjected to drying in a freeze dryer [36, 37].
Miscellaneous:
Encapsulation by Coating and Agglomeration
In this method, solid form of core material is kept in motion in a specially designed vessel (Figure 5) [38].
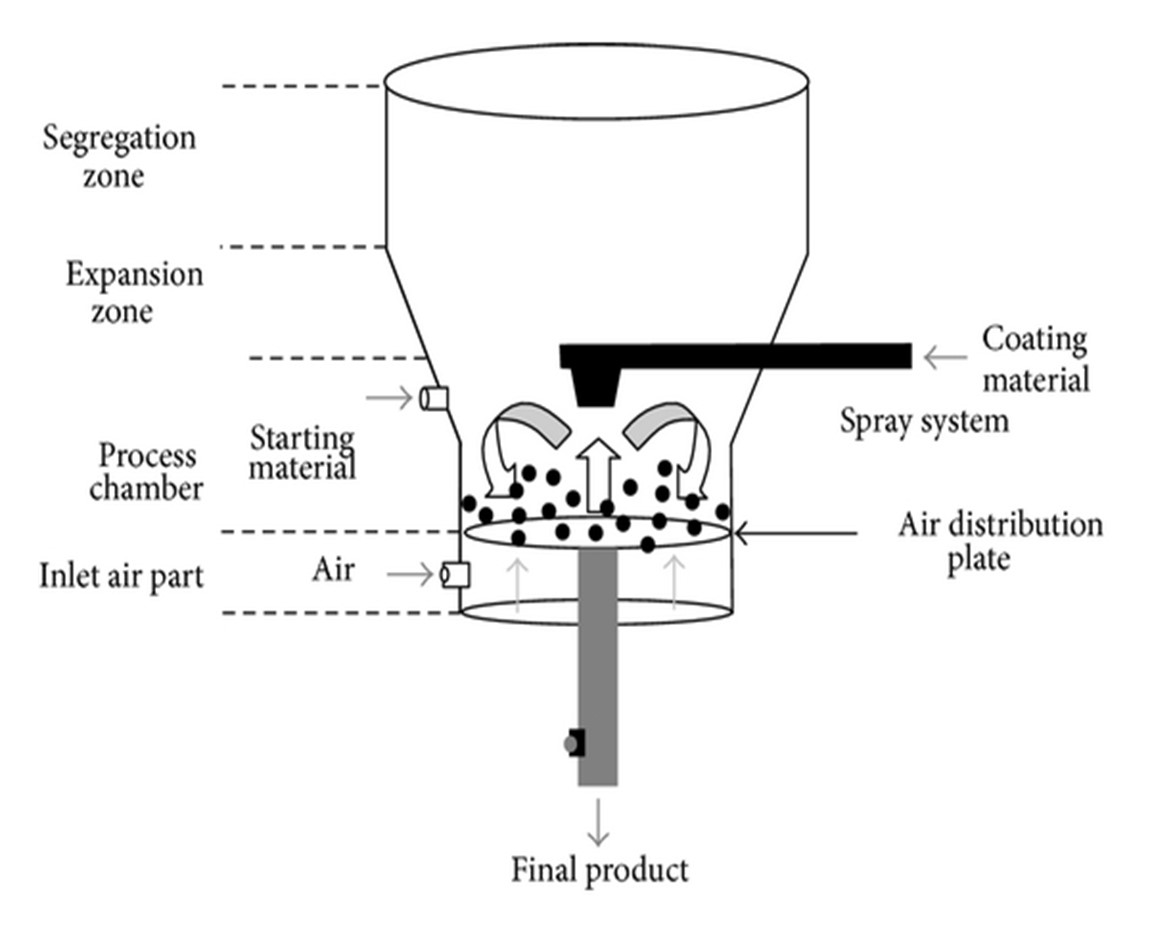
Fig 6 Encapsulation by coating and agglomeration
Coacervation Technique for Encapsulation
In coacervation process, colloidal particle is separated from a solution and deposited around core material. It is a three-step process comprising of phase separation, deposition, and solidification [39]. In the first step, coating material containing one or more polymer goes through a phase separation process and forms a coacervate. Suspended or emulsified form of core material remains, and as soon as wall material particles coalesce, it causes a decrease in surface area and total free interfacial energy of the system. In this process, coacervate nuclei adsorption to the surface of core material and form uniform layer around the core particles. Finally, solidification of coating material is done by cross-linking using chemical, thermal, or enzymatic method. The formed microparticles are then collected by filtration or mild centrifugation followed by drying.
