Abstract
Objective: This study aims to formulate and evaluate oral disintegrating tablets (ODTs) incorporating clove powder as an analgesic for toothache. Clove is rich in eugenol and has shown analgesic activity, antimicrobial, and anti-inflammatory, and has been used as a traditional remedy for tooth pain. This study included Clove powder in ODTs to guarantee quick dissolving and timely toothache treatment for patients. The difficulty, though, is in creating a dose form that is both efficient and patient-friendly. Among the many benefits of oral disintegrating pills are their quick onset of action and convenience of administration without water. The active substance in the tablets was clove powder, and crosprovidone was used as a super-disintegrants. Methodology: Direct compression was used to prepare oral disintegrating tablets containing clove powder as active Pharmaceutical Ingredients and crospovidone, mannitol, aspartame, magnesium stearate, and talc as excipients. Evaluation parameters were evaluated, including thickness, disintegration time, weight variation, hardness, and friability. Pre-compression assessments were also carried out such as angle of repose, bulk, and tapped density. Results: The results demonstrate that clove powder might be successfully added to oral disintegration tablets and might be a good substitute for treating toothaches by offering immediate, localized pain relief in a handy, simple-to-give dosage form. Additionally, the study indicates that the prepared tablets may be a good substitute for treating conditions where Clove's therapeutic qualities are advantageous.
Keywords
Oral disintegrating tablets, clove powder, eugenol, super-disintegrants, antimicrobial activity, formulation, evaluation.
Introduction
-
- Oral Disintegrating Tablets
Orally disintegrating tablets are solid dosage forms that are formulated with the aim of improving the disintegrating and dissolution rates of a pharmaceutical product. A significant disadvantage of conventional solid dose forms is difficulty swallowing (dysphagia) or chewing in some patients (particularly geriatric and paediatric patients), gastrointestinal enzymatic degradation, and a slow commencement of action. To address this issue, tablet formulations that may quickly dissolve or disintegrate in the oral cavity are the best option. (1) Oral delivery is currently considered the gold standard in the pharmaceutical industry since it is deemed to be the most patient-friendly, safest, practical, and cost-effective method of administering medications. They enable immediate tablet disintegration following placement on the tongue, ensuring medication release in saliva. Drugs absorbed through the "oral cavity" instantly enter systemic circulation via the jugular vein, resulting in the instant commencement of the action, avoidance of pre-systemic metabolism, drug degradation in the gastric region, and enzymatic hydrolysis in the intestine. (2) Although ODTs have various advantages, they remain a niche product in the market due to their additional requirements. Flavour perception is a crucial factor to consider; developing bitter medications as ODTs is difficult, and flavour masking materials should be used since they are compressed with low force and have a porous matrix. (3)
-
- Toothache
Toothache is a pain around the teeth, which is due to irritation of the nerve present in the root of a tooth. It is mainly due to tooth decay (cavities), gum disease, dental abscess, tooth fracture or crack, teeth grinding (bruxism), sinus infection, and impacted wisdom teeth. Other causes are dental trauma, periodontal disease, dental cavities, inflammation of the pulp, or injuries to the teeth.(4–6) The intense pain linked to toothaches can greatly affect a person's overall well-being, leading to discomfort, challenges in eating and speaking, and disruptions in sleep. The main reasons for toothaches are frequently linked to Timely and effective relief from pain is essential for those experiencing toothaches, not only to ease their suffering but also to tackle the root dental problems and prevent additional issues from arising. For treatment of toothache, natural product which has analgesic and anti-inflammatory activity can be used. Clove (Eugenia caryophyllus) is well known for its analgesic and anti-inflammatory action, thus has encouraging potential for creating a tooth analgesic formulation using natural components that as clove,
-
- Clove - Its analgesic and other activity
Herbal Medicine is the oldest known healthcare system for humans, having been employed by a number of tribes throughout history. Herbal medical products are specifically defined as those having one or more active substances for medicinal purposes. (7) Clove (Eugenia caryophyllus) is one of the most precious spices, having been used for millennia as a food preserver and for a variety of therapeutic uses. It is a nutraceutical that provides health and therapeutic benefits, such as disease prevention and treatment. It has been used for ages as food preserves and medicinal plants, owing to its antioxidant and antibacterial properties (8). Many studies have confirmed the antibacterial, antifungal, antiviral, and anti-carcinogenic effects of spice plants. Clove, in particular, has drawn attention because of its high antioxidant and antibacterial effects that stand out among the other spices. Clove is a key vegetal source of phenolic chemicals such as flavonoids, hydroxybenzoic acids, hydroxycinnamic acids, Beta-Caryophyllene, Triterpenes, Tannins, and hydroxyphenyl propenes. Eugenol is the major bioactive ingredient of clove. (9).
