Abstract
This article highlights the critical role that metals play in biological processes and increases environmental concerns about heavy metal contamination brought on by urbanisation and industrialisation. People, animals, and plants can all suffer major health problems as a result of heavy metals like arsenic, lead, cadmium, and mercury that can enter the food chain through contaminated air, water, and soil. Iron, copper, and zinc are currently required in small quantities for biological functions, whereas non-essential metals can be extremely poisonous. The research highlights the critical need for effective removal techniques by highlighting the detrimental effects of heavy metals on ecosystems, humans, and plants. There involves discussion of methods for detecting heavy metals, such as atomic absorption spectroscopy, X-ray fluorescence spectrometry, and neutron activation analysis. Removal methods include membrane technology, adsorption, and phytoremediation are also being researched to lessen contamination in plants, water, and soil. Additionally, practical solutions are proposed, emphasising simple ways to combat heavy metal toxicity, like soaking fruits and vegetables in vinegar to reduce concentrations of heavy metals..
Keywords
Heavy metals, Environmental contamination, Heavy metal toxicity, human health effect, Removal of heavy metals, or heavy metals detection techniques.
Introduction
Metals has important role in biological systems because a living cell cannot exist without metal ions [1]. Urbanization is taking place at a rapid pace resulting in an increased amount of pollution. Eventually, the extraction of precious metals and minerals releases hazardous metallic substances into the atmosphere increasing their existing quantity. Many health problems and illnesses in humans are associated with heavy metal toxicity. Plants, microorganisms and aquatic organisms are also affected [2]. In recent years, there has been an increasing ecological and global public health concern associated with environmental contamination by these metals. Also, human exposure has risen dramatically as a result of an exponential increase of their use in several industrial, agricultural, domestic and technological applications [3]. Contamination of water and air by toxic metals is an environmental concern and hundreds of millions of people are being affected around the world. Food contamination with heavy metals is another concern for human and animal health [4]. Heavy metals are conventionally defined as a group of metallic chemical elements that have relatively high densities, atomic weights, and atomic numbers. The common heavy metals/metalloid includes: arsenic (As), Nickel (Ni), Cadmium (Cd), Chromium (Cr), Copper (Cu), Mercury (Hg), Lead (Pb), and Zinc (Zn) etc. Metals are natural components in soil [5]. Although heavy metals are naturally occurring in the soil, geologic and anthropogenic activities increase the concentration of them to amounts that may be harmful for both plants and animals due to their potential toxicity which disturbs their physiology and development. Metals are taken up by plant roots and translocated to shoot system and then pose a potential threat to human health as they enter the food chain [6]. It has been reported that metals such as cobalt (Co), copper (Cu), chromium (Cr), iron (Fe), magnesium (Mg), manganese (Mn), molybdenum (Mo), nickel (Ni), selenium (Se) and zinc (Zn) are essential nutrients that are required for various biochemical and physiological functions. Inadequate supply of these micro nutrients results in a variety of deficiency diseases or syndromes, while other metals such as aluminium (Al), antinomy (Sb), barium (Ba), beryllium (Be), bismuth (Bi), cadmium (Cd), gallium (Ga), germanium (Ge), gold (Au), indium (In), lead (Pb), lithium (Li), mercury (Hg), nickel (Ni), platinum (Pt), silver (Ag), strontium (Sr), tellurium (Te), thallium (Tl), tin (Sn), titanium (Ti), and uranium (U) have no established biological functions and are considered as non-essential metals[3]. According to their toxicity to living organisms, the heavy metals are arranged in the following order: Hg > Cu > Zn > Ni > Pb > Cd > Cr > Sn > Fe > Mn > Al [7].
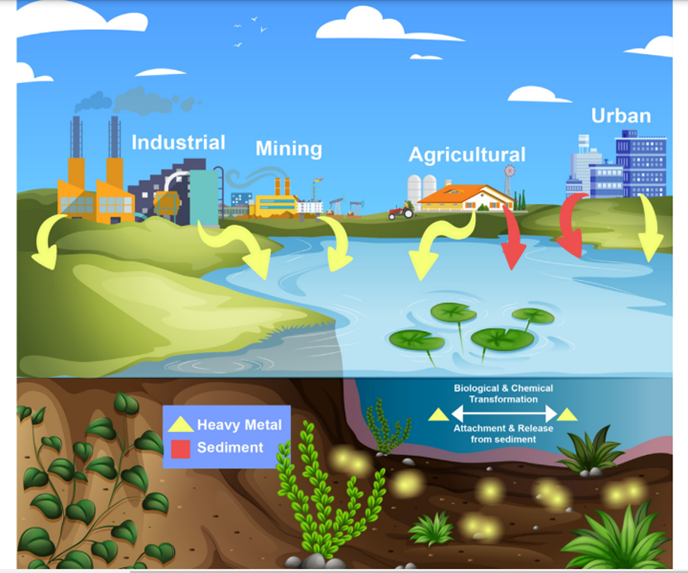
Figure 1: Diagrammatic explanation about heavy metals in the environment [13]
Development of industrialization and urbanization, the abundance of heavy metals in the environment has increased enormously during the past decades, which raised significant concerns throughout the world [8]. Some of these activities include the mining and smelting of metals, the burning of fossil fuels, the use of fertilizers and pesticides in agriculture, and the production of batteries and other metal products in industries, sewage sludge, and municipal waste disposal [9].
In addition, the issue of soils contaminated with heavy metals has become an increasingly significant environmental concern worldwide [10]. Once plants are grown in the land polluted with municipal, domestic or industrial wastes can absorb heavy metals in the form of mobile ions from the soil solution through their roots or foliar absorption. The absorbed metals get bio accumulated in the roots, stems, fruits, grains, and leaves of plants. Some heavy metals like as, Cd, Hg, and Pb are particularly hazardous to plants, animals, and humans. In dumpsites, the municipal wastes contain heavy metals such as As, Cd, Co, Cu, Fe, Hg, Mn, Pb, Ni, and Zn which end up in the soil as the sink when they are leached out from the dumpsites [11]. Heavy metals, unlike organic pollutants, are not biodegradable and tend to accumulate in living organisms when they are released into the environment, which can negatively impact the health of all forms of life, including humans, animals, and plants. Therefore, it is crucial to remove heavy metals in water, plants, fruits and vegetables to mitigate their detrimental impacts on the environment [12]. Vegetables and fruits are the major components of the human diet, as it is the source of essential micronutrients like copper (Cu), zinc (Zn), calcium (Ca), iron (Fe), magnesium (Mg), iodine (I), sodium (Na), potassium (K), antioxidants, vitamins, and various metabolites. They are consumed in both cooked and raw forms, thus vegetables containing toxic metals can cause detrimental effects on human health. Consumption of heavy metals through the food chain has been thoroughly documented worldwide. Plants can absorb heavy metals from soil and build up in higher concentrations in them [14]. Therefore, the heavy metals contaminated vegetables will no longer serve as a nutritional source only but also introduce dangerous pollutants into the human body. The heavy metals are not easily removed from the body. Instead, they will accumulate in the blood and tissue for an extended period. The exposure of heavy metals inside the body will lead to many types of disease such as brain damage, kidney failure and infertility [15]. To remove these heavy metals from fruits and vegetables, some home remedies are used, in which fruits and vegetables are shocks in vinegar and other solutions which are discussed further in this review. Phytoremediation techniques for removal of heavy metals from plants (use of plants to restore the polluted environments through phytostabilisation, phytoextraction and phytovolatilization) have been regarded as useful tools for remediate metals, yet the application of those are only reported in developed countries. Today, as heavy metal accumulation becomes a worldwide concern, application of those methods would be timely important. More specifically, hyper accumulators (plants that accumulate heavy metals at 100 to 1000-fold in their tissues than the non-accumulators) would be a safe and simple strategy to reduce the impact of heavy metal [16]. Phytoremediation categories, phytoextraction, can be used to remove heavy metals from soil using its ability to uptake metals which are essential for plant growth (Fe, Mn, Zn, Cu, Mg, Mo, and Ni). Some metals with unknown biological function (Cd, Cr, Pb, Co, Ag, Se, Hg) can also be accumulated [5]. Many treatment procedures have been developed to counteract the toxicity of heavy metals. Natural products are being efficiently used to treat the adverse consequences [13]. All life forms on earth depend on water for their presence in the ecosystem. Water is the second most important element required by human for survival after the air we breathe [17]. In recent years, there has been increasing water pollution. Water pollution is contamination of quality of water. It is usually contaminated with solid, human and animal activities, effluents from chemical industries and dissolved gases [18]. Industrial wastes contain heavy metals, solvents, cyanides, minerals and organic acids, fats, oil, bleaching agents, sulphides, ammonia, etc. many of which are known to be toxic [19]. Deposition of excess heavy metal ions in the soil leads to poor quality of soil and water, and due to their long-term persistence in the environment, can be a major problem for potable water. Accumulation of heavy metals in the freshwater ecosystem is a problem of global concern [20]. To remove these toxic metals there are some conventional methods used to treat wastewater, such as adsorption, flotation, bio sorption, coagulation, ion exchange and bioremediation. Although these have been well established, restrictive environmental legislation, more extensive space requirements, labour-intensive operations, lower selectivity, lower separation efficiency, and the high cost of these conventional methods have resulted in the search for more promising and unconventional techniques. Membrane technology in industrial pollution abatement has attracted research interest worldwide due to its high efficiency, smooth operation, and less space requirements [21]. Heavy metals are detected with some specific instruments like Atomic Absorption Spectroscopy (AAS), Neutron Activation Analysis (NAA), Inductively Coupled Plasma-Mass Spectroscopy (ICP-MS), and X-ray Fluorescent Spectrometer (XRF) are used. Among all these instruments, atomic absorption spectroscopy gives the precise quantitative determination [7].
