Abstract
Recent developments in floating delivery systems for pharmaceuticals (FDDS), which included the uniform dispersion of multiparticulate dose forms along the GIT, led to the development of gastro-retentive floating microspheres. This may result in less chance of local discomfort and more reliable drug absorption. As a dose form with remarkable buoyancy in the stomach, microballoons (MB), a multi-unit prolonged release with a sphere-shaped hollow wrapped in a strong polymer shell, have been developed. Because stomach acid has a lower relative density than this preparation for limited intestinal absorption, it is designed to float on top of it.The emulsion solvent diffusion method and enteric acrylic polymers are used to create and fill microballoons with drugs in their outer polymer casings. For medications with site-specific absorption, microballoons drug delivery systems have proven to be more significant in regulating release rate. As a viable strategy for stomach retention, the floating microballoons demonstrated gastroretentive controlled release distribution with effective ways to increase the bioavailability. By reducing the frequency of dosages, floating microspheres can increase patient compliance and improve the therapeutic efficacy of medications with short half-lives. Improved absorption of medications that only dissolves in the stomach; buoyancy lengthens the duration that food is retained in the stomach. Solvent diffusion is used to create floating microspheres. Optimized hollow microspheres will play a key role in innovative drug administration, especially in safe, efficient, and targeted in vivo distribution, which holds promise as a possible strategy for gastric retention.
Keywords
Gastric retention time, novel technique, floating drug delivery system, polymers, peptic ulcer.
Introduction
The oral method of drug administration has received the most focus out of all the routes of drug administration. Administering oral dosage forms with ease allows for greater flexibility in their design compared to many other options and different pathways that have led to the success of administering drugs orally. In order to enhance the predictability and increase the availability of drugs, short gastric residence times and unpredictable gastric emptying must be considered.
Challenges posed by digestion, metabolism, and various bodily conditions must be addressed. This led to the development of prolonged gastric residence time in oral controlled drug delivery systems. This is especially crucial for medications that have an absorption window in the stomach and duodenum, as well as for medications with stability issues.1 The gastro-retentive dosage form is one of the most preferable methods since one of the goals is to control the time spent in the stomach and thus achieve an extended and predictable drug delivery in the intestinal tract. These are mainly long acting; time released drug delivery systems which are also drug controlled. Therefore, employing this dosage form in the target area of the GI tract is beneficial especially for specializing the drug which targets the window of GI tract absorption.2
Basic GIT physiology3
The stomach is anatomically separated into three regions: the fundus, body, and antrum (pylorus). While the antrum is the primary location for mixing motions and serves as a pump for stomach emptying through pushing activities, the proximal portion, which is composed of the fundus and body, serves as a reservoir for undigested materials. Both when fasting and when eating, the stomach empties.

Figure.1 Anatomy of stomach
The interdigestive myloelectric cycle, also known as the migrating myloelectric cycle (MMC) is a sequence of interdigestive electrical events that occur during the fasting state and cycle through the stomach and intestine every two to three hours. It is further subdivided into four phases:
Phase 1 (Basic phase), which lasts 30 to 60 minutes and has occasional contractions.
Phase 2, also known as the pre-burst phase, lasts 20 to 40 minutes and is characterized by periodic contractions and action potentials.
Phase 3, the third phase, known as the burst phase, lasts 10 to 20 minutes and is characterized by brief, strong contractions.
Phase 4: Between phases 2 and 1 of two consecutive cycles, phase 4 lasts for 0–5 minutes.
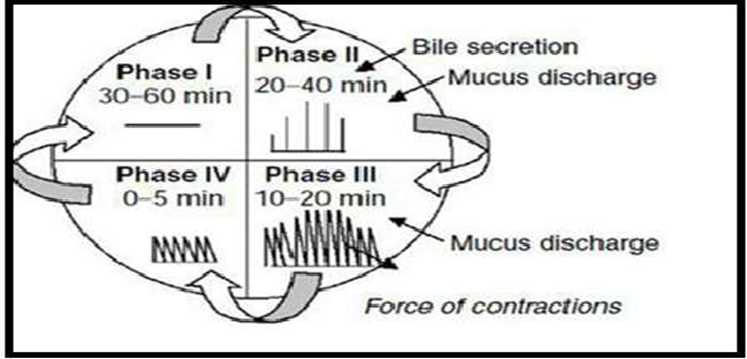
Figure.2.-Gastrointestinal motility pattern
Following the consumption of a mixed meal, the contraction pattern, also known as the digestive motility pattern-shifts from the fasted to the fed state.
Suitable Drug Candidates for Gastroretention4
Generally speaking, molecules with poor colonic absorption but superior absorption qualities at the higher regions of the GIT make good candidates for GRDDS.
- Drugs which are absorbed from the proximal part of gastro-intestinal tract(GIT)
- Drugs that are majorly absorbed from stomach and upper part of GIT
- Drugs which are less soluble or are degraded by alkaline pH.
Different approaches of the GRDDS5
Several strategies have been tried to improve oral dosage forms' stomach retention. While some are designed as multicomponent dosage forms, others are made as single components. GRDDS can be broadly divided into two types: floating and non-floating systems.
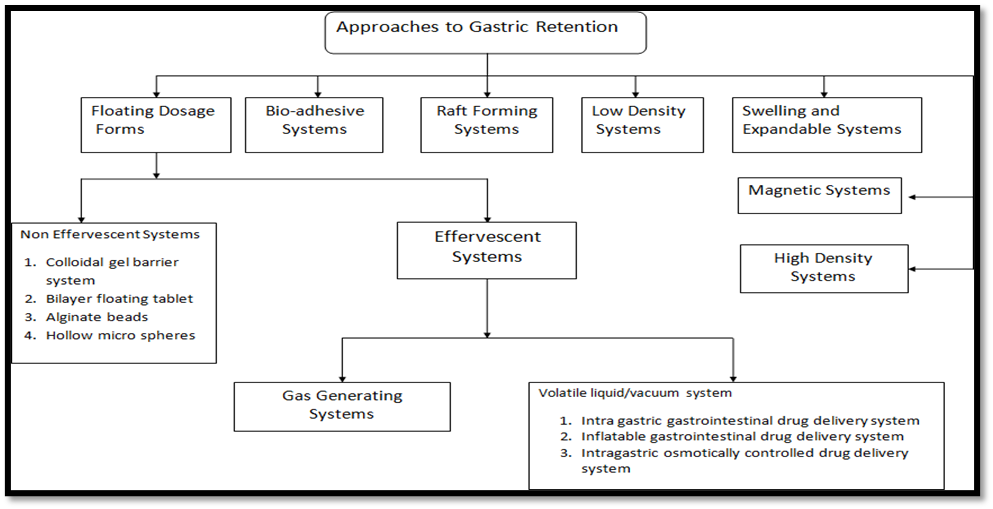
Figure 3: Classification of FDDS
Table 1: Various approaches for Gastroretentive Drug Delivery
|
S.no.