Co crystallization
In this method, core material is dispersed in supersaturated sucrose solution maintained at high temperature. The heat is gradually released allowing the solution to crystallize with the core material. Finally, the product is dried and sieved as per the particle size requirement [40].
Molecular Inclusion
This method involves entrapment of smaller molecule inside the hollow cavity of a larger molecule [41,42]. Cyclodextrins are commonly used.
Centrifugal Extrusion Technique
In this technique, core and coating materials are pumped through a separate tube to the surface of rotating cylinder. With the rotational motion of the cylinder, both materials are mixed and extruded as a fluid rod which is broken by the centrifugal force. The coating over the core material forms capsules caused by the difference in surface tension. Finally, formed capsules are placed on a moving bed of starch, which absorbs excess moisture and cushion the impact [43].
Biomaterials Used for Microencapsulation of Probiotics
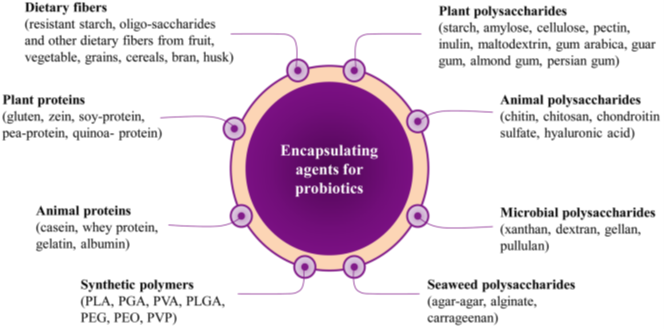
It includes natural and synthetic polymers which are directly in contact with living cell so they should be biocompatible and biodegradable [44]. Encapsulation of probiotics in biodegradable polymer matrix has a number of advantages. Finally, microcapsules are dried. Microcapsule produced by using polymer is easy on a lab scale. But the scaling process is very difficult and processing cost is very high.
Alginate System for Encapsulation of Probiotics
Alginate is a naturally derived polysaccharide extracted from various species of algae and composed of two monosaccharide units: -L-guluronic acid (G) and -D-mannuronic acid (M) linked from (1–4) glycosidic bond [45,46]. Usually, alginate is used in concentration range of 0.5–4%
Chitosan for Encapsulation of Probiotics
Chitosan is a linear polysaccharide with negative charge arising from its amine groups obtained by deacetylation of chitin. It can be isolated from crustacean shells, insect cuticles, and the membranes of fungi. It is a copolymer of two monomer residues anhydro-N-acetyl-D-glucosamine and anhydrous-D-glucosamine. It is soluble at pH < 6>
Starch for Encapsulation of Probiotics
Starch consists of D-glucose unit joint together with glycosidic bonds. It has been used as a material for coating of alginate capsules. High-amylose corn starch (HACS) can be applied for enhancing functions of capsule or shell/coat formation [48].
Xanthan-Gellan Gum for Encapsulation of Probiotics
Gellan gum is an anionic polysaccharide derived from Sphingomonas elodea which is constituted of a repeating unit of four monomers that are glucose, glucuronic acid, glucose, and rhamnose [49].