Sources Of Heavy Metals
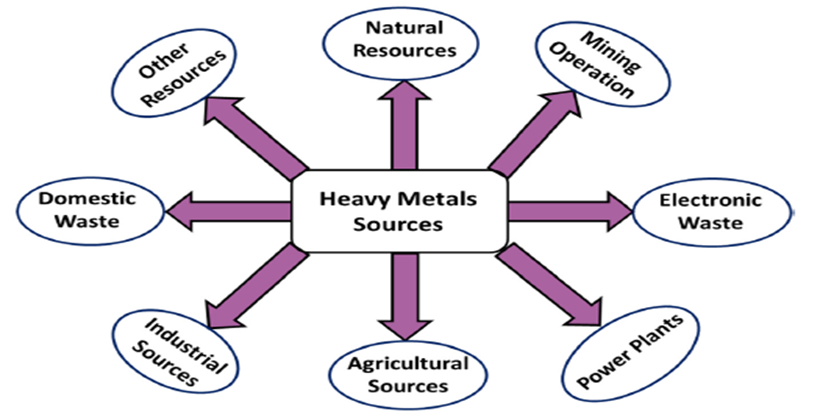
Figure 2: Main sources of heavy metals [22]
Natural sources:
The heavy metals occur naturally in many soils, in different concentrations [2]. The metals in natural, unpolluted soils originate reclusively from the lithosphere, accordingly from the mineral part of the soil that forms the rocks and minerals that make up the Earth's crust [23]. The natural sources such as volcanic eruptions, sea-salt sprays, forest fires, rock weathering, biogenic sources and windblown soil particles are included [24].
-
- Electronic wastes [25]:
E-waste affects not only the environment but has various adverse effects on human health due to improper disposal, as e-wastes contain various heavy and toxic metals. These toxic elements either can be released directly or generated during the recycling process. They will eventually end up untreated in soil or in water affecting the ecosystems causing harmful effects on environment and Human health.
-
- Mining operations:
Mining is considered the prime source of heavy metal contamination in the surrounding environment. Vigorous extraction of minerals produces a huge amount of waste minerals along with tailings and if not properly managed, they produce highly polluting acid mine drainage (AMD) that precipitates when exposed to water and can inflict serious environmental damage. Tailings are substances that are left over after the separation process during mining and settle at the bottom due to their large size and heavy weight.
-
- Power plants:
The work was envisaged to understand the rising environmental and multiple health issues from coal power plants. Studies on the adverse impacts of coal power plants on the environment, including soil, surface water, groundwater and air, were critically evaluated. The health risk from exposure to different pollutants and toxic metals released from the power plant was also demonstrated. The study also highlighted the government initiatives and policies regarding coal power operation and generation. Lastly, the study focused on guiding coal power plant owners and policymakers in identifying the essential cues for the risk assessment and management.
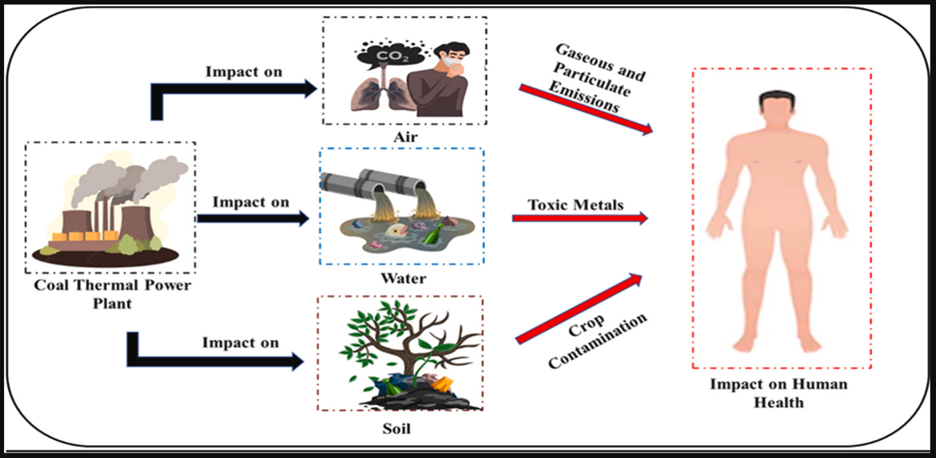
Figure 3: Power plants as a source of heavy metals [26]
Agricultural sources:
Agricultural soils are not only source of nutrients for plant life, but also transfer many contaminants such as heavy metals to cultivated plants through their roots. Contamination of agricultural soils with heavy metals, such as Pb, Cd, Cu, and Ni increased dramatically during the last years. The agricultural soils receive many of toxic metals from both natural and anthropogenic sources. Accumulation of heavy metals in agricultural soil may be by through emissions from the industrial activities, petrochemicals, wastewater irrigation, atmospheric deposition and agricultural activities such as fertilizers and pesticides. Heavy metals can accumulate from contaminated soil to the different parts of food chain [24]. The other agricultural sources include application of inorganic fertilizers, application of fungicide, pesticide, and herbicide, agricultural waste water [27].
-
- Industrial sources:
Industrial and municipal sewage is also an important source of heavy metals [7]. Industrial sources include metal smelting and refining, coal and fossil fuel plants and its refining, mining and smelting, electroplating paints and chemical wastes [27].
-
- Domestic waste:
Coupled with the improvement of life quality, many kinds of living goods have been entered into people's daily lives. Correspondingly, the municipal solid waste components became complicated and disordered, which result in the great variety of heavy metal sources. Batteries, paints, dyes and inks in paper, pesticides and fertilizers in yard waste, which are examples of household and commercial wastes, contain high quantities of heavy metals. However, the municipal solid waste components mentioned above are not weighted equally. For example, the battery is always reported to have high heavy metal contents, but it always has very low proportion in municipal solid waste. While component like paper seems low but the total amount takes high percentage in municipal solid waste. Moreover, it must also be noted that the discharge of metals from a landfill is not limited to a short period. For a long period after the dumping of the municipal solid waste at a landfill, and also after the closure of a landfill, the leaching of heavy metals will continue [28].
-
- Other sources:
Some other sources of heavy metals include pharmaceutical, Environmental sources, Foundries, [3] Fertilizers, Pesticides, Herbicides and so on.
- Effects Of Heavy Metals
2.1) Effects of heavy metal on plants:
Plants growing in a Municipal Solid Waste landfill and its surroundings are associated with heavy metals contamination that can affect the food chain. Heavy metals can affect the plant in so many ways (Table 4).
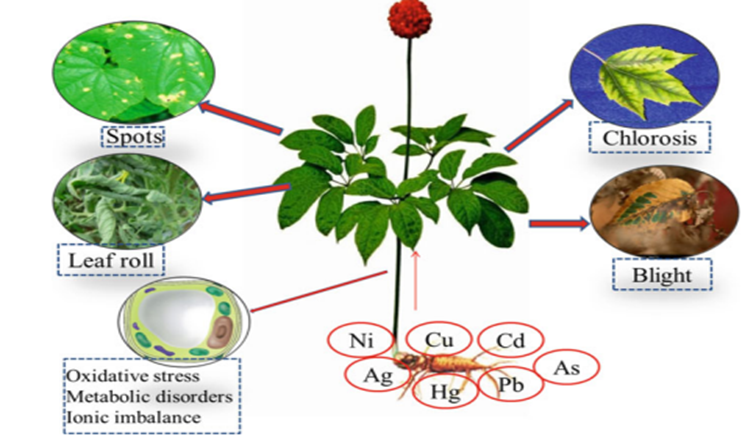
Figure 4: Heavy metals toxicity on plants
Table 1: Effects of Heavy Metals On Plants [11]
|
Heavy metals
|
Effect on plants
|
|
Lead (Pb)
|
Seed germination by gradually slowing down.