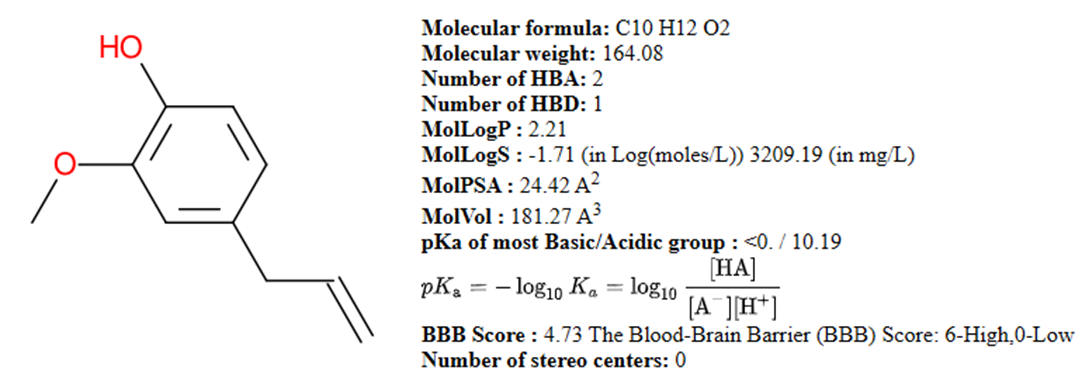
Fig 1: Drug Likeness Properties of Eugenol (Obtained from Molsoft L.L.C)
Analgesic activity
Because clove contains eugenol, it has strong analgesic effects. Cloves exhibit analgesic effects through a variety of mechanisms, such as TRP channel regulation, COX inhibition, and antioxidant activity. (10). Eugenol found in cloves has been demonstrated to suppress the cyclooxygenase (COX) enzymes, especially COX-2, which is involved in the manufacture of prostaglandins. Inflammation and pain are mediated by prostaglandins. Like NSAIDs, clove may help reduce the level of pain by blocking the COX enzymes. The phenolic and flavonoid chemicals found in cloves have potent antioxidant properties. Cloves help scavenge damaging free radicals and reduce oxidative stress, which in turn lessens discomfort linked with oxidative tissue damage. (11) It has been demonstrated that clove eugenol affects serotonin and dopamine, two neurotransmitters that control mood and pain perception. Clove may change how pain is perceived and lessen certain types of pain, such as headaches and migraines, by changing these molecules.
-
-
- Antimicrobial activity
The volatile oils in cloves may have antibacterial qualities, especially when it comes to bacterial infections. By disrupting the bacterial cell membrane and growth, eugenol's presence produces antibiotic activity that eventually results in cell death. (12)
Because its extracts also inhibit the growth of dental plaque and oral bacteria, clove is used in mouthwash and toothpaste.
Furthermore, clove oil has antifungal qualities against a variety of fungal strains, such as those that cause ringworm, athlete's foot, and candidiasis.
-
-
- Anti-oxidant Activity
Numerous phenolic components with potent antioxidant properties, including flavonoids, eugenol, and other volatile oils, are found in cloves. These substances have the potential to be analgesics and help prevent cell damage by reducing oxidative stress and neutralizing free radicals (13).
-
-
- Anti-inflammatory Activity
Clove's bioactive components, especially eugenol, a phenolic molecule with strong anti-inflammatory properties, are primarily responsible for its anti-inflammatory properties.(14)
- Material and Preparation
- Materials
- Clove (Eugenia caryophyllus) was Obtained from the local market. Collected from the local area of Akurdi.