?-Carrageenan for Encapsulation of Probiotics
Carrageenan is polymer having linear structure consisting of D-galactose units alternatively linked by ?-(1–3) and ? (1–4) bonds. Types of carrageenan are kappa (?), iota (?), and lambda (?) [237]. Monosulfated ?-carrageenan and bisulfated ?-carrageenan contain oxygen bridge between 3 and 6 of the D-galactose, which is responsible for the conformational transition and gelatin. The ?-carrageenan is trisulfated and does not have this bridge required for gel formation [50]. Usually, it is used in concentration such as 2–5%
Gelatin
Gelatin is used as a thermally reversible gelling agent for encapsulation. Because of its amphoteric nature, it is an excellent candidate to incorporate with anionic-gel-forming polysaccharides, such as gellan gum. It is a protein derived by partial hydrolysis of collagen of animal origin. It has versatile functional properties, and forms a solution of high viscosity in water which set to a gel on cooling.
Milk Protein
Milk proteins are natural vehicles for probiotic cells, and owing to their structural and physicochemical properties, they can be used as a delivery system [51]
Whey Protein
It easily heats denatured which affect aggregation and reduction in emulsion stability. Whey proteins are heat sensitive and show inferior surface activities.
Cellulose Acetate Phthalate (CAP)
Because of its safe nature, CAP is used for controlling drug release in the intestine. It is not soluble at pH less than 5 but it is soluble at pH higher than 6 . This property is essential for probiotic encapsulation because the bilateral must not dissolve in the stomach but only in the gut. The disadvantage of CAP is that it cannot form gel beads by ionotropic gelation so capsules have been developed by emulsification. CAP is widely used as a coating agent because it provides better protection for microorganisms in simulated GI conditions.
REFERENCES
- The Encyclopedia of Food and Health, Volume 5 (2016)
- Advanced drug delivery reviews vol 161-162,2020, pg 1-21, Shadi Asgari, Ali Pourjavadi, Tine Rask Licht et al; Polymeric carriers for enhanced delivery of probiotics.
- Douillard FP, de Vos WM (2019) Biotechnology of health-promoting bacteria. Biotechnol Adv 37:107369. https:// doi. org/ 10. 1016/j.biote chadv. 2019. 03. 008
- Journal of Controlled Release, Volume 162, Issue 1, 20 August 2012, Pages 56-67, Microencapsulation of probiotics for gastrointestinal delivery, Michael T.CookabGeorgeTzortziscDimitrisCharalampopoulosa
- B. F. Gibbs, S. Kermasha, I. Alli, and C. N. Mulligan, “Encapsulation in the food industry: a review,” International Journal of Food Sciences and Nutrition, vol. 50, no. 3, pp. 213–224, 1999 View at: Publisher Site | Google Scholar
- C. P. Champagne and K. Kailasapathy, “Encapsulation of probiotics,” in Delivery and Controlled Release of Bioactives in Foods and Nutraceuticals, N. Garti, Ed., pp. 344–369, Woodhead, Cambridge, UK, 2008.View at: Google Scholar
- N. J. Zuidam and E. Shimoni, “Overview of microencapsulates for use in food products or processes and methods to take them,” in Encapsulation Technologies for Active Food Ingredients and Food Processing, N. J. Zuidam and V. Nedovic, Eds., pp. 3–29, Springer, New York, NY, USA, 2009.View at: Google Scholar)