|
|
Abnormality of plant metabolism, morpho-physiological features, plant growth, and productivity
|
|
Reduce plant growth, resulting in malformation of cellular structure, lowering chlorophyll biosynthesis, imbalance hormones, and induce over-production of reactive oxygen species (ROS); which can cause oxidative stress within plant cells and readily attack biological structures and biomolecules, thus result in metabolic dysfunction
|
|
Reduce soil productivity
|
|
Cadmium (Cd)
|
Cause many abnormalities in different parts of the plant such as roots, shoots, leaf, fruit, and also increased dry to fresh mass ratio (DM / FM) in all organs
|
|
Can exhibits adverse effects on sugar content and amino acids in some plant species by strengthening their concentration, indicating inhibition of starch hydrolysis
|
|
Imbalance the macro and micronutrients by augmenting the macronutrients and reducing micronutrients
|
|
Poisoning the soil and this affects the production of phytochelatins due to obstruction of the transporter/channel for loading other elements and an imbalance of plant nutrients
|
|
Zinc (Zn)
|
Phytotoxic and can directly affect crop yield
|
|
Affects the growth of pea plants
|
|
Copper (Cu)
|
Reduced the availability of soil Nitrogen and Sulphur for crop production
|
2.2) Effects of heavy metals on human
Plant uptake of heavy metals from soils at high concentrations may lead to great health risks into considering the food chain implications. Consumption of food crops contaminated with heavy metals is a major food chain route for human exposure [1]. Some of the heavy metals are having so much of biological importance in trace amounts. The biological importance of these metals is enzyme functioning (vanadium and manganese), hormone functioning, production (selenium), cellular growth (nickel), and metabolic growth (arsenic). But these metals are required for human in trace amounts only, if their amount in the body increases, they cause adverse effects on human health and produce toxicity [29]. The toxicity of these heavy metals in the human body reduces energy levels, disrupts brain functioning, disturbs the functioning of various other organs such as the brain, lungs, liver, and kidney, and also hinders blood composition. If the contact with heavy metals continues, then it can hinder the physical, neurological, and muscular functioning. And due to these diseases like multiple sclerosis, Parkinson’s disease, muscular dystrophy, and Alzheimer’s disease. Chronic exposure to some of the heavy metals and their compounds may even cause cancer [30]. Some of these adverse effects of specific heavy metals are mentioned below.
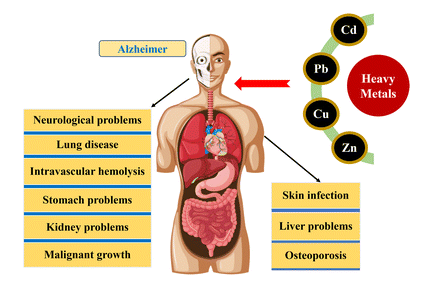
Figure 5: Toxic effects of heavy metals on human [12]
Cadmium (Cd): Bone disease, coughing, emphysema, headache, hypertension, itai-itai, kidney diseases, lung and prostate cancer, lymphocytosis, microcytic, hypochromic anaemia, testicular atrophy, vomiting [32], irritations of respiratory [31].
Arsenic (As): Brain damage, cardiovascular and respiratory disorder, conjunctivitis, dermatitis, skin cancer [32], loss of appetite, nausea, vomiting, liver damage, kidney damage [31].
Lead (Pb): Anorexia, chronic nephropathy, damage to neurons, high blood pressure, hyperactivity, insomnia, learning deficits, reduced fertility, renal system damage, risk factor for Alzheimer’s disease, shortened attention span [32], lung damage, liver damage, loss of appetite, nausea [31].
Chromium (Cr): Bronchopneumonia, chronic bronchitis, diarrhea, emphysema, headache, irritation of the skin, itching of respiratory tract, liver diseases, lung cancer, nausea, renal failure, reproductive toxicity, vomiting [31].
Mercury (Hg): Ataxia, attention deficit, blindness, deafness, decrease rate of fertility, dementia, dizziness, dysphasia, gastrointestinal irritation, gingivitis, kidney problem, loss of memory, pulmonary edema, reduced immunity, sclerosis [32], liver damage, kidney damage, loss of hearing, muscle coordination [31].
Copper (Cu): Abdominal pain, diarrhea, headache, liver damage, metabolic disorders, nausea, vomiting [32], Brain damage, chronic anemia, Kidney damage, Intestine irritation, Liver cirrhosis, Spontaneous abortions and gestational diabetes [33].
Nickel (Ni): Lung cancer, Immuno-toxic Allergic disease, neurotoxin, geotaxis, Infertility [33], Cardiovascular diseases, chest pain, dermatitis, dizziness, dry cough and shortness of breath, headache, kidney diseases, nasal cancer, nausea [32].
Zinc (Zn): Dizziness, fatigue, vomiting, renal damage, decreased Immune function, [33] its fumes have corrosive effect on skin, causes damage to the nervous system.
Manganese (Mn): Inhalation or contact causes damage to central nervous system.
- Methods For Removal of Heavy Metals
Removal of heavy metals from fruits, vegetables, plants, and water can be done by using following methods
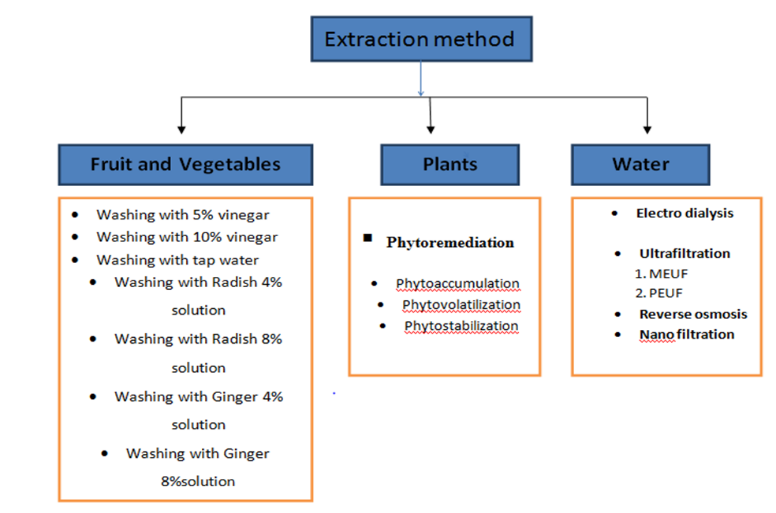
Figure 6: Classification of Methods of Removal of Heavy Metals
3.1) Removal of Heavy Metals in Fruits and Vegetables:
1.1) By Soaking with 5% Vinegar:
To reduce the heavy metal concentrations in fruit and vegetable samples, the following samples were washed with 5% vinegar: fruiting vegetables (tomato, yellow cherry tomato, red cherry tomato, long cucumber, and zucchini), fruits (strawberry, pear, and grapes), leafy vegetables (cabbage, lettuce), bulbus (garlic), and root vegetables (carrot) [34].

Figure 7: Fruits and vegetables soaked in vinegar solution
After the fruit and vegetable samples had been washed with 5% vinegar for 3–5 min, a decrease in heavy metal concentrations was observed compared to the initial analyses [34]. A considerable reduction in heavy metals was detected in the case of fruiting vegetables (tomato, yellow cherry tomato, red cherry tomato, and long cucumber), leafy vegetables (lettuce), fruits (strawberry, pear, grapes), and root vegetables (potato). Bulbus (garlic) showed a reduction in as and Zn concentrations. The results revealed that heavy metals in washed tomato (tomato, yellow cherry tomato, red cherry tomato) and long cucumber were decreased, with values between 30% and 93% which also showed a significant reduction in heavy metals compared to the initial analyses [34]. The concentrations of as in fruit and vegetable samples decreased in the order of red cherry tomato > long cucumber > tomato > garlic > yellow cherry tomato > zucchini > grapes > strawberry > pear > cabbage > lettuce > potato > carrot. Based on the studies and on the statistical analysis performed, washing with 5% vinegar can be recommended for fruit and vegetables, regardless of their origin (vegetable and fruit market or amateur farmers); this operation considerably reduces the concentrations of As, Cd, and Pb [34].
Table 2: The Concentrations of Heavy Metals Analysed in Sample of Fruit and Vegetables Washed with Tap Water and 5% Vinegar [34]
|
Element
|
Washing with water
|
Washing with Vinegar (5?etic acid)
|
|
As (µg/kg)
|
121.94
|
90.49
|
|
Cd (µg/kg)
|
15.78
|
11.48
|
|
Pb (µg/kg)
|
42.71
|
32.15
|
|
Zn (µg/kg)
|
2983.51
|
2836.13
|
Results showed a reduction in as of up to 35% (from 121.94 µg/kg FW to 90.49 µg/kg FW), a reduction in Cd of 37% (from 15.78 µg/kg FW to 11.48 µg/kg FW), and a reduction in Pb of 33% (from 42.71 µg/kg FW to 32.15 µg/kg FW) in samples washed with acetic acid compared to those washed with water. Regarding Zn concentrations, the results showed a reduction of up to 5% in samples washed with acetic acid (2836.13 µg/kg FW) compared to those washed only with water (2983.51 µg/kg FW). (FW = Fresh weight) [34].
1.2) By soaking with 10% vinegar:
Similarly, to the samples washed with 5% vinegar, samples washed with 10% vinegar showed a decrease in heavy metal concentrations as compared to the initial analyses. A considerable reduction in heavy metals can be observed in the case of fruiting vegetables (tomato, yellow cherry tomato, red cherry tomato, long cucumber, and zucchini), fruits (strawberry, grapes), and leafy vegetables (lettuce, garlic) [34].