|
System
|
Description
|
|
1
|
High density /
Sinking system
|
These systems formulated by coating the drug on a heavy core or mixing it with inert materials, density exhibited higher than the density of gastric content. GI transit time of pellets can be enhanced by this method.
|
|
2
|
Swelling
System
|
Systems undergo swelling up to an extent which prevents their exit from the pylorus and the formulation is retained for a prolonged period of time.
|
|
3
|
Magnetic
System
|
In this type of dosage form, it comprises a smaller magnetic material(internal magnet) and another external magnet is applied over the position of the stomach.
|
|
4
|
Bioadhesive/
Mucoadhesive
System
|
Gastric retention increased by adhering the bioadhesive system to the gastric mucosal membrane, thus improving the bioavailability.
|
|
5
|
Expandable
System
|
Gastric emptying can be delayed when formulation is larger in size than the pyloric sphincter. Thus, gastro retention can be increased via a combination of substantial dimensions with high rigidity of formulations to withstand mechanical contractions of the stomach.
|
|
6
|
Unfoldable system
|
These systems undergo unfolding and enlarge in size after swallowed and remain lodged at sphincter thus, delaying gastric emptying.
|
|
7
|
Low density/ floating system
|
These systems are formulated with density less than gastric fluids and so that it remains buoyant/float in the stomach without affecting gastric emptying.
|
|
a.
|
Effervescent
|
Comprises of the swellable polymers like chitosan, and effervescent agents like sodium bicarbonate, disodium glycine carbonate, citric acid, and tartaric acid
|
|
b.
|
Non-effervescent
|
Formulated using highly swellable or gel-forming cellulose type hydrocolloids, matrix-forming polymers, and polysaccharides
|
|
c.
|
Microporous
|
Drug reservoir is encapsulated within the microporous compartment containing pores. The floating chamber contains entrapped air that provides the ability to remain buoyant over the gastric content to the delivery system. The gastric fluid penetrates through the aperture, dissolves the drug and carries the dissolved drug for absorption in the stomach and upper part of the small intestine
|
|
8
|
Ion-exchange resin
system
|
Here ion-exchange beads are treated with bicarbonate and the negatively charged drug is then bound to the resin. To prevent the loss of carbon dioxide, the beads are entrapped in a semipermeable membrane. On oral administration, the exchange of bicarbonate and chloride occurs in the stomach.
|
|
9
|
Raft system
|
Raft-forming system is one of the most extensively used systems due to the advantage of prolonged and predictable drug delivery provided by this system. This system is effective in releasing the drug in a sustained manner. Raft forming systems are hydrogels at room temperature and undergo gelation on contact with body fluids or with a change in pH. The main objective behind the development of this system is to minimize the dosing frequency and to enhance the efficacy of drug via localization at the desired site of action.
|
A. Non-floating system: These gastro-retentive drug delivery systems are retained in the stomach through a variety of mechanisms, but they do not float there. The non-floating system is further separated into:
- High density (sinking) drug delivery system
- Bioadhsive or mucoadhesive system
c. Magnetic system
d. Unfoldable system
B. Floating system: Unlike high density drug delivery systems, FDDSs have a density lower than the gastric contents, which allow them to stay buoyant in the stomach for an extended amount of time without affecting the gastric contents. Floating Drug Delivery Systems (FDDS) is a method devised to extend the gastric residence time of dosage forms. FDDS is beneficial for medications that work within the proximal gastrointestinal tract and has a lower bulk density compared to gastric fluids, allowing it to float in the stomach for an extended duration without impacting stomach emptying(5). Figure 1 illustrates the mechanism of floating of the floating drug delivery system (FDDS), also known as a low density system
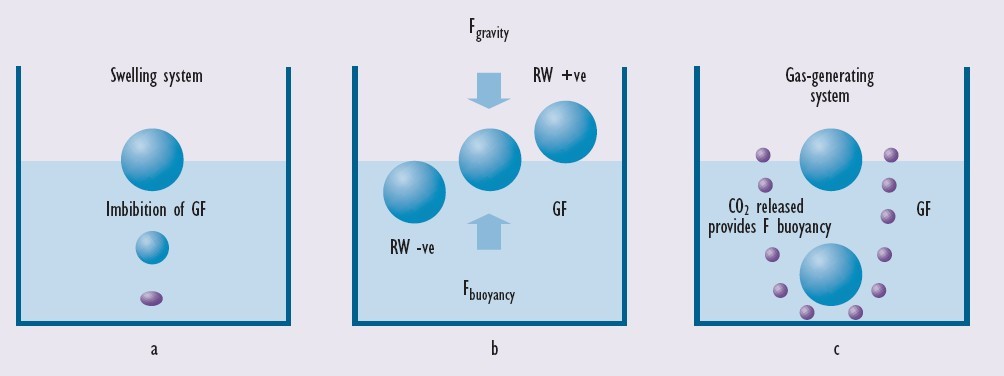
Figure 4: The mechanism of floating system7
Floating drug delivery system can be divided into:
-
- Effervescent system
- Non-effervescent system
- Hydrodynamically balanced system
- Microbaloons or hollow microspheres
- Alginate beads
- Microporous compartment
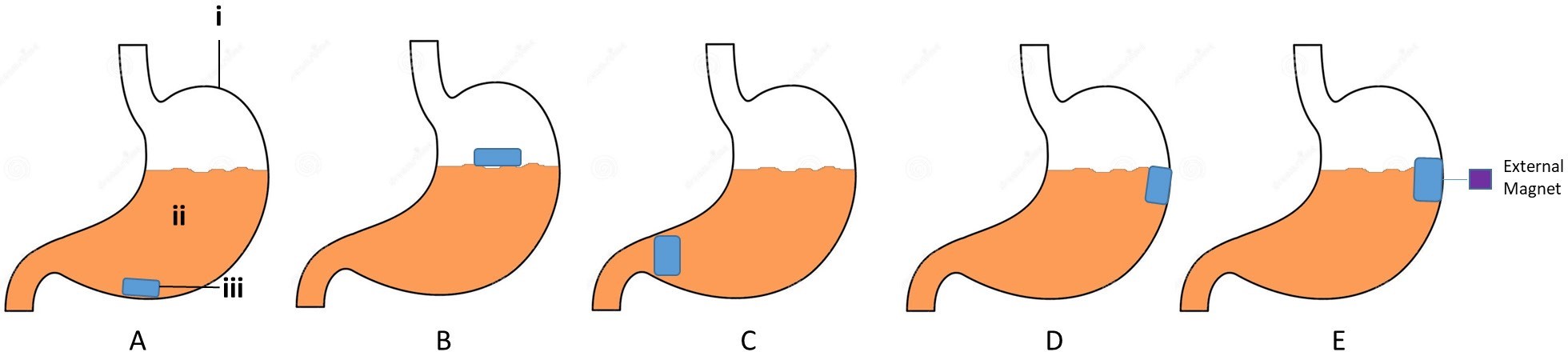
Figure 5. Different types of gastro-retentive drug delivery system.
