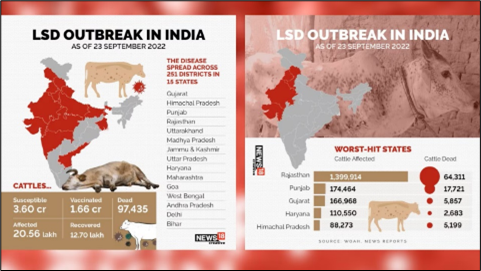
Lumpy Skin Disease (LSD) is a frequent disease of bovines which is a member of Capripoxvirus genus. It is common in Africa, Middle Eastern nations, Asia and Europe. In Gujarat and Rajasthan, the illness has killed around 97,000 people and caused 1850,000 cases between July and September. Direct economic losses incurred by livestock producers and industries caused by LSD include death, milk drop, the financial burden posed by treatment, labour costs involved in treating affected animals or clusters of sick animals, expenses associated with induced sterility and new restrictions on movement across cattle. Multiple vaccine types such as live attenuated, inactivated and recombinant have been developed to fight LSD. There are promising live attenuated but adverse reaction inducing vaccines. Inactivated vaccines have a narrow spectrum of immunity and genetically mutant recombinant vaccines have more than pathogen protective ability. More research is needed to improve compositions and lessen negative effects.
Lumpy Skin Disease, Capripoxvirus, Live attenuated vaccine, Inactivated vaccine, Recombinant vaccine.
The Neethling virus causes LSD. It is an infectious illness in cattle characterized by fever, numerous nodules and swollen lymph nodes on the skin and mucus membranes, including the gastrointestinal and respiratory systems (Fig. 1). Additionally, lameness and edematous swelling in the limbs are possible symptoms of infected cattle. It transmits through skin lesions, scabs, gashes, milk, nasal concealment, blood, and semen and lasts for five weeks causing secondary pneumonia, bacterial infections, anaemia, infertility, miscarriage, and the decline in lactation (1). This disease was first discovered in Zambia in 1929 and spread to Botswana, South Africa, Kenya, Mauritania, Mali, Ghana, and Liberia in 1977. It migrated west to Nigeria in 1974 and north into Sudan in 1970. A second epizootic struck Tanzania, Kenya, Zimbabwe, Somalia, and Cameroon between 1981 and 1986, killing 20% of infected livestock. In 1988 and 1989, Egypt and Israel confirmed LSD's existence outside northern Sahara and African area. It was reported again in 2006, coinciding with the Sheep-pox outbreak(2). Some techniques for detecting the effects of LSD include PCR, viral isolation, and serological testing. The most sensitive real-time PCR is used to identify the virus on the skin, and the circular ELISA system is the appropriate method to diagnose the LSD contagion in cow serum samples. Techniques such as Loop-mediated isothermal amplification (LAMP), Immuno-peroxidase Monolayer Assay (IPMA), and immunohistochemistry help identify populations at danger for LSD exposure and give an exact diagnosis (3). Vaccination is still the most efficient preventive measure, even if controlling vector populations and putting strong biosecurity measures in place are also essential to halting the transmission of illness. Although attenuated live vaccines, like the Neethling strain of LSDV, are altered to lessen pathogenicity, they have disadvantages like as toxicity, adverse clinical reactions, and the possibilities of viral recombination (4). Inactivated vaccines, made by killing viruses or bacteria, require booster shots for long-term protection but have limitations (5)Recombinant vaccines, produced through genetic alteration, target specific virulence components and show promise for protection opposing LSDV and similar viruses. Recombinant vaccines may reduce environmental and safety concerns comparing to live vaccinations, as some viruses do not replicate in animals, offering a safer alternative (6).
1.1 What causes LSD?
Cattle with are susceptible to an infectious virus, characterized by high fever, unusual lumps and swollen lymph nodes. This condition can be lethal, particularly in animals with compromised immune systems or who are yet to be exposed. LSD is spread by bloodsucking insects like ticks, mosquitoes, and flies. The morbidity rate is higher in cattle compared to buffalo and calves and heifers are more affected than mature animals. There is little epidemiological evidence to support the notion that small ruminants act as a reservoir for LSDV, in spite of the fact that certain strains may multiply in cattle and sheep.
The Geographic Dynamics of LSD Impacts