Figure 8: Fruits and vegetables soaked in 10% vinegar solution
A considerable reduction in heavy metals was also identified in those samples where 5% vinegar had not resulted in any significant decrease. Heavy metals in washed tomato (tomato, yellow cherry tomato, red cherry tomato) and long cucumber were decreased, with values between 10% and 100%. Washing with 10% vinegar can be recommended for fruit and vegetables, regardless of where they were purchased (vegetable and fruit market or amateur farmers) as this operation reduces considerably the concentration of as, Cd, and Pb. The analysed heavy metals follow the same reduction trend as in the case of washing with 5% vinegar. The results of the analysis showed reductions of up to 45% for as, 65% for Cd, and 33% for Pb [34].
Table 3: The Concentration of Heavy Metals Analysed in Samples of Fruit and Vegetables Washed with Tap Water and 10% Vinegar [34]
|
Element
|
Washing with water
(µg/kg)
|
Washing with Vinegar (10?etic acid) (µg/kg)
|
|
As (µg/kg)
|
121.94
|
84.16
|
|
Cd (µg/kg)
|
15.78
|
9.55
|
|
Pb (µg/kg)
|
42.71
|
32.07
|
|
Zn (µg/kg)
|
2983.13
|
2629.32
|
Based on the results obtained in this research, it can be seen that a simple wash with tap water ensures a low concentration of heavy metals, especially for as, Cd, and Pb. However, washing samples with 5% vinegar resulted in 33% to 37% lower concentrations in as, Cd, and Pb as compared to washing with tap water. Fruit and vegetable samples washed with 10% vinegar recorded an even more pronounced reduction in heavy metal concentrations, with values ranging between 33% and 65% [34].
Table 4: The Difference Between the Heavy Metal Concentrations in Sample of Fruit and Vegetables Washed with 5% Vinegar and 10% Vinegar [34]
|
Element
|
Washing with Vinegar (5?etic acid)
(µg/kg)
|
Washing with Vinegar (10?etic acid)
(µg/kg)
|
|
As (µg/kg)
|
90.49
|
84.16
|
|
Cd (µg/kg)
|
11.48
|
9.55
|
|
Pb (µg/kg)
|
32.15
|
32.07
|
|
Zn (µg/kg)
|
2836.13
|
2629.32
|
1.3) By using different solutions:
In different vegetables concentrations of arsenic and cadmium are shown in the Table 5 and 6 while chromium and lead in Table 7 and 8. Unwashed samples of all the vegetables were found to be heavily contaminated with arsenic, cadmium and lead as their contents were more than their established MRLs values by FAO. Whereas washing of vegetables with different biological solutions resulted in significant reductions of heavy metals [35].
Table 5: Arsenic Contents in Different Vegetables (Ppm) [35].
|
Treatment
|
Cauliflower
|
Spinach
|
Okra
|
Brinjal
|
|
Unwashed
|
0.130±0.08
|
0.122± 0.01 0.01
|
0.113 ± 0.08
|
0.105 ± 0.11
|
|
Tap water washed
|
0.119± 0.09 0.09
|
0.113 ± 0 .21 .21
|
0.106 ± 0.01
|
0.097 ± 0.23
|
|
Radish 4% solution
|
0.113± 0.04
|
0.107 ± 0.13
|
0.097 ± 0.03
|
0.089 ± 0.12
|
|
Radish 8% solution
|
0.106 ± 0.09
|
0.097 ± 0.05
|
0.087 ± 0.45
|
0.077 ± 0.05
|
|
Ginger 4% solution
|
0.107 ± 0.13
|
0.102 ± 0.23
|
0.093 ± 0.03
|
0.086 ± 0.11
|
|
Ginger 8% solution
|
0.089 ± 0.04
|
0.087 ± 0.44
|
0.078 ± 0.04
|
0.074 ± 0.05
|
Similarly, unwashed samples of vegetables were found to be heavily contaminated with cadmium (Table 5), chromium (Table 6) and lead (Table 7) [35].
Table 6: Cadmium Contents in Different Vegetables (Ppm) [35]
|
Treatment
|
Cauliflower
|
Spinach
|
Okra
|
Brinjal
|
|
Unwashed
|
0.054± 0.03
|
0.061 ± 0.01
|
0.057 ± 0.09
|
0.059 ± 0.11
|
|
Tap water washed
|
0.050 ± 0.04
|
0.058 ± 0.09
|
0.052 ± 0.05
|
0.055 ± 0.33
|
|
Radish 4% solution
|
0.047 ± 0.01
|
0.055 ± 0.03
|
0.049 ± 0.22
|
0.050 ± 0.52
|
|
Radish 8% solution
|
0.044 ± 0.08
|
0.050 ± 0.11
|
0.044 ± 0.19
|
0.045 ± 0.31
|
|
Ginger 4% solution
|
0.045 ± 0.06
|
0.047 ± 0.07
|
0.046 ± 0.03
|
0.049 ± 0.11
|
|
Ginger 8% solution
|
0.039 ± 0.04
|
0.042 ± 0.03
|
0.037 ± 0.043
|
0.042 ± 0.21
|
Table 7: Chromium Contents in Different Vegetables (Ppm) [35]
|
Treatment
|
Cauliflower
|
Spinach
|
Okra
|
Brinjal
|
|
Unwashed
|
0.994 ± 0.03
|
0.907 ± 0.19
|
1.011 ± 0.10
|
0.901 ± 0.20
|
|
Tap water washed
|
0.0904 ± 0.09
|
0.843± 0.09
|
0.950 ± 0.05
|
0.837 ± 0.11
|
|
Radish 4% solution
|
0.864 ± 0.04
|
0.798 ± 0.04
|
0.859 ± 0.05
|
0.783 ± 0.45
|
|
Radish 8% solution
|
0.785 ± 0.04
|
0.725 ± 0.10
|
0.768 ± 0.19
|
0.702 ± 0.29
|
|
Ginger 4% solution
|
0.834 ± 0.05
|
0.752 ± 0.06
|
0.818 ± 0.04
|
0.774 ± 0.07
|
|
Ginger 8% solution
|
0.725 ± 0.08 0.08
|
0.671 ±0.07
|
0.717 ± 0.11
|
0.675 ±0.01
|
Table 8: Lead Contents in Different Vegetables (Ppm) [35]
|
Treatment
|
Cauliflower
|
Spinach
|
Okra
|
Brinjal
|
|
Unwashed
|
0.491 ± 0.045
|
0.770 ± 0.02
|
0.392 ± 0.16
|
0.475 ±0.21
|
|
Tap water washed
|
0.461 ± 0.03
|
0.732 ± 0.12
|
0.364 ± 0.09
|
0.446 ±0.11
|
|
Radish 4% solution
|
0.427 ± 0.02
|
0.701 ± 0.09
|
0.348 ± 0.05
|
0.408 ± 0.09
|
|
Radish 8% solution
|
0.397 ± 0.16
|
0.670 ± 0.05
|
0.321 ± 0.11
|
0.370 ±0.22
|
|
Ginger 4% solution
|
0.407 ± 0.08
|
0.647 ± 0.16
|
0.333 ± 0.13
|
0.389 ± 0.13
|
|
Ginger 8% solution
|
0.378 ± 0.09
|
0.608 ± 0.09
|
0.294 ± 0.07
|
0.351 ±0.05
|
MRLs= maximum residual limits as established by FAO
FAO = Food and agricultural organization
MRLs value for Arsenic: 0.10ppm
MRLs value for Cadmium: 0.05ppm
MRLs value for Chromium: 1.30ppm
MRLs value for Lead: 0.10ppm [35].
During preliminary trials (data not shown) when biological solutions were used in concentration < 4>8% with the intensions to get more reduction in heavy metals were found to be adversely affect the sensory properties of these vegetables as it imparts taste and flavour [35].
3.2) Removal of heavy metals from plant
3.2.1) Phytoremediation method:
The term of phytoremediation is relatively new, started from 1991. The term “phytoremediation” consists of the Greek prefix Phyto which is means ‘plant’ and the Latin root remedies which is means ‘to correct or remove evil’ [36]. Phytoremediation is defined as the method in which green plants use for cleaning up contaminated hazardous wastes sites. Phytoremediation has applied Ex-situ and In-Situ, continually and induces to clean up contaminated terrain of toxic metals. Phytoremediation (phytoextraction) is a promising technology for cleaning the polluted sites by using plants to extract the heavy metals from the contaminated soil and accumulates them in roots, stems, and branches. Edible crops are not recommended for such a process for the fear of introducing these potentially toxic metal ions into the food chain; thus, fibre crop would be the best suited candidate for use in phytoremediation. The efficiency of phytoremediation differs depending on plant morphological, physiological, and anatomical characteristics that affect the mechanisms of ion uptake [37].