A) High density system, B) floating/low density system, C) inflatable system, D) muco-adhesive system, E) magnetic system (i- stomach, ii- gastric fluid, iii- dosage form)6
Microballoons
Due to the center hollow region inside the microsphere, microballoons are regarded as one of the most advantageous buoyant systems, offering the special benefits of multiple unit systems together with improved floating capabilities. Simple solvent evaporation, emulsion-solvent diffusion, single and double emulsion techniques, phase separation coacervation techniques, polymerization techniques, spray drying and spray congealing techniques, and hot melt encapsulation techniques are some of the innovative methods used in their preparation. The kind of polymer, plasticizer, and solvents used in the preparation process primarily determine the gradual release of medications at the appropriate rate and improved floating qualities. Hollow microspheres are made from polymers such polylactic acid, Eudragit S, hydroxypropyl methylcellulose, and cellulose acetate and the polymer-plasticizer ratio and polymer concentration can be modified to control the drug's release.
Mechanism of Flotation of Microballoons7
Because of their low density and adequate buoyancy, Microballoons can float above gastric fluid and stay in the stomach for an extended amount of time. Increased gastric retention and less variation in plasma drug concentration are the results of the drug being released gradually at the desired rate while the system floats over the stomach fluid. A colloidal gel barrier that regulates the pace of fluid penetration into the device and, in turn, the release of drugs is created when microballoons come into contact with gastric fluid. The hydration of the nearby hydrocolloid layer keeps the gel layer intact as the dosage form's outer surface dissolves. The air that the expanded polymer traps lowers the density compared to the stomach juice, giving the microspheres buoyancy. A small amount of stomach content is necessary, though, in order to achieve buoyancy.If the mucoadhesive qualities of the particles have not been altered by the stomach contents, specifically non-adherent mucus, adherence to the stomach wall will be achievable during the emptying process in both fed and fasted states. The most recent developments include Gelucire floating granules, acrylic resin hollow microspheres (microballoons), Eudragit, hypromellose, polyethylene oxide, cellulose acetate, polystyrene floatable shells, and polycarbonate floating balloons.
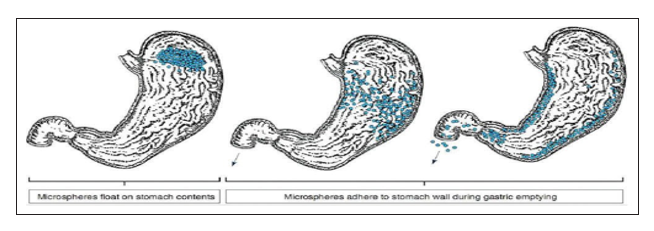
Figure 6: Mechanism of floatation of microballoons1
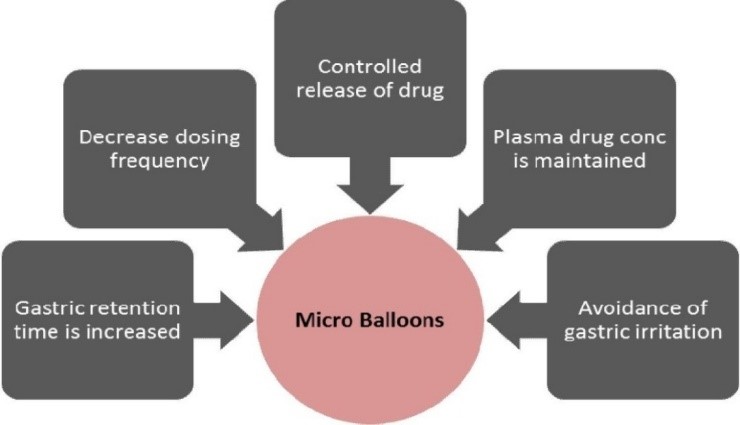
Figure 7: Rationale behind microballoons8
Mechanism of Drug Release5
The following mechanisms can lead to drug release from microballoons:
Diffusion: Drug diffusion begins as gastrointestinal fluid is absorbed to the interior of the drug delivery system, which is followed by dissolution of the drug and slow release from the delivery system to the exterior by diffusion mechanism.
Erosion: The drug contained within the particle can be released when certain coatings are made to erode progressively over time,there by the drug action may be sustained.
Osmosis: osmotic pressure is built on uptake of water within the drug delivery system, by suitable means, which pushes the drug out of delivery system in a sustained manner.
Advantages8
- By reducing the frequency of doses, it enhances patient compliance,
- Due to variations in drug plasma, bioavailability improves even with the first pass effect,
- Avoiding concentration, continuous medication release maintains a desired plasma drug concentration,
- Due to buoyancy, the gastric retention duration is prolonged,
- More effective absorption of medications that dissolve solely in the stomach,
- Controlled drug releases over an extended length of time,
- Site-specific drug delivery to stomach can be possible,
- Better than single unit floating dosage forms,microballoons distribute the medicine equally and eliminate the possibility of dose dumping,
- Gastric irritation can be avoided due to the extended release action,
- Better therapeutic effect can be achieved for drugs with short half-life.