- N. J. Zuidam and E. Shimoni, “Overview of microencapsulates for use in food products or processes and methods to take them,” in Encapsulation Technologies for Active Food Ingredients and Food Processing, N. J. Zuidam and V. Nedovic, Eds., pp. 3–29, Springer, New York, NY, USA, 2009.View at: Google Scholar
- M. J. Chen and K. N. Chen, “Applications of probiotic encapsulation in dairy products,” in Encapsulation and Controlled Release Technologies in Food Systems, J. M. Lakkis, Ed., pp. 83–107, Wiley-Blackwell, New York, NY, USA, 2007.View at: Google Scholar
- A. Mortazavian, S. H. Razavi, M. R. Ehsani, and S. Sohrabvandi, “Principle’s and method of microencapsulation of probiotic microorganisms,” Iranian Journal of Biotechnology, vol. 5, no. 1, pp. 1–18, 2007.View at: Google Scholar
- J. Klein, J. Stock, and K.-D. Vorlop, “Pore size and properties of spherical Ca-alginate biocatalysts,” European Journal of Applied Microbiology and Biotechnology, vol. 18, no. 2, pp. 86–91, 1983.View at: Publisher Site | Google Scholar
- H. Eikmeier and H. J. Rehm, “Stability of calcium-alginate during citric acid production of immobilized Aspergillus niger,” Applied Microbiology and Biotechnology, vol. 26, no. 2, pp. 105–111, 1987.View at: Google Scholar
- C. P. Champagene and P. Fustier, “Microencapsulation for delivery of probiotics and other ingredients in functional dairy products,” Functional Dairy Products, vol. 2, pp. 404–426, 2007. View at: Google Scholar
- N. J. Zuidam and E. Shimoni, “Overview of microencapsulates for use in food products or processes and methods to take them,” in Encapsulation Technologies for Active Food Ingredients and Food Processing, N. J. Zuidam and V. Nedovic, Eds., pp. 3–29, Springer, New York, NY, USA, 2009. View at: Google Scholar
- K. M. K. Kebary, S. A. Hussein, and R. M. Badawi, “Improving viability of Bifidobacteria and their effect on frozen ice milk,” Egyptian Journal of Dairy Science, vol. 23, pp. 319–337, 1998.View at: Google Scholar
- W. K. Ding and N. P. Shah, “Effect of various encapsulating materials on the stability of probiotic bacteria,” Journal of Food Science, vol. 74, no. 2, pp. M100–M107, 2009.View at: Publisher Site | Google Scholar
- C. P. Champagene and P. Fustier, “Microencapsulation for delivery of probiotics and other ingredients in functional dairy products,” Functional Dairy Products, vol. 2, pp. 404–426, 2007.View at: Google Scholar
- G. K. Gbassi and T. Vandamme, “Probiotic encapsulation technology: from microencapsulation to release into the gut,” Pharmaceutics, vol. 4, no. 1, pp. 149–163, 2012. View at: Publisher Site | Google Scholar
- T. Heidebach, P. Först, and U. Kulozik, “Microencapsulation of probiotic cells by means of rennet-gelation of milk proteins,” Food Hydrocolloids, vol. 23, no. 7, pp. 1670–1677, 2009.View at: Publisher Site | Google Scholar
- G. K. Gbassi, T. Vandamme, S. Ennahar, and E. Marchioni, “Microencapsulation of Lactobacillus plantarum spp in an alginate matrix coated with whey proteins,” International Journal of Food Microbiology, vol. 129, no. 1, pp. 103–105, 2009.View at: Publisher Site | Google Scholar
- Li B, Tian F, Liu X et al (2011) Effects of cryoprotectants on viability of Lactobacillus reuteri CICC6226. Appl Microbiol Biotechnol 92:609–616. https:// doi. org/ 10. 1007/s00253- 011- 3269-4
- Marcial-Coba MS, Cieplak T, Cahú TB et al (2018) Viability of microencapsulated Akkermansia muciniphila and Lactobacillus plantarum during freeze-drying, storage andin vitro simulated upper gastrointestinal tract passage. Food Funct 9:5868–5879.https:// doi. org/ 10. 1039/ C8FO0 1331D