The following are the steps involved in the phytoremediation process:
1. Identification of area.
2. Chemical analysis of the soil before application of the phytoremediation.
3. Sowing the plant phytoaccumulators.
4. Usage of agricultural and technical measures and Inspection of vegetative development.
5. Picking and drying the plants.
6. Chemical analysis of the soil near the root after finished phytoremediation.
7. Chemical analysis of green leaves of plants [38].
Mechanism of phytoremediation:
The mechanism of phytoremediation method includes [39].
Phytoaccumulation (inorganic contaminants)
- Phytovolatilization (organic and inorganic contaminants)
- Phytostabilization (organic and inorganic contaminants)
- Phytoaccumulation
Phytoaccumulation has been the most popular phytoremediation approach for removing heavy metals from plants. Phyto accumulation involves using plants (hyper accumulators) to absorb pollutants from water or the soil, then translocate and accumulate these pollutants in the aboveground plant biomass [40]. A list of some plant hyper accumulators are given in Table 8.
Table 9: Some Metal Hyper Accumulating Plants [41]
|
Plant
|
Metal
|
Concentration (mg/kg)
|
|
Dicotyledons
|
|
Cystusladanifer
|
Cd
Co
Cr
Ni
Zn
|
309
2667
2667
4164
7695
|
|
Thlaspicaerulescens
|
Cd
Zn
|
10000 – 15000
10000 - 15000
|
|
Arabidopsis halleri
|
Cd
|
5900 – 31000
|
|
Alyssum sp.
|
Ni
|
4200 – 24000
|
|
Brassica junica
|
Pb
Zn
|
10000 – 15000
2600
|
|
Betula
|
Zn
|
528
|
|
Grasses
|
|
Vetiveriazizaniodes
|
Zn
|
0.03
|
|
Paspalum notatum
|
|
Stenotaphrumsecundatum
|
|
Pennisetum glaucum
|
In this methodology, plants uptake the metal contaminants and store them in the harvestable regions such as stem, roots and leave.
The phytoaccumulation of metals involves several steps [40]:
- Metal mobilization in the rhizosphere.
- Uptake of metal by plant roots.
- Translocation of metal ion from roots to the aerial parts of the plant
- Sequestration and compartmentation of metal ion in plant tissues
Continuous phytoextraction and Induced phytoextraction are the two approaches used in phytoaccumulation. Continuous phytoextraction takes advantage of indigenous plants that can naturally collect high levels of metals (hyper-accumulators). Induced phytoextraction increases the accumulation of metals by plants using chemical compounds such as chelates to improve metal bioavailability and root uptake. Chelating agents including amino carboxylic acids and synthetic carboxylic and their salts are used. Plants employed in phytoaccumulation should generally have the following characteristics: tolerance to high metal concentrations, rapid growth, a high biomass yield, a root system that is extensive, easy to cultivate and harvest, high extraction ability with high metal accumulation in aerial tissues, very resistant to pests and infections, and be unattractive to herbivores (so as to prevent metals from entering the food chain).
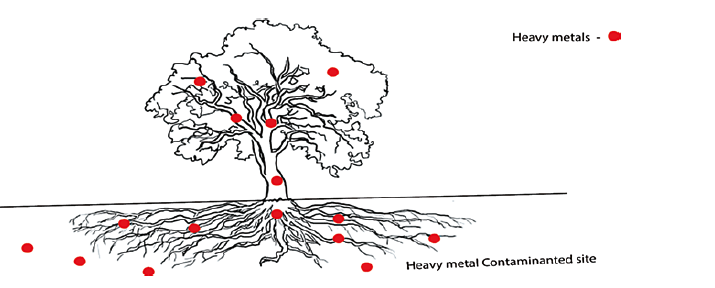
Figure 9: Phytoaccumulation mechanism: plants absorb the contaminants and stored them in root, stem and leaves [39]
Example: Heavy metal contaminants such zinc, copper, chromium, manganese, cadmium and lead are extracted by plants by their roots via this process [39].
- Phytostabilization [39]: In this method, the heavy metals were immobilized at contaminated sites itself and prevent the spreading of the contaminants to nearby lands.
The processes involved in the phytostabilization are:
- Metals adsorbed in root and root hair surface.
- Stabilization of contaminants in the root zone: The plant produces enzymes and proteins within the root zone and precipitates or immobilizes the contaminants at the root zone itself.
- The stabilization of contaminants in root membrane: The root membrane contains enzymes and proteins which can bind with the contaminants and stabilize the contaminants on the exterior portion of the root membrane.
- Stabilization of contaminants inside the root cells: Once contaminants entered into the root membrane, few enzymes facilitate the transport of contaminants across the cell membrane and arrest it within the root vacuoles and prevent it from reaching the shoot system.
- Soil amendments such as alkalizing agents, bio solids, organic matter, and phosphates are used to reduce metal solubility in the soil and their leaching to groundwater, as well as to prevent their transport. In phytostabilization, plants can change the pollutant factor’s form into non-resoluble or non-transportable in water.
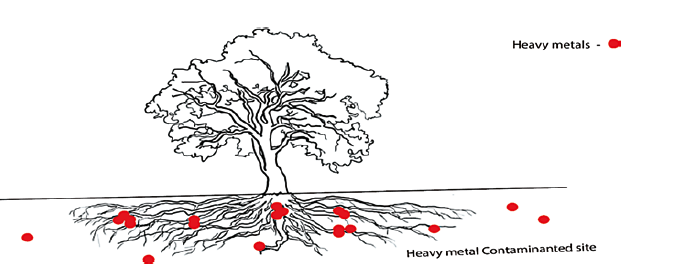
Figure 10: Phytostabilization mechanism: plants stabilize the contaminants in the root zone itself and prevent them from entering into the food chain [39]
Example: Phytostabilization has been employed to remove cadmium and mercury [39]
- Phytovolatilization [39]:
In phytovolatilization, the contaminants from the soil were absorbed by the plants and released into the atmosphere after biotransformation into less toxic forms; this method was not considered as complete remediation solution because there is a chance for redeposition of the released products (less toxic forms) in nearby lands.
The steps involved in the phytovolatilization process are:
- Translocation of adsorbed contaminants (organic or inorganic) through the root modifies them to less toxic form within plant tissues.
- The modified compounds were translocated to the leaves.
- In leaves, they released into the atmosphere either using the process of transpiration (volatile gaseous form) or evaporation (released through the stomata in liquid form and gets evaporated)
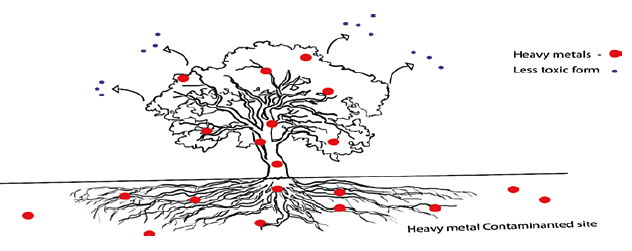
Figure 11: Phytovolatilization mechanism: plants absorb and convert the toxic contaminants into less toxic form [39]
Example: Phytovolatilization has been used to remove toxic mercury by converting it into less toxic elemental mercury. It has also been used to remove Arsenic from polluted soils. The metals such as selenium and mercury were absorbed by the plants from the soil and converted into volatile forms such as dimethyl selenide and mercuric oxide and released into the atmosphere, the volatile compounds (dimethyl selenide, mercuric oxide) were less toxic than metallic form, but they still affect human beings [39]
Advantages of phytoremediation [36]:
- A cost-effective.
- Low energy required.
- High efficiency.
- environment
- Eco-friendly technology.
Disadvantages of phytoremediation [ 42]:
- Long length of time required for remediation (usually more than one growing season).
- Treatment is generally limited to soils at less than 3 feet from the surface and groundwater within 10 feet of the surface.
- Climatic or hydrologic conditions (e.g., flooding, drought) may restrict the rate of growth of types of plants that can be utilized.
- Ground surface at the site may have to be modified to prevent flooding or erosion.
- Contaminants may still enter the food chain through animals/insects that eat plant material containing contaminants.
- Soil amendments may be required, including chelating agents to facilitate plant uptake by breaking bonds binding contaminants to soil particles.
- Removal of heavy metals from water:
Heavy metal removal from waste water can be achieved by conventional treatment processes such as chemical precipitation, ion exchange, and electrochemical removal. These processes have significant disadvantages, which are for instance incomplete removal, high energy requirements, and production of toxic sludge. Recently, membrane separation processes such as Electro dialysis (ED). Ultrafiltration (UF), Reverse Osmosis (RO), and Nanofiltration (NF) have been increasingly used for the treatment of waste water [43]. When compared with the conventional separation technologies, Membrane separation methods i.e. RO, UF, ED, NF have demonstrated their competitiveness in the removal of heavy metals from waste water [44].
3.3.1 Membrane Filtration:
Membrane processes are the best available techniques for water and wastewater treatment. The capability of separating a wide variety of components from the aqueous stream done by the membrane filtration [45].
The processes related to membrane filtration have been separated into Electro dialysis, Ultrafiltration, Reverse osmosis, and Nano filtration [46].