Disadvantages8
- FDDS is not an appropriate candidate for drugs that irritate the stomach mucosa.For instance, digoxin, theophylline, corticosteroids, iron (ferrous sulfate), oral contraceptives, tricyclic antidepressants, NSAIDs, and some antibiotics,
- Drugs that undergo first-pass metabolism and are absorbed throughout the GIT, such as Nifedipine, may not be desirable.
- They are also not favorable candidates for drugs that have stability or solubility issues in the stomach.For example Ranolazine
- The "all or none concept" that is connected to single unit floating capsules or tablets can be avoided by creating multiple unit systems, such as floating microballoons or microballoons.
Factors impacting gastric retention9,10,11
Size of the dosage form
Gastric retention is significantly impacted by the dosage form's size. The GRT was longer for dosage forms with a diameter more than 7.5 mm than for those with a diameter of 9.9 mm.
Shape of the dosage form
One crucial element to take into account while creating a dosage form is its shape. With a 24-hour GRT of 90–100%, ring- and tetrahedron-shaped devices function better than other types in terms of stomach retention.
Density of a dosage form
When it comes to gastroretention, a dosage form's density matters. In gastric fluids, dose forms with a lower density than the contents of the stomach may float, allowing for gastroretention. Conversely, high-density systems have a propensity to sink to the bottom of the stomach. The drug or treatment is effectively separated from the pylorus by both dosage schedules.
Nature of food intake
The kind of food ingested has a significant impact on gastroretention. The calorie content, feeding frequency, meal viscosity, and meal volume all affect dosage form retention. In general, gastroretention is prolonged when food is present. For instance, a substantial breakfast rich in lipids and proteins might increase gastroretention by 4–10 hours. Fatty acid salts or indigestible polymers can alter the stomach's motility pattern to a fed condition, extending the duration of medication release and lowering the rate of stomach emptying. GRT can be increased
by four to 10 hours with a meal that is high in proteins and fats
Gender, posture, and age
Compared to men, women often have slower stomach emptying rates.GRT is not much different between upright, ambulatory, and supine positions. Gastric emptying slows down in the elderly.
Single or multiple unit formulation
Comparing multiple unit formulations to single unit dosage forms, the former allow co-administration of units with different release profiles or containing incompatible substances, allow a larger margin of safety against dosage form failure, and demonstrate a more predictable release profile with negligible performance impairment due to unit failure.
Fed or unfed state
GI motility during fasting is typified by bursts of vigorous motor activity or the migration of the myoelectric complex (MMC), which happens every 1.5 to 2 hours. If the timing of the formulation's administration aligns with that of the MMC, the unit's GRT should be extremely brief as the MMC removes undigested material from the stomach. However, MMC is delayed and GRT is significantly longer in the fed condition.
Feed frequency
Because of the low frequency of MMC, giving consecutive meals instead of a single meal can result in a GRT rise of more than 400 minutes.
Concomitant drug administration
Anticholinergics like Atropine and Propantheline, Opiates like Codeine and Prokinetic agents like Metoclopramideand Cisapride.
Biological factors – Diabetes and Crohn’s disease.
Materials for Preparation of Microballoons12,13
Drugs
Drugs with narrow therapeutic window in GI tract, mainly absorbed from stomach and upper part of GIT, locally act in the stomach, degrade in the colon, disturb normal
colonic bacteria. E.g. Aspirin, Salicylic acid,Ethoxybenzamide, Indomethacin and Riboflavin, Para amino benzoic acid, Furosemide, Calcium supplements, Chlordiazepoxide, Scinnarazine, Riboflavin, Levodopa,Antacids, Misoprostol, Ranitidine HCl, Metronidazole and Amoxicillin trihydrate.
Polymers
Cellulose acetate, chitosan, eudragit, acrycoat, methocil, polyacrylates, polyvinyl acetate, carbopol, agar, polyethylene oxide, polycarbonates, acrylic resins and polyethylene
Solvents
It should have good volatile properties, so that it should easily come out from the emulsion leaving hollow microspheres eg ethanol, dichloromethane (DCM),acetonitrile,acetone, isopropyl alcohol(IPA),dimethylformamide (DMF).
Processing Medium
It is used to harden the drug polymer emulsified droplets when the drug polymer solution is poured into it, should not interact with the former; mainly used processing medium are liquid paraffin, polyvinyl alcohol and water.
Surfactant
They are stabilizers or emulsifiers, play the role of hardening the microspheres as well. E.g. tween 80, span 80 and SLS.
Cross linking agent
Chemical cross-linking of microspheres can be achieved using cross linking agents such as formaldehyde,glutaraldehyde or by using di acid chlorides such as terephthaloyl chloride. The method is limited to drugs that do not have any chemical interaction with the cross-linking agent.
Hardening agent
This helps to harden the microspheres formed in the processing medium eg n-hexane, petroleum ether (in case the processing medium is liquid paraffin)
Table 2: Components needed to prepare Micro balloons12
|
Components
|
Goal
|
Examples
|
|
Polymers
|
control the drugs release rate
|
Polyacrylates, Polyvinyl Acetate, Carbopol, Agar,Polyethylene oxide, Polycarbonates, Acrylic resins and Polyethylene etc. Cellulose acetate, Chitosan, Eudragit, Acrycoat, Methocel
|
|
Solvents
|
Should have high volatile characteristics, allowing them to quickly separate from of the emulsion, having left hollow microspheres.