- Heidebach T, F.rst P, Kulozik U (2009) Microencapsulation of probiotic cells by means of rennet-gelation of milk proteins. Food Hydrocoll 23:1670–1677. https:// doi. org/ 10. 1016/j. foodh yd. 2009.01. 006
- Mokarram RR, Mortazavi SA, Najafi MBH, Shahidi F (2009) The influence of multi stage alginate coating on survivability of potential probiotic bacteria in simulated gastric and intestinal juice. Food Res Int 42:1040–1045. https:// doi. org/ 10. 1016/j.foodr es. 2009. 04. 023
- T. Y. Sheu and R. T. Marshall, “Microencapsulation of lactobacilli in calcium alginate gels,” Journal of Food Science, vol. 54, pp. 557–561, 1993.View at: Google Scholar
- T. Heidebach, P. Först, and U. Kulozik, “Transglutaminase-induced caseinate gelation for the microencapsulation of probiotic cells,” International Dairy Journal, vol. 19, no. 2, pp. 77–84, 2009.View at: Publisher Site | Google Scholar
- K. Kailasapathy, “Microencapsulation of probiotic bacteria: technology and potential applications,” Current Issues in Intestinal Microbiology, vol. 3, no. 2, pp. 39–48, 2002.View at: Google Scholar
- Raghavi LM, Moses JA, Anandharamakrishnan C (2018) Refractance window drying of foods: a review. J Food Eng 222:267–275. https:// doi. org/ 10. 1016/j. jfood eng. 2017. 11. 03
- Zotarelli MF, Carciofi BAM, Laurindo JB (2015) Effect of process variables on the drying rate of mango pulp by refractance window. Food Res Int 69:410–417. https:// doi. org/ 10. 1016/j.foodr es. 2015. 01. 013
- Nindo CI, Feng H, Shen GQ et al (2003) Energy utilization and microbial reduction in a new film drying system. J Food Process Preserv 27:117–136. https:// doi. org/ 10. 1111/j. 1745- 4549. 2003.tb005 06.x
- Ochoa-Martínez CI, Quintero PT, Ayala AA, Ortiz MJ (2012) Drying characteristics of mango slices using the refractance windowTM technique. J Food Eng 109:69–75. https:// doi. org/ 10.1016/j. jfood eng. 2011. 09. 032
- Gover Antoniraj M, Maria Leena M, Moses JA, Anandharamakrishnan C (2019) Cross-linked chitosan microparticles preparation by modified three fluid nozzle spray drying approach. Int J Biol Macromol 147:1268–1277. https:// doi. org/ 10. 1016/j. ijbio mac. 2019. 09.25
- Maria Leena M, Gover Antoniraj M, Moses JA, Anandharamakrishnan C (2020) Three fluid nozzle spray drying for co-encapsulation of curcumin and resveratrol and controlled release. J Drug Deliv Sci Technol 57:101678. https:// doi. org/ 10. 1016/j. jddst. 2020. 101678
- A. H. King, “Encapsulation of food ingredients: a review of available technology, focussing on hydrocolloid,” in Encapsulation and Controlled Release of Food Ingredients, J. R. Sara and A. R. Gary, Eds., vol. 590 of ACS Symposium Series, pp. 26–39, American ChemicalSociety, Washington, DC, USA. View at: Google Scholar
- K. Kailasapathy, “Encapsulation technologies for functional foods and nutraceutical product development,” CAB Reviews, vol. 4, no. 6, pp. 1–19, 2009. View at: Publisher Site | Google Scholar
- Martín MJ, Lara-Villoslada F, Ruiz MA, Morales ME (2015) Microencapsulation of bacteria: a review of different technologies and their impact on the probiotic effects. Innov Food Sci Emerg Technol 27:15–25. https:// doi. org/ 10. 1016/j. ifset. 2014.09. 010
- Rajam R, Anandharamakrishnan C (2015) Spray freeze drying method for microencapsulation of Lactobacillus plantarum. J Food Eng 166:95–103. https:// doi. org/ 10. 1016/j. jfood eng. 2015.