3.3.1.1 Electro dialysis [47]:
Some industrial processes require large amounts of water. Thus, reducing, recycling, recovering materials, and reusing the produced wastewater provide a major economic benefit.
One good method used to treat industrial wastewater is electrodialysis (ED). Electrodialysis is a membrane process that can separate charged metal ions from a fluid solution using charged ion exchange membranes. As electrodialysis is a simple and efficient process, a series of studies have been performed to determine the ability of using this process to remove contaminants from wastewater. This process only works for substances that are ionized in solution, such as sodium chloride which becomes sodium and chloride ions in solution. When terminals associated to appropriate coordinate current supply are drenched in salt arrangement, current stream carried by ions.
Particles that are positively charged and attracted to the negative cathode are called cations. Negatively charged anions stream towards the positive anode. In electrodialysis, layers or a channel of particular anions and cations are placed between cathodes. Cation channels allow a stream of anions but act as an obstruction for cations, while anions are obstructed by an anion channel but cations are allowed to pass through.
Advantages of Electro dialysis [49]:
-
-
-
- High separation efficiency of positively and negatively charged ions.
- Can deal with low concentrations of metals.
- Low operating pressure.
- Simple setup and scale treatment.
- Easy scale-up.
- Potential to reuse the secondary streams.
Disadvantages of Electro dialysis [49]:
-
- High energy consumption.
- Separation efficiency influenced by operational parameters.
- Low selectivity.
- Generation of secondary streams.
- Metal precipitation can occur.
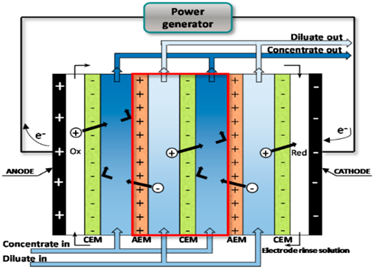
Figure 12: Schematic diagram of electro dialysis [48]
Table 10: Removal of Heavy Metals and Other Pollutants by ED Process [50]
|
Metals or other compounds
|
Concentration (mg/L)
|
Anode – Cathode
|
Removal efficiency ( % )
|
|
Cr3+, Cr6+
|
887.2, 1495.2
|
Fe – Fe
|
100
|
|
Cu2+, Cr, Ni2+
|
45, 44.5, 394
|
Al – Fe
|
100
|
|
Cd2+
|
20
|
Al – Al
|
AC:97.5,
DC: 96.2
|
|
No3-
|
150
|
Fe - Fe, Al – Al
|
90, 89.7
|
|
Pb2+, Zn2+, Cd2+
|
170, 50, 1.5
|
Al – SS
|
95, 68, 66
|
|
As
|
150
|
Al – Al, Fe – Fe
|
93.5,94
|
|
Toc, Ni2+, Zn2+
|
173, 248, 232
|
SS304 – SS304
|
66, 90, 100
|
|
Humic acid
|
20
|
Fe – Fe
|
92.69
|
3.3.1.2 Ultrafiltration [47]:
Ultrafiltration (UF) removes dissolved and colloidal particles at low pressures.
There are two types of ultrafiltration:
- Micellar-enhanced ultrafiltration (MEUF)
- Polymer-enhanced ultrafiltration (PEUF)
- Micellar-enhanced ultrafiltration (MEUF)
MEUF is a process in which wastewater is treated with an anionic surfactant prior to physicochemical membrane separation. Anionic surfactant monomers combine and form micelles and the electrostatic forces trap heavy metals on the outer surface of the micelles. The excess surfactant and heavy metals not trapped on the external surface of the micelles pass through the UF membrane.
MEUF can be used to remove single metal ion as well as mixture of metal ions with high removal efficiency [51].
Ci = Initial concentration
PS = Polysulfone
PA = Polyamide Membrane
PES =Polyethersulfone Membrane
PVA = Polyvinyl alcohol
Table 11: Performance of MEUF in Heavy Metal Removal. [52]
|
Membrane
|
Surfactant
|
Metal ion
|
Ci (mg/L)
|
Removal efficiency (%)
|
|
PA
|
Sodium dodecyl sulfate ( SDS )
|
Co2+
|
50
|
99.8
|
|
Cr3+
|
50
|
99.7
|
|
Cs2+
|
50
|
83.3
|
|
Cu2+
|
50
|
99.2
|
|
Mn2+
|
50
|
99.8
|
|
Sr2+
|
50
|
99.9
|
|
Zn2+
|
50
|
98
|
|
PES
|
Cetylpyridium Chloride
( CPC )
|
Cr3+
|
|
99
|
|
Ni2+
|
|
91
|
|
As5+
|
|
98
|
|
PS
|
Sodium dodecyl sulfate (SDS)
|
Cd 2+
|
50
|
99
|
|
Cu 2+
|
50
|
99
|
|
Pb 2+
|
50
|
99
|
|
Zn 2+
|
50
|
99
|
2. Polymer-enhanced ultrafiltration (PEUF):
In PEUF, a water-soluble polymer is added to wastewater. These polymers are retained by the membrane if their size is greater than the membrane’s molecular weight cut off. PEUF aids in the removal of small solute molecules, which conventional UF cannot remove.
Table 12: Performance of PEUF in Heavy Metal Removal [52]
|
Membrane
|
Surfactant
|
Metal ion
|
Ci (mg/L)
|
Removal efficiency (%)
|
|
PES
|
Carboxy methyl cellulose
|
Cu2+
|
10
|
97.6
|
|
Cr3+
|
10
|
99.5
|
|
Ni2+
|
10
|
99.1
|
|
Poly (acrylic acid)
|
Ni2+
|
|
93.2
|
|
Zn2+
|
|
99.2
|
|
Polyethyleneimine
|
Cu2+
|
|
94
|
|
Ni2+
|
50
|
98.1
|
|
Zn2+
|
|
91.6
|
|
Polyvinylamine
|
Cu2+
|
10
|
97
|
|
Fe3+
|
10
|
99
|
|
Pb2+
|
10
|
99
|
|
PS
|
Poly ( ammonium acrylate )
|
Cd2+
|
112.4
|
99
|
|
Polyethyleneimine
|
Cr3+
|
10
|
99
|
|
PVA
|
Polyethyleneimine
|
Cd2+
|
100
|
99.5
|
|
Cu2+
|
100
|
99.5
|
|
Pb2+
|
100
|
99.5
|
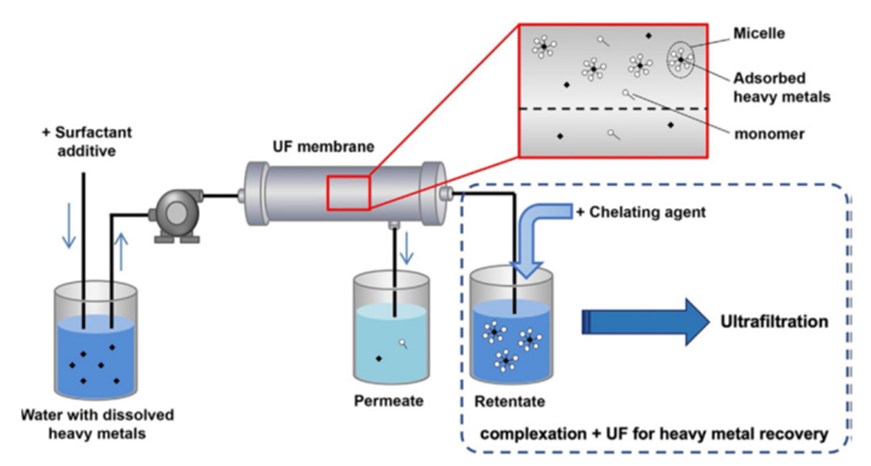
Figure 13: Schematic diagram of ultrafiltration method [52]
Advantages of ultrafiltration:
- high flux.
- high removal.
- High permeability.
- Have a longer lifetime (More than 10 years).
- Disadvantage of ultrafiltration:
- Increased running costs.
- Brittleness: sensitive to crack formation.
3.3.1.3) Reverse osmosis [53]:
Reverse osmosis (RO) can be used to reduce many heavy metals in water, such as chromium, copper, lead, and arsenic. RO technology uses added pressure to push water through a semipermeable membrane, which blocks contaminants larger than 0.0001 micrometres from passing through while allowing water molecules free passage. Removal rates depend on several factors including the post and pretreatment steps, but RO removal efficiencies are high for metals, with upwards of 99.4% removal for metals like cadmium and copper. Though reverse osmosis is a very effective method, it is expensive (systems cost anywhere from $150-$1000) and creates a high volume of wastewater. Not only will this likely increase your water bill (by about $50/month), but the wastewater stream contains a high concentration of contaminants can harm the environment if not properly disposed of.