- The Excipients (Table 1) were collected from the laboratory of Dr. D.Y Patil College of Pharmacy, akurdi, Pune.
Table no. 1. List of material used
|
Sr No.
|
Material Used
|
|
1
|
Clove Powder
|
|
2
|
Mannitol
|
|
3
|
Crospovidone
|
|
4
|
Aspartame
|
|
5
|
Magnesium Stearate
|
|
6
|
Surfactants
|
|
7
|
Talc
|
-
-
Instruments used
The Instruments used for the formulation and evaluation are given in Table 2.
Table no. 2. List of instruments used
|
Sr No.
|
Instruments Used
|
|
1
|
Electronic Weighing balance
|
|
2
|
Sieve shaker
|
|
3
|
Mortar Pestle
|
|
4
|
Hot air oven
|
|
5
|
Tablet Punching Machine
|
|
6
|
Disintegration Apparatus
|
|
7
|
Friabilator
|
-
-
Preparation
- For the preparation of the oral disintegrating tablets containing clove powder, direct compression was employed.
- Steps Involved in the preparation of the tablet is as follows:
- The clove buds were dried and were cleaned to remove dust (Fig 1).
- After cleaning the clove buds were pulverized.
- All other ingredients such as magnesium stearate, talc, crospovidone, and aspartame were weighed accurately using an electronic weighing balance.
- After that all the ingredients were mixed excluding glidants and lubricant for 15 minutes.
- The powder blend was mixed with the lubricant and gliding agent.
- The tablets were compressed into 500 mg tablets using a tablet punching machine by direct compression (Fig 2).
Table no. 3. Formulation Table
|
Sr No
|
Ingredients
|
F1 (mg)
|
F2 (mg)
|
Role
|
|
1
|
Clove Powder
|
170
|
170
|
API
|
|
2
|
Mannitol
|
310
|
310
|
Filler, Diluent
|
|
3
|
Aspartame
|
6
|
6
|
Sweetener
|
|
4
|
Crospovidone
|
4
|
6
|
Super-Disintegrants
|
|
5
|
Magnesium Stearate
|
4
|
4
|
Lubricant
|
|
6
|
Surfactant
|
2
|
2
|
To increase the solubility of clove powder
|
|
7
|
Talc
|
2
|
2
|
Glidants
|

Fig 1: Clove buds

Fig 2: ODT containing Clove Powder
Evaluation of the Tablets
- Pre-Compression Studies
- Angle of Repose
The fixed funnel method was employed to determine the results. A specific amount of powdered medicine was placed into the funnel, which was first stopped by a thumb. After the powder was expelled from the funnel, the angle of repose was measured and recorded in degrees (?).
(?)= tan-1 h/r
|
Excellent
|
<25>
|
|
Good
|
25-30
|
|
Moderate
|
30-40
|
|
Poor flow
|
>40
|
-
-
-
Bulk Density
To determine the bulk density of powder, measure the volume of a known mass of powdered sample in a graduated cylinder (100 mL).
Bulk Density = Weight of powder / Bulk Volume
-
-
- Tapped Density
To determine tapped density, weigh the powder combination accurately and pour it into a 100 mL glass cylinder. The Powder was tapped 100 times. The volume of the tapped powder combination was recorded and computed using an equation.
Tapped Density = Weight of powder / Tapped Volume
-
-
- Carr’s Index
The Carr’s Index determines the compressibility of a powder based on its tapped and bulk densities.
Carr’s Index= [(Tapped Density - Bulk Density) /Tapped Density] x 100
|
Excellent
|
5 - 15
|
|
Good
|
12 – 16
|
|
Fair
|
18 – 21
|
|
Slightly Poor
|
23 – 28
|
|
Poor
|
28 – 35
|
|
Very poor
|
35 – 38
|
|
Extremely poor
|
> 40
|
-
-
-
Hausner Ratio
The Hausner ratio compares a powder's tapped density to its poured (loose) bulk density.