- A. H. King, “Encapsulation of food ingredients: a review of available technology, focussing on hydrocolloid,” in Encapsulation and Controlled Release of Food Ingredients, J. R. Sara and A. R. Gary, Eds., vol. 590 of ACS Symposium Series, pp. 26–39, American Chemical Society, Washington, DC, USA.View at: Google Scholar
- C. Desmond, R. P. Ross, E. O'Callaghan, G. Fitzgerald, and C. Stanton, “Improved survival of Lactobacillus paracasei NFBC 338 in spray-dried powders containing gum acacia,” Journal of Applied Microbiology, vol. 93, no. 6, pp. 1003–1011, 2002. View at: Publisher Site | Google Scholar
- B. R. Bhandari, N. Datta, B. R. D'Arcy, and G. B. Rintoul, “Co-crystallization of honey with sucrose,” Food Science and Technology, vol. 31, no. 2, pp. 138–142, 1998.
- C. Desmond, R. P. Ross, E. O'Callaghan, G. Fitzgerald, and C. Stanton, “Improved survival of Lactobacillus paracasei NFBC 338 in spray-dried powders containing gum acacia,” Journal of Applied Microbiology, vol. 93, no. 6, pp. 1003–1011, 2002.
- A. Hedges and C. McBride, “Utilization of ?-cyclodextrin in food,” Cereal Foods World, vol. 44, no. 10, pp. 700–704, 1999.View at: Google Scholar
- K. G. H. Desai and H. J. Park, “Recent developments in microencapsulation of food ingredients,” Drying Technology, vol. 23, no. 7, pp. 1361–1394, 2005. View at: Publisher Site | Google Scholar
- A. Hedges and C. McBride, “Utilization of ?-cyclodextrin in food,” Cereal Foods World, vol. 44, no. 10, pp. 700–704, 1999.View at: Google Scholar
- Z. Dong, Q. Wang, and Y. Du, “Alginate/gelatin blend films and their properties for drug controlled release,” Journal of Membrane Science, vol. 280, no. 1-2, pp. 37–44, 2006.View at: Publisher Site | Google Scholar
- K. I. Draget, K. Steinsvåg, E. Onsøyen, and O. Smidsrød, “Na+ and K+alginate; effect on Ca2+-gelation,” Carbohydrate Polymers, vol. 35, no. 1-2, pp. 1–6, 1998.View at: Google Scholar
- D. Semyonov, O. Ramon, Z. Kaplun, L. Levin-Brener, N. Gurevich, and E. Shimoni, “Microencapsulation of Lactobacillus paracasei by spray freeze drying,” Food Research International, vol. 43, no. 1, pp. 193–202, 2010. View at: Publisher Site | Google Scholar
- N. T. Annan, A. D. Borza, and L. T. Hansen, “Encapsulation in alginate-coated gelatin microspheres improves survival of the probiotic Bifidobacterium adolescentis 15703T during exposure to simulated gastro-intestinal conditions,” Food Research International, vol. 41, no. 2, pp. 184–193, 2008.View at: Publisher Site |Google Scholar
- M. J. Chen and K. N. Chen, “Applications of probiotic encapsulation in dairy products,” in Encapsulation and Controlled Release Technologies in Food Systems, J. M. Lakkis, Ed., pp. 83–107, Wiley-Blackwell, New York, NY, USA, 2007. View at: Google Scholar
- S. Gaaloul, S. L. Turgeon, and M. Corredig, “Influence of shearing on the physical characteristics and rheological behaviour of an aqueous whey protein isolate-?appa-carrageenan mixture,” Food Hydrocolloids, vol. 23, no. 5, pp. 1243–1252, 2009.View at: Publisher Site | Google Scholar
- Y. D. Livney, “Milk proteins as vehicles for bioactives,” Current Opinion in Colloid and Interface Science, vol. 15, no. 1-2, pp. 73–83, 2010.View at: Publisher Site | Google Scholar


 PREETHA PUTHEZHATH*
PREETHA PUTHEZHATH*
 C K DHANAPAL
C K DHANAPAL







 10.5281/zenodo.13764819
10.5281/zenodo.13764819