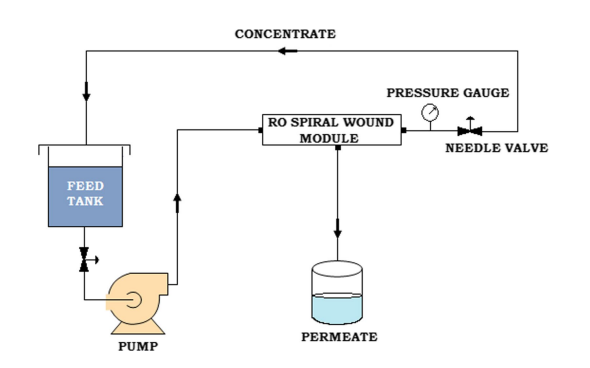
Figure 14: Schematic diagram of reverse osmosis [54]
Advantages of RO Process [55]:
- RO systems are simple to design and operate.
- Both inorganic and organic pollutants can be removed simultaneously by RO membrane processes.
- RO systems allow recovery/recycle of waste process streams with no effect on the material being recovered.
- RO systems require less energy as compared to the technology.
- The specific energy requirement is significantly low 3- 9.4 kW h/m3 product.
- The process is electrically driven hence it is readily adaptable to powering by solar panels.
Disadvantage of RO [55]:
- Removes Healthy Minerals Present in Water and Decreases ph.
- Requires Expensive Maintenance.
3.3.1.4) Nano filtration (NF):
Nano filtration (NF) is used to concentrate constituents whose molecular weight is >1000?Da and remove solutes whose size of 0.0005–0.007??m with molecular weights >200?Da. Thus, the operating range of NF is between UF and reverse osmosis (RO) processes. The NF membranes are composed of polymer composites of multiple-layer thin-film of negatively charged chemical groups [40]. It used simultaneous co-extrusion of polybenzimidazole (PBI) to form a membrane. PBI offers the advantage of excellent chemical resistance and a unique charge characteristic, whereas polyethersulfone (PES) / polyvinylpyrrolidone (PVP) has a low cost, superior spinner ability, hydrophilic nature, better mechanical properties, and ease of porous membrane formation [47].
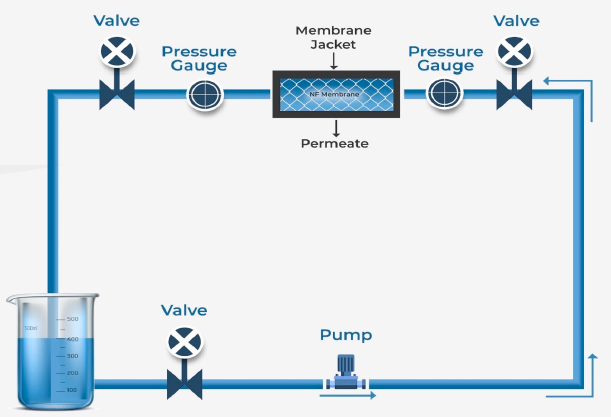
Figure 15: Schematic diagram of Nano filtration [43]
Advantages of Nano filtration [49]:
- High separation efficiency.
- Small space requirements.
- Continuous operation.
Disadvantages of Nano filtration [49]:
- High operational costs due to pressure and fouling.
- Low production volume.
- NF may need pH adjustments for high removal efficacy.
- Backwash requirements. (chemical addition, time, stop operation…)
Example [47]:
Cadmium, chromium, and lead were all rejected at 95%, 98%, and 93%, respectively, by this membrane. If use polyamide NF membrane for lead removal, achieved 97.5% removal of lead by increasing the pressure and initial feed concentration.
3.1.5) Advantages of membrane filtration:
- Such as low energy requirement as comparing to distillation/evaporation and electrochemical process.
- Small volume of retentive that needs to be handled.
- Selective removal of pollutants with complexing agents or by membrane surface modification.
- Possibilities of achieving zero discharge with reuse of permeate water and removed compounds.
- Continuous operation.
- Minimal labour requirement [44].
- High efficacy.
- Low space occupancy and
- Simple design [46].
3.1.6) Disadvantage of membrane filtration [56]:
- Investment costs are often too high for small and medium industries.
- High energy requirements.
- The design of membrane filtration systems can differ significantly.
- High maintenance and operation costs.
- Rapid membrane clogging (fouling with high concentrations).
- Low throughput.
- Limited flow rates.
- Not interesting at low solute feed concentrations.
- The choice of the membrane is determined by the specific application.
- Specific processes.
- Elimination of the concentrate.
4. Analytical methods for heavy metal detection
Some analytical method for detection of heavy metals are used such as,
- Atomic absorption spectroscopy
- Inductively Coupled Plasma Mass Spectrometry (ICPMS)
- X-ray Fluorescent Spectrometer (XRF)
- Neutron Activation Analysis (NAA) [15].
CONCLUSION
The urbanisation all over the world, the pollution rate in the environment is also increasing. Without proper remediation, these wastes are discarded in the soil or water bodies which then harm the environment. Heavy metals are normally present at very low concentrations in the environment, but human activities in the ecosystem increase their quantities. Mining is regarded as a major source of heavy metal emissions. When any precious mineral or metal is extracted from the crust, it releases certain harmful metals that linger in the surrounding soil or travel to the bodies of water (including groundwater, rivers, streams), plants, and soil and enters into the human and animals. In this review, we also focused on the impacts of some heavy metals, for example arsenic, lead, mercury, cadmium, chromium, aluminium and iron, on nature, plants and animals. Sources and hazardous effects of heavy metals were mentioned in this review and different methods for removal of heavy metals from fruits, vegetables, soil, plant, and waste water were discussed. Based on the studies and on the statistical analysis performed, washing with either 5%or 10% vinegar can be recommended for fruit and vegetables, as both procedures resulted in a significant reduction in heavy metal concentrations as compared to washing with tap water. Heavy metal removal from contaminated sites using different phytoremediation methods has been discussed in this review elaborately. Membrane filtration methods for heavy metal removal from water has been discussed. By using these methods toxic heavy metals can be removed successfully. Inability to control the presence of heavy metals will bring about extreme inconveniences in future due to the unfriendly impacts forced by them.
REFERENCES
- Lawal KK, Ekeleme IK, Onuigbo CM, Ikpeazu VO, Obiekezie SO. A review on the public health implications of heavy metals. World Journal of Advanced Research and Reviews. 2021;10(3):255-65.
- Karn R, Ojha N, Abbas S, Bhugra S. A review on heavy metal contamination at mining sites and remedial techniques. In IOP Conference Series: Earth and Environmental Science 2021 Jun 1 (Vol. 796, No. 1, p. 012013). IOP Publishing.
- Paul BT, Clement GY, Anita KP, Dwayne JS. Heavy metals toxicity and the environment. EXS. 2012;101: 133-64.
- Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Frontiers in pharmacology. 2021 Apr 13;12: 643972.
- Tangahu BV, Sheikh Abdullah SR, Basri H, Idris M, Anuar N, Mukhlisin M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. International journal of chemical engineering. 2011;2011(1):939161.
- Shehata SM, Badawy RK, Aboulsoud YI. Phytoremediation of some heavy metals in contaminated soil. Bulletin of the National Research Centre. 2019 Dec; 43:1-5.
- Kumari S, Mishra A. Heavy metal contamination. In Soil contamination-threats and sustainable solutions 2021 Apr 6. IntechOpen.
- Yan A, Wang Y, Tan SN, Mohd Yusof ML, Ghosh S, Chen Z. Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Frontiers in plant science. 2020 Apr 30; 11:359.
- Sperdouli I. Heavy metal toxicity effects on plants. Toxics. 2022 Nov 23;10(12):715.
- Abd Elnabi MK, Elkaliny NE, Elyazied MM, Azab SH, Elkhalifa SA, Elmasry S, Mouhamed MS, Shalamesh EM, Alhorieny NA, Abd Elaty AE, Elgendy IM. Toxicity of heavy metals and recent advances in their removal: a review. Toxics. 2023 Jul 3;11(7):580.
- Nyiramigisha P. Harmful impacts of heavy metal contamination in the soil and crops grown around dumpsites. Reviews in Agricultural Science. 2021; 9:271-82.
- Aziz KH, Mustafa FS, Omer KM, Hama S, Hamarawf RF, Rahman KO. Heavy metal pollution in the aquatic environment: efficient and low-cost removal approaches to eliminate their toxicity: a review. RSC advances. 2023;13(26):17595-610.
- Mitra S, Chakraborty AJ, Tareq AM, Emran TB, Nainu F, Khusro A, Idris AM, Khandaker MU, Osman H, Alhumaydhi FA, Simal-Gandara J. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. Journal of King Saud University-Science. 2022 Apr 1;34(3):101865.
- Mawari G, Kumar N, Sarkar S, Daga MK, Singh MM, Joshi TK, Khan NA. Heavy metal accumulation in fruits and vegetables and human health risk assessment: findings from Maharashtra, India. Environmental Health Insights. 2022 Aug; 16:11786302221119151.
- Ruzaidy NI, Amid A. Heavy metal contamination in vegetables and its detection: A review. Science Heritage Journal (GWS). 2020;4(1):1-5.
- Priyashantha AK, Sugirtharan M. Heavy metals and pathogenic contamination in vegetable crops through wastewater irrigation and risk reduction in developing countries. Science, Engineering and Health Studies. 2021 Dec 29:21010008.