|
Ethanol, Dichloromethane (DCM), Acetonitrile, Acetone,Isopropyl alcohol (IPA), Dimethylformamide (DMF)
|
|
Processing Medium
|
When the drug liquid solution is began pouring into it, it is used to harden the drug polymer dispersed droplets and must not interact with both the former
|
Liquid paraffin, Polyvinyl alcohol and Water
|
|
Surfactant
|
they seem to be stabilisers or dispersants that also serve to toughen the microspheres
|
Tween 80, Span 80 and SLS
|
|
Hardening agent
|
aids inside the hardening of microspheres constructed inside the processing medium
|
n-hexane, Petroleum ether
|
Development14
Floating microspheres are non-effervescent, gastro-retentive drug delivery methods. In the strictest sense, hollow microspheres are spherical, coreless particles. These microspheres, which are ideally smaller than 200 micrometers, are characterized as free-flowing powders made of proteins or synthetic polymers. The use of solid biodegradable microspheres with a drug dissolved or disseminated throughout the particle matrix may allow for controlled drug release. Microballoons are often made using straightforward solvent evaporation or solvent diffusion/evaporation procedures. Eudragit S, polycarbonate, cellulose acetate, Agar, low methoxylated pectin, and calcium alginate are frequently utilized as polymers. The amount of polymer, the plasticizer–polymer ratio, and the solvent utilized all affect buoyancy and drug release. The medication is either dissolved or dispersed in the polymer solution after the polymer is dissolved in an organic solvent. To create an oil in water emulsion, the drug-containing solution is subsequently emulsified into an aqueous phase that contains polyvinyl alcohol. The organic solvent is removed once a stable emulsion has formed, either by stirring constantly or by raising the temperature under pressure. Polymer precipitation results from the solvent removal at the o/w interface of droplets, creating a cavity and making them hollow to give them the ability to float.15
Method of preparation
Solvent Evaporation Method16
Among the polymers used in the development of these systems include ethyl cellulose, HPMC KM4, and Eudragit. To create a homogenous polymer solution, polymers are combined with drugs and then dissolved in ethanol, acetone, or dichloromethane, either alone or in combination. Pouring the resultant solution into 100 milliliters of liquid paraffin while it rotates at 1500 rpm. After three hours of heating to 35°C, the emulsion is created. The acetone or dichloromethane is totally evaporated once a stable emulsion has formed, and the resulting solidified microspheres are then filtered through Whattman filter paper. The floating and sustained properties are attributed to these hollow microspheres.
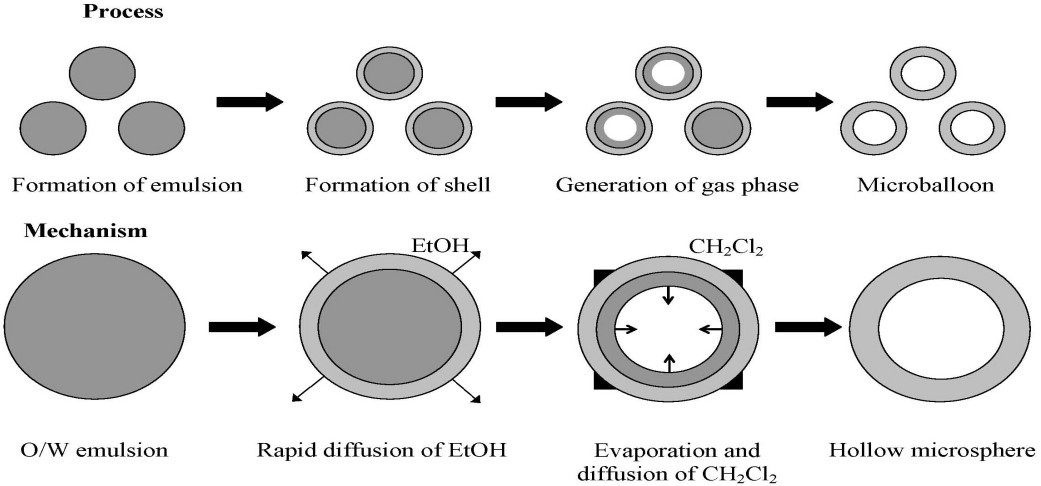
Figure 8: Solvent evaporation method
Emulsion solvent diffusion method17
This technique involves adding a polymer and drug solution in ethanol, methylene chloride, to an agitated polyvinyl alcohol aqueous solution. The ethanol quickly separates into the external aqueous phase, and the polymer precipitates around the methylene chloride droplets. When trapped methylene chloride evaporates, internal cavitis are created inside the microparticles.
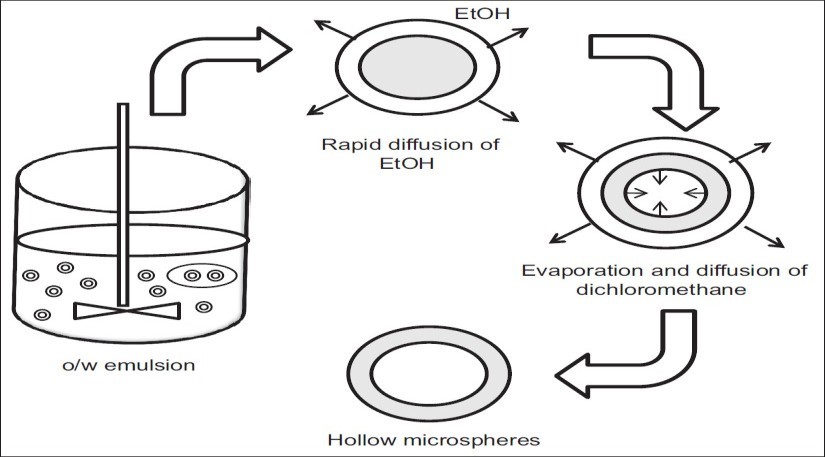
Figure 9: Emulsion solvent diffusion method
Single Emulsion Technique

Figure 10: Single Emulsion Technique
Double Emulsion Technique
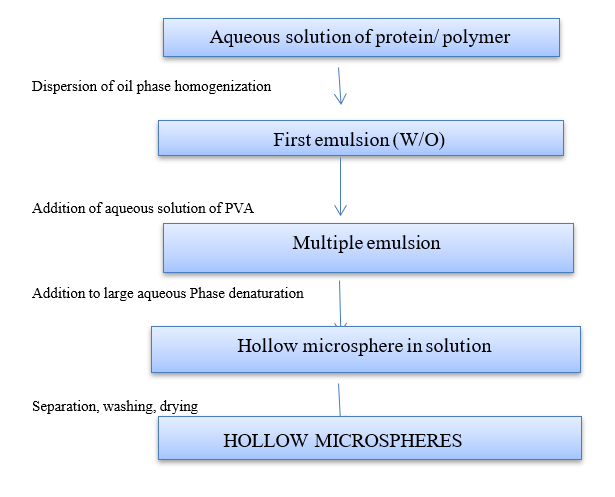
Figure 11: Double Emulsion Techniques
Coacervation phase separation technique18
The primary steps in this procedure are as follows:
Step 1: A coating polymer solution is used to spread the core material.
Step 2: In the liquid manufacturing vehicle phase, the coating is achieved by carefully combining the coating solution and core material.
Step 3: Use the following techniques to solidify the coated polymer.
- Thermal Change:
At 80 ºC, the polymer is vigorously stirred to dissolve it in cyclohexane. The drug is then added to the aforementioned solution while being constantly stirred. The microsphere is produced by putting it in an ice bath to lower the temperature. After being twice cleaned with cyclohexane, the product is allowed to air dry.