Hausner Ratio = Tapped Density / Bulk Density
|
Excellent
|
1.00 – 1.11
|
|
Good
|
1.12 – 1.18
|
|
Fair
|
1.19 – 1.25
|
|
Passable
|
1.26 – 1.34
|
|
Poor
|
1.35 – 1.45
|
|
Very poor
|
1.46 – 1.59
|
|
Extremely poor
|
> 1.60
|
-
-
Post-Compression Studies
- Thickness of tablet
The tablet dimensions are measured with a calibrated dial caliper. Five tablets are randomly chosen from the sample formulation and their thickness is measured individually. The average thickness is then computed.
-
-
- Hardness test
The Hardness test was performed using a Monsanto hardness tester which determines the crushing strength of tablets. To shatter the tablet, place it lengthwise between the upper and lower plungers and apply stress with a threaded bolt. The tablet's hardness was measured as Kg/cm2.
-
-
- Weight Variation
The weight variation test involves weighing 20 tablets, computing the average weight, and then comparing each tablet's weight to that average. This approach is efficient in determining the consistency of medication content in tablets.
-
-
- Friability
Tablets are tested for friability using a Roche friabilator, which rotates at 25 rpm. Tablets are dropped from a certain height for 100 revolutions. After dusting and reweighing pre-measured pills, reliability is assessed to ensure less than 1?viation from the norm.
Friability = [(Initial weight - Final Weight) / Initial weight] X 100
-
-
- Disintegration test
Disintegration time measures how long it takes for a tablet to break down into little grains or bits. The test involves employing a basket rack assembly with six glass tubes measuring 7.75 cm long and 2.15 mm in diameter, each fitted with a 10-mesh sieve at the bottom. The assembly goes up and down 28 to 32 times per minute in 900 cc of medium at 37°C. Each tube holds six tablets, and disintegration time is measured by how long it takes for all fragments to pass through the sieve.
- RESULT
- Pre-compression
- Angle of repose
The angle of repose of both formulations F1 and F2 was found to be 28.34 + 0.12 and 29.56 + 0.16 respectively.
-
-
- Bulk Density
The Bulk density of both formulations F1 and F2 was found to be 0.576 + 0.13 and 0.589 + 0.15 respectively.
-
-
- Tapped Density
The tapped density of both formulations F1 and F2 was found to be 0.652 + 0.16 and 0.678 + 0.17 respectively.
-
-
- Carr’s Index
The Carr’s Index of both the formulations F1 and F2 was found to be 11.656 + 0.11 and 13.126 + 0.13 respectively.
-
-
- Hausner Ratio
The Hausner Ratio of both the formulations F1, and F2 was found to be 1.131 and 1.151 respectively.
|
Formulations
|
Angle of repose (?)
|
Bulk Density
|
Tapped Density
|
Carr’s Index
|
Hausner Ratio
|
|
F1
|
28.34 + 0.12
|
0.576 + 0.13
|
0.652 + 0.16
|
11.656 + 0.11
|
1.131
|
|
F2
|
29.56 + 0.16
|
0.589 + 0.15
|
0.678 + 0.17
|
13.126 + 0.13
|
1.151
|
-
- Post-Compression
- Thickness of tablet
The thickness of the tablets was found to be 5 + 0.11, the thickness of the tablet depends upon the size of the die punch.
-
-
- Hardness
The hardness of the tablets was found to be 4 + 0.3 to 3.8 + 0.5, in the which hardness of the tablet depends upon the punching force of the machine.
-
-
- Weight Variation
The weight variation of the tablets was found to be 0.495 + 0.031 to 0.497 + 0.023 which is done by taking the weight of 20 tablets.
-
-
- Friability
The Friability of the tablets was found to be 0.154 + 0.04 to 0.162 + 0.03, which is in an acceptable limit that is less than 1%.