- Beniah Obinna I, Christian Ebere E. A review: Water pollution by heavy metal and organic pollutants: Brief review of sources, effects and progress on remediation with aquatic plants. Analytical Methods in Environmental Chemistry Journal. 2019 Sep 1;2(3):5-38.
- Kannan L, Gokulprasath M, Gurusamy R, Selvam R, Palaniswamy R. Analysis of heavy metals contamination in water: A review. SSRN Electron. J. 2021;8(4):201-13.
- Nwajel GE. ? distribution of heavy metals in the sediments of lagos lagoon. Biological Sciences-PJSIR. 2000 Dec 18;43(6):338-40
- Jamshed Zaidi JZ, Amit Pal AP. Review on heavy metal pollution in major lakes of India: remediation through plants.2017, 11(6), 255-265.
- Verma B, Balomajumder C, Sabapathy M, Gumfekar SP. Pressure-driven membrane process: a review of advanced technique for heavy metals remediation. Processes. 2021 Apr 24;9(5):752.
- Das TK, Poater A. Review on the use of heavy metal deposits from water treatment waste towards catalytic chemical syntheses. International Journal of Molecular Sciences. 2021 Dec 13;22(24):13383.
- Abdullahi A, Lawal MA, Salisu AM. Heavy metals in contaminated soil: source, accumulation, health risk and remediation process. Bayero Journal of Pure and Applied Sciences. 2021 Dec 17;14(1):1-2.
- Abdel-Rahman GN. Heavy metals, definition, sources of food contamination, incidence, impacts and remediation: A literature review with recent updates. Egyptian Journal of Chemistry. 2022 Jan 1;65(1):419-37.
- Manikandan S, Inbakandan D, Nachiyar CV, Namasivayam SK. Towards sustainable metal recovery from e-waste: A mini review. Sustainable Chemistry for the Environment. 2023 Aug 24;2: 100001.
- Vig N, Ravindra K, Mor S. Environmental impacts of Indian coal thermal power plants and associated human health risk to the nearby residential communities: A potential review. Chemosphere. 2023 Sep 7:140103.
- Jayakumar M, Surendran U, Raja P, Kumar A, Senapathi V. A review of heavy metals accumulation pathways, sources and management in soils. Arabian Journal of Geosciences. 2021 Oct;14(20):2156.
- Long YuYang LY, Shen DongSheng SD, Wang HongTao WH, Lu WenJing LW, Zhao Yan ZY. Heavy metal source analysis in municipal solid waste (MSW): case study on Cu and Zn.
- Jyothi NR. Heavy metal sources and their effects on human health. Heavy metals-their environmental impacts and mitigation. 2020 Dec 30:1-2.
- Anubhav SG, Anuj SA, Rohit K. VA, Rushikesh L. CE, Pritam P. PT, Varad NR, Vinay AI, Sumit K. CY, Garima AI, Kumud K. AI and Mahipal S. SA, “Heavy metal contamination of water and their toxic effect on living organisms.” Intech. 2022, 10, 1-20.
- Abdel-Raouf MS, Abdul-Raheim AR. Removal of heavy metals from industrial waste water by biomass-based materials: A review. j pollut eff cont 5: 180. doi: 10.4172/2375-4397.1000180 page 2 of 13 volume5 • issue 1• 1000180 j pollut eff cont, an open access journal issn: 2375-4397 the top six toxic threats: Estimated population at risk at identified sites*(million people) estimated global impact**(million people) 1. lead 10 18-22 2. Mercury. 2017; 8:15-9.
- Shukla L, Jain N. A review on soil heavy metals contamination: effects, sources and remedies. TIDEE: TERI Information Digest on Energy and Environment. 2022 Mar 1;21(1):83-.
- Manwani S, Vanisree CR, Jaiman V, Awasthi KK, Yadav CS, Sankhla MS, Pandit PP, Awasthi G. Heavy metal contamination in vegetables and their toxic effects on human health. Sustainable Crop Production: Recent Advances. 2022 Jul 6;181.
- Bora FD, Bunea A, Pop SR, Bani?? SI, Du?a D?, Chira A, Bunea CI. Quantification and reduction in heavy metal residues in some fruits and vegetables: A case study Gala?i County, Romania. Horticulturae. 2022 Nov 5;8(11):1034.
- Sattar MU, Anjum FM, Sameen A. Mitigation of heavy metals in different vegetables through biological washing techniques. International Journal of Food and Allied Sciences. 2015 Dec 25;1(2):40-4.
- Sumiahadi A, Acar R. A review of phytoremediation technology: heavy metals uptake by plants. InIOP conference series: earth and environmental science. (2018) Mar 1 (Vol. 142, p. 012023). IOP Publishing
- Shehata SM, Badawy RK, Aboulsoud YI. Phytoremediation of some heavy metals in contaminated soil. Bulletin of the National Research Centre. 2019 Dec; 43:1-5.
- Vasavi A, Usha R, Swamy PM. Phytoremediation–an overview review. J Ind Pollut Control. 2010 Jan 1;26(1):83-8.
- Muthusaravanan S, Sivarajasekar N, Vivek JS, Vasudha Priyadharshini S, Paramasivan T, Dhakal N, Naushad M. Research updates on heavy metal phytoremediation: enhancements, efficient post-harvesting strategies and economic opportunities. Green materials for wastewater treatment. 2020:191-222.
- Nnaji ND, Onyeaka H, Miri T, Ugwa C. Bioaccumulation for heavy metal removal: a review. SN Applied Sciences. 2023 May;5(5):125.
- Wuana RA, and Okieimen FE “Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation.International Scholarly Research Notices 2011; 2011(1):402647.
- RalindaMR, and P.G, “A review on phytoremediation.” Technol.overv.1996, 3, 1-26.
- Qasem NA, Mohammed RH, Lawal DU, “Removal of heavy metal ions from wastewater: a comprehensive and critical review.” Npj Clean water 2021 Jul 8;4(1), 1-5
- Pandya J, “Nanofiltration for recovery of heavy metal from waste water RsearchGate 2015; 10, 1-20.
- Verma B, Balomajumder C, Sabapathy M, Gumfekar sp. “Pressure driven membrane process: a review of advanced technique for heavy metals remediation. Processes 2021; Apr 24;9(5):752
- Vidu, R., Matei, E., Predescu, AM., Alhalaili, B., Pantilimon, C, Tarcea, C. and Predescu, C. Removal of heavy metals from wastewaters: A challenge from current treatment methods to nanotechnology applications. Toxics,2020 Nov 10; 8(4):101
- Razzak SA, Faruque MO, Alsheikh Z, Alsheikhmohamad L, Alkuroud D, Alfayez A, Hossain S.Z, and Hossain MM, A comprehensive review on conventional and biological driven heavy metals removal from industrial wastewater.” Environmental Advances 2022 Apr 1; 7:100168
- Gurreri L, Tamburini A, Cipollina A, and Micale G. “Electrodialysis applications in wastewater treatment for environmental protection and resources recovery: A systematic review on progress and perspectives.” Membranes 2020 Jul 9; 10(7):146.
- Juve JM, Christensen. FM., Wang Y, Wei. Z. “Electrodialysis for metal removal and recovery: A review.” Chemical Engineering Journal 2022 May 1;435: 134857
- Bazrafshan E, Mohammadi L, Ansari-Moghaddam A, Mahvi AH. “Heavy metals removal from aqueous environments by electro coagulation process - a systematic review. Journal of environmental health science and engineering 2015 Dec; 13: 1-16.
- Mungray AA, Kulkarni SV, and Mungray AK. “Removal of heavy metals from wastewater using micellar enhanced ultrafiltration technique: a review.” Central European Journal of Chemistry 2012Feb; 10: 27-46.
- El Batouti M, Al-Harby NF, Elewa MM. A review on promising membrane technology approaches for heavy metal removal from water and wastewater to solve water crisis. Water. 2021 Nov 16;13(22):3241.
- Isabelle KH, and Samantha BR, “Heavy metals in water and soil: methods for treatment.” Hum.Eng.2022, 2, 1-5.
- Govardhan B, Fatima S, Madhumala M, Sridhar S, “Modification of used commercial reverse osmosis membranes to nanofiltration modules for the production of mineral rich packaged drinking water. Applied Water Science 2020 Nov;10(11):1-7
- Garud RM, Kore VS, and Kulkarni GS, “A short review on process and applications of reverse osmosis.” Universal Journal of Environmental Research & Technology,2011 Oct 1(3),233-238.
- Crini G, Lichtfouse E. Advantages and disadvantages of techniques used for wastewater treatment. Environmental chemistry letters 2019; Mar 1;17: 145-55.


 Dr. Hina Bagada*
Dr. Hina Bagada*
 Bhupendra Mahla
Bhupendra Mahla




















































 10.5281/zenodo.14848134
10.5281/zenodo.14848134