- Non-Solvent Addition:
First, the drug is distributed throughout a closed beaker filled with toluene and propyl isobutylene after the polymer has been dissolved in it for six hours at 500 rpm. With constant stirring, the resulting solution is added to benzene. The microcapsules are allowed to air dry for two hours after being cleaned with n-hexane.
- Polymer addition
Methylene blue is used as a core material after the polymer (ethylcellulose) is dissolved in toluene to create microspheres. The addition of liquid polybutadiene achieves coacervation. Hexane is added as a nonsolvent to solidify the polymer covering. After that, the final product is cleaned and allowed to air dry.
- Salt addition
Corn oil is used to dissolve the oil-soluble vitamin, which is then added to the gelatin solution at 50ºC. Adding sodium sulphate causes coacervation, which gives the gelatin a consistent coating. After being gathered, the microspheres are cleaned, refrigerated, and dried.
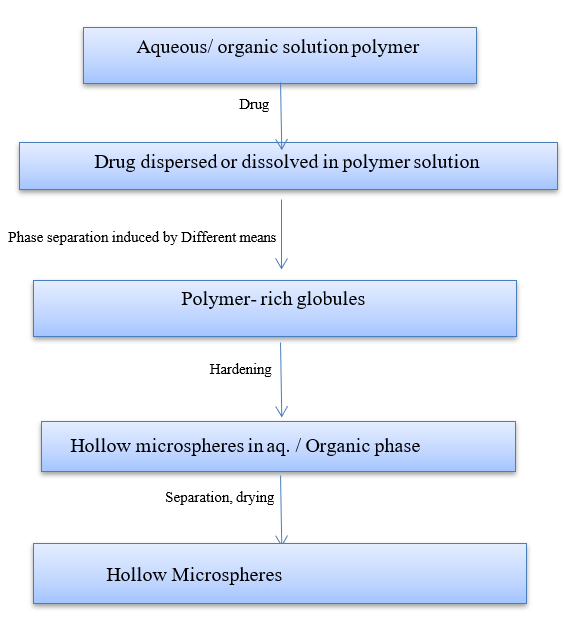
Figure 12: Coacervation Phase Separation Method
Spray drying technique17,19
The medication is introduced while the polymer solution is being homogenized at a high speed. When upward dispersion in a hot air stream is atomized, tiny droplets or fine mist are created. Rapid solvent evaporation creates microspheres that range in size from 1 to 100 ?m. It uses a cyclone separator to separate the microspheres from the heated air. The ability to operate in aseptic circumstances, which results in the quick and porous creation of microparticles that can be employed for poorly soluble medications, is a significant benefit of this process.
Spray drying: - The coating solidification can be done by rapid evaporating of solvent in which coating material is dissolved
Spray congealing: - The coating solidification can be done by thermally congealing a molten coating material. The removal of solvent is done by sorption, extraction or evaporation
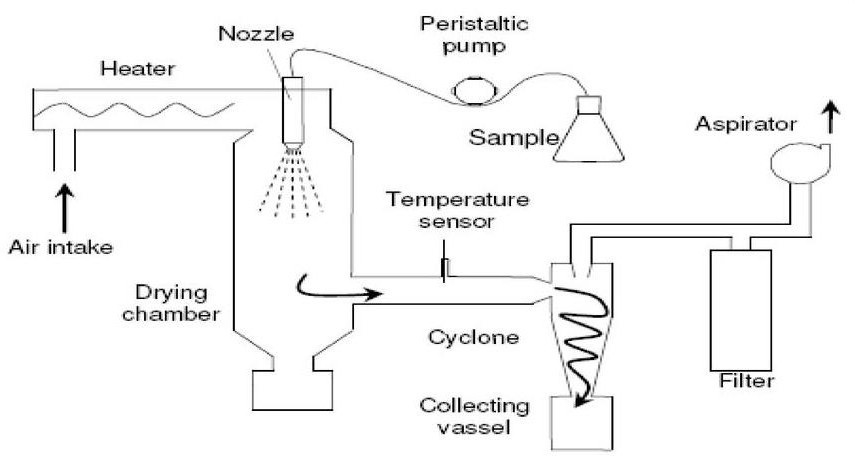
Figure 13: Spray drying and spray congealing
Evaluation of hollow microspheres16,20
Percentage Yield
The percentage yield of the hollow microspheres is determined
for drug and is calculated using the following equation
Yield=M/Mo x 100
Where M = weight of beads
Mo = total expected weight of drug and polymer
b) Micromeritic properties
The micromeritic characteristics of microballoons, including particle size and shape, bulk density, tapped density, Carr's index and angle of repose establish Hausner's ratio and flow characteristics. An optical microscope is used to measure the particle size, and a calibrated ocular micrometer is used to calculate the average particle diameter (by measuring 200 to 300 particles). The liquid displacement method determines true density; a bulk density instrument measures the change in volume to derive tapped density and compressibility index; and the fixed funnel method determines angle of repose. Scanning electron microscopy confirms that microspheres are hollow. The compressibility/carr’s index was calculated using following
formula:
I = Vb –Vt / Vb x 100
Where, Vb is the bulk volume and
Vt is the tapped volume.
The value given below 15% indicates a powder with usually give rise
to good flow characteristics, whereas above 25% indicate poor
flow ability.
True density is determined using a Helium densitometer. Angle of repose of the micro balloons are determined by the fixed funnel method.
Porosity (e) is calculated using the following equation:
e = {1- (tapped density/true density)} ×100
c) In vitro buoyancy
A suitable number of empty or hollow microspheres are added to 900 milliliters of 0.1N HCl. In the dissolving equipment, the mixture is agitated for 8–10 hours at 100 rpm. The buoyant microsphere layers are pipetted and filtered out after 8 to 10 hours. Filtration separates the particles that are in the sinking particulate layer. Both types of particles—settled and buoyant microspheres—are dried in a desiccator until their weight remains constant. Both the empty and hollow microsphere fractions are weighed, and the weight ratio of floating microspheres to the total of floating and sinking microspheres is used to calculate in vitro buoyancy.