-
-
- Disintegration time
The disintegration of the tablets was found to be 193 + 3.11 to 136 + 2.28.
|
Formulations
|
Thickness (mm)
|
Hardness (kg/cm2)
|
Weight Variation (g)
|
Friability (% wt. Loss)
|
Disintegration Time (sec)
|
|
F1
|
5 + 0.11
|
4 + 0.3
|
0.495 + 0.031
|
0.154 + 0.04
|
193 + 3.11
|
|
F2
|
5 + 0.13
|
3.8 + 0.5
|
0.497 + 0.023
|
0.162 + 0.03
|
136 + 2.28
|
-
CONCLUSION
The conclusion is that the formulation and evaluation of oral disintegrating (ODTs) with clove powder as an analgesic for toothache shows promising results in terms of quick onset of action and ease of use. Due to clove’s characteristic analgesic and anti-inflammatory activity, clove powder was used in the ODT formulation to provide efficient treatment for pain with quick disintegration. The tablets meet the required quality standards for disintegration time, hardness, and friability, which assures that the formulation will provide effective, localized relief for people suffering from toothache. The results of this study suggest that ODT based on clove powder may be a practical and effective substitute as an oral analgesic for the treatment of toothache and other dental problems. However, further clinical research and trials are required to validate the formulations in long-term efficacy, safety, and effectiveness in real-world situations.
REFERENCES
- Dhakal B, Thakur JK, Mahato RK, Rawat I, Rabin DC, Chhetri RR, et al. Formulation of Ebastine Fast-Disintegrating Tablet Using Coprocessed Superdisintegrants and Evaluation of Quality Control Parameters. SoonMin H, editor. Sci World J. 2022 May 19;2022:1–13.
- Battu SK, Repka MA, Majumdar S, Rao Y M. Formulation and Evaluation of Rapidly Disintegrating Fenoverine Tablets: Effect of Superdisintegrants. Drug Dev Ind Pharm. 2007 Jan;33(11):1225–32.
- Ghourichay MP, Kiaie SH, Nokhodchi A, Javadzadeh Y. Formulation and Quality Control of Orally Disintegrating Tablets (ODTs): Recent Advances and Perspectives. Yuksel N, editor. BioMed Res Int. 2021 Jan;2021(1):6618934.
- Cohen LA, Bonito AJ, Akin DR, Manski RJ, Macek MD, Edwards RR, et al. Toothache Pain. J Am Dent Assoc. 2008 Sep;139(9):1205–16.
- Bastos J, Gigante D, Peres K. Toothache prevalence and associated factors: a population?based study in southern Brazil. Oral Dis. 2008 May;14(4):320–6.
- Erratum: Managing tooth pain in general practice. Singapore Med J. 2023;64(6):413.
- Dhanraj Patil*1 AP. Formulation And Evaluation Of Clove-Based Chewable Tablet For Toothache Management. 2024 Aug 10 [cited 2025 Jan 12]; Available from: https://zenodo.org/doi/10.5281/zenodo.13293039
- Cortés-Rojas DF, De Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014 Feb;4(2):90–6.
- Neveu V, Perez-Jimenez J, Vos F, Crespy V, Du Chaffaut L, Mennen L, et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database. 2010 Jul 30;2010(0):bap024–bap024.
- Kamkar Asl M, Nazariborun A, Hosseini M. Analgesic effect of the aqueous and ethanolic extracts of clove. Avicenna J Phytomedicine. 2013;3(2):186–92.
- Chandrika UG, Karunarathna U. Anesthetics and analgesic activities of herbal medicine: Review of the possible mechanism of action. In: Features and Assessments of Pain, Anaesthesia, and Analgesia [Internet]. Elsevier; 2022 [cited 2025 Jan 13]. p. 47–56. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128189887000030
- Prabuseenivasan S, Jayakumar M, Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med. 2006 Dec;6(1):39.
- Lee KG, Shibamoto T. Antioxidant property of aroma extract isolated from clove buds [Syzygium aromaticum (L.) Merr. et Perry]. Food Chem. 2001 Sep;74(4):443–8.
- Han X, Parker TL. Anti-inflammatory activity of clove ( Eugenia caryophyllata ) essential oil in human dermal fibroblasts. Pharm Biol. 2017 Jan;55(1):1619–22..


 Rohit Khandare*
Rohit Khandare*
 Sarika Nikam
Sarika Nikam




 10.5281/zenodo.14880896
10.5281/zenodo.14880896