Buoyancy (%) = {Wf / (Wf + Ws)} × 100
Where, Wf and Ws are the weights of the floating and settled microspheres
d) Electron scanning microscopy
A gold-coated ion sputter is used to implant dry hollow microspheres on an electron microscope brass stub. After that, spectro random scanning of the stub is used to capture images of the microsphere. Microspheres are observed with a 20KV accelerating voltage.
e) Buoyancy Lag time
It is the amount of time it takes for gastroretentive formulations to adhere to the dissolving medium's surface. It is established utilizing a USP dissolution apparatus with a testing media of 900 mL of 0.1 N HCl solution kept at 37°C. Floating lag time is the amount of time needed to float various dose forms.
f) Floating Time
This establishes the dose form's buoyancy. 900 mL of dissolving medium maintained at 37°C is utilized in this test, along with a particular dissolution apparatus based on the kind of dosage form. Visual observation is used to establish the dose form's floating time or floating duration.
g) Density/specific gravity
Estimates of specific gravity are crucial for GRDDS of both low and high densities. Specific gravity is determined using the displacement method.
h) In vivo studies
In vivo studies are carried in healthy male albino rabbits which weigh 2-2.5 kg. The animals are kept fasted for 24 hours before the experiment begins; however, they can be given free access to food and water during the experiments. Collect blood samples (2 mL) from the marginal ear vein into heparinized centrifuge at different time intervals.
Applications of floating microspheres21
1. Floating microspheres are used as carriers for drugs with so-called absorption windows for example antiviral, antifungal and antibiotic agents(sulphonamides, quinolones, penicillins, cephalosporins,aminoglycosides and tetracyclines) are taken up only from very specific sites of the GI mucosa.
2. Hollow microspheres of non-steroidal anti-inflammatory drugs provide controlled release and reduce the major side effect of gastric irritation. For example, floating microspheres of indomethacin are quite beneficial for rheumatic patients.
3. Sparingly soluble and insoluble drugs can be easily formulated as floating microspheres,Weakly basic drugs which are poorly soluble at an alkaline pH the rate-limiting step in release is solubility, hollow microspheres may avoid this risk by restricting such drugs to the stomach.Thus gastroretentive floating microspheres will enhance the bioavailability by altering the absorption profile of the active agent.
4. Hollow microspheres are known to improve the pharmacotherapy of the stomach by releasing drug locally and providing high drug concentrations at the gastric mucosa, thus help in causing eradication of Helicobacter pylori from the sub-mucosal tissue of the stomach. It also help in treatment of stomach and duodenal ulcers, gastritis and oesophagitis.
CONCLUSION
Microballoons have low-density and sufficient buoyancy to float over gastric contents and remain in stomach for prolonged period, without causing any irritation to gastro intestinal tract and releasing drug in controlled manner at desired rate, resulting in the reduced fluctuations in plasma drug concentration.Microballoons are considered to be more advantageous than the other floating gastroretentive drug delivery systems as they do not require lag time and floating time for the buoyancy.Hollow spheres promises to be a potential approach for the gastric retention. Microballoons possess efficient means of enhancing the bioavailability. Optimized microballoons are novel drug delivery, particularly in diseased cell sorting, diagnostics, gene & genetic materials, safe, targeted and effective in vivo delivery.
REFERENCES
- Grandhi Srikar, Dadi Shanthi, J. Ramesh, V. Kalyani, I. Nagamma: Floating Microspheres: A Prevailing Trend in the Development of Gastroretentive Drug Delivery System, Asian Journal of Pharmaceutics • Oct-Dec 2018 • 12 (4),235-242
- Sudhir Maddela, Ch. Bhavani Devi, K. Sumalatha, V Harshitha, D. Chandana, Sk. Afrin, M. Saranya,D. Priyanka, M. Saranya, and T. L. N. S. Anjana : Microbaloons: An Incredible Gastro Retentive Drug Delivery System, Journal of Cardiovascular Disease Research Vol12,Issue 05,2021,1764-1773
- Gayatri Verma, Dr. Anand Mahalwar, Ashwanee Kumar Sahu, Yugal Kishor Rajput, Rajendra Kumar Sahu, Omprakash Sahu,Poonam Rajput: The Microballoons Drug Delivery System Its Inhacment Of Bioavailability Of Ramipril Drug, Journal of Pharmaceutical Negative Results Volume 13,Special Issue 10,2022,4031-4045
- Vinay Kumar Katakam, Jagan Mohan Somagoni, Sunil Reddy, Chandra Mohan Eaga, Bala Ramesha Chary Rallabandi, Madhusudan Rao Yamsani: Floating Drug Delivery Systems: A Review, Current Trends in Biotechnology and Pharmacy Vol. 4 (2) 610-647 April 2010,610-647
- Anamika Saxena, Kalpesh Gaur, Virendra Singh, Rajneesh Kant Singh, and Ashok Dashora: Floating Microspheres as Drug Delivery System, AJPPS 2014, 1(2),27-36
- Pranit P. Hajare, Punit R. Rachh: Gastroretentive Microballoons: A Novel Approach For Drug Delivery, IJPSR (2020), Volume 11, Issue 3,1075-1083
- Himal Paudel Chhetri, Panna Thapa: An Overview on Gastroretentive Drug Delivery System,Journal of Science,Engineering,Technology, Vol.10, No.I, November, 2014, pp 90-103
- Snehal Patel, Chintan Aundhia, Avinash Seth, Nirmal Shah, Kartik Pandya, Chainesh Shah, Vinod Ramani, Ankur Javia: Microballoons: A Novel Approach in Gastro-retention Floating Drug Delivery System (FDDS), Pharma Science Monitor 7(2), Apr-Jun, 2016,332-345
- Payal B. Chavan, Prashant S. Malpure, Gokul S. Talele, Abhishek R. Kadam: The Latest Advancements: A Comprehensive Review of Microballoons for Enhanced Gastroretention, Asian Journal of Pharmaceutical Research and Development. 2024; 12(3), 222-229
- Natasha Sharma, Dilip Agarwal, M.K. Gupta and Mahaveer Pr. Khinchi: A Comprehensive Review on Floating Drug Delivery System, International Journal of Research in Pharmaceutical and Biomedical Sciences Vol. 2 (2) Apr – Jun 2011,428-441
- Malini Chandra S, Shaiju S Dharan, Athira Ajikumar: Microballoon: Novel Gastroretentive Floating Drug Delivery System, J. Pharm. Sci. & Res. Vol. 12(10), 2020, 1345-1348
- Sachin Namdeo Kothawade et al : A Floating System for Drug Delivery using Microballoons, Preprints (www.preprints.org)
- M S Kawade, Ashwini V: A Review of Microballoons: An Advance Technique for Gastroretentive Drug Delivery System, International Journal of Pharmaceutical and Clinical Research 2019; 11(2): 84-89
- Joshi Vishal Kumar,Jamini Manish:Microballoons for Drug delivery: A Review Asian Journal of Pharmaceutical Research and Development Vol.1 (1) Jan – Feb 2013:07 –17
- Nilesh K. Gorde, Mohan Kale: Microballoons as a Gastro-retentive Floating Drug Delivery System: An Overview, Int.J.Pharm.Phytopharmacol.Res. 2013, 2(4): 230-239
- Ritesh Kumar et al: Microballoons: An Advance Avenue for Gastroretentive Drug Delivery System- A Review, UK Journal of Pharmaceutical and Biosciences Vol. 4(4), 29-40, 2016
- Ankita Srivastava,Ruchi Shukla et al: Microballoons: A Gastro Retentive Drug Delivery System, Journal of Drug Delivery & Therapeutics. 2019; 9(4-s):625-630
- Preeti Sudheer, Hemanth Kumar, Litha Thomas and Nethravathi D R: Floating Microspheres - An Excellent Approach For Gastric Retention, Journal of Pharmaceutical Research Vol. 14, No. 4, October - December 2015 : 71-80
- Krishna, Abhishek Kumar, Rajat Srivastava: In Vitro In Vivo Studies on Floating Microspheres for Gastroretentive Drug Delivery System: A Review, Asian J Pharm Clin Res, Vol 14, Issue 1, 2021, 13-26
- Kuldeep Vinchurkar Jitendra Sainy, Masheer Ahmed Khan, Sheetal Mane, Dinesh K Mishra, Pankaj Dixit: Features and Facts of a Gastroretentive Drug Delivery System-A Review, Turk J Pharm Sci 2022;19(4):476-487
- Jagtap Yogesh Mukund, Bhujbal Rohan Kantilal, Ranpise Nisharani Sudhakar: Floating microspheres: A review, Brazilian Journal of Pharmaceutical Sciences Vol. 48, n. 1, Jan./Mar., 2012
- Bansal H, Kaur SP, Gupta AK, Microspheres: Methods of preparation and applications: A comparative study, Int. J. Pharm. Sci. Rev. Res, 2011; 10: 69-78
- Deng Y et al., Preparation, Characterization, and Application of Multistimuli?Responsive Microspheres with Fluorescence? Labeled Magnetic Cores and Thermoresponsive Shells, Chem. Eur. J, 2005; 11(20): 6006-13
- Chaurasia H, Chaurasia D, Singh S, Formulation and In-Vitro Evaluation of Floating Microballoons of Indomethacin, Acta Poloniae Pharmaceutical Drug Research, 2010; 67(3): 291 – 298
- Sarkar BS, Tanwar SS, Soni P, Jain P, Formulation, characterization and in vitro evaluation of floating microspheres of Esomeprazole, Int. J. of Bioassay, 2012; 21(2): 120-124.
- Pusp RN, Myung KC, Hoo KC, Preparation of floating microspheres for fish farming, International Journal of Pharmaceutics, 2007; 341: 85-90
- Sato Y, Kawashima Y, Takeuchi H, Yamamoto H, In vitro evaluation of floating and drug releasing behaviors of hollow microspheres (microballoons) prepared by the emulsion solvent diffusion method, European Journal of Pharmaceutics and Biopharmaceutics, 2004; 57(2): 235-43.
- Amrutha R, Lankapalli S, Suryadevara V, Formulation and evaluation of controlled release floating Microballoons of stavudine, Scientia Pharmaceutica, 2015; 83(4): 671-682. Tripathi M, Formulation and evaluation of Glipizide hollow Microballoons for floating drug delivery, Bull. Pharm. Res, 2011; (1): 67-74.
- Kumar DJ, Formulation and Evaluation of Gastro retentive floating microballoons of Anti diabetic drugs, Asian Journal of Pharmacy and Life Science, 2011; 1(2): 402-408.
- Swami G, Saraf SA, Preparation and Evaluation of sustained release micro balloons of propranolol, Daru Journal of Pharmaceutical Sciences, 2011; 19(3): 502-507.
- Saneshan GV, Kanth K, Preparation and in vitro evaluation of microballoon drug delivery system of Telmisartan, Int. J. Pharm. Sci. and Drug Res, 2013; 5(4): 141-145.
- Sharma M, Kohli S, Dinda A, In vitro and in vivo evaluation of Repaglinide loaded floating microspheres prepared from different viscosity grades of HPMC polymer, Saudi Pharm J, 2015; 23: 675-682.
- Malapati Sandhya rani*, K. Anil Kumar “Formulation and Evaluation of Floating Tablets of Ramipril” International Journal of Trends in Pharmacy and Life Sciences Vol. 1, Issue: 3, 2015: 373-384.
- Prakash Goudanavar et al, “ Development and in vitro characterization of esomeprazole floating gastro retentive microspheres” Journal of Applied Pharmaceutical Science Vol. 3 (03) PP 071-077, March 2013
- Vyas, S.P. & Khar., “Targeted and Controlled Drug Delivery Novel Carrier System”, Ist ed., CBS Publishers and Distributors, New Delhi, 2002, pp.417-54.
- Reddy L.H., Murthy R.S., Floating dosage systems in drug delivery. Crit.Rev. Ther. Drug Carr. Syst. 2002; 19:553–585. Streubel, A., Siepmann, I., Bodmeier, R.floating microparticles based on low density foam powder. Int. J. Pharm., 2002; 241:279-292
- Rouge N. , Buri P., Doelker E., Drug absorption sites in gastrointestinal tract and dosage forms for site delivery. Int. J. Pharm. 1996; 136:117–139.
- Chen GL, Hao WH, In vitro performance of floating sustained release capsules of verapamil. Drug Dev Ind Pharm. 1998; 24:1067-1072.
- Thanoo BC, Sunny MC, Jayakrishnan A. Oral sustained release drug delivery systems using polycarbonate microspheres capable of floating on the gastric fluids. J Pharm Pharmacol. 1993; 45, 21-24


 Gurpreet Kaur*
Gurpreet Kaur*
 Ashita Pawaiya
Ashita Pawaiya













 10.5281/zenodo.14501085
10.5281/zenodo.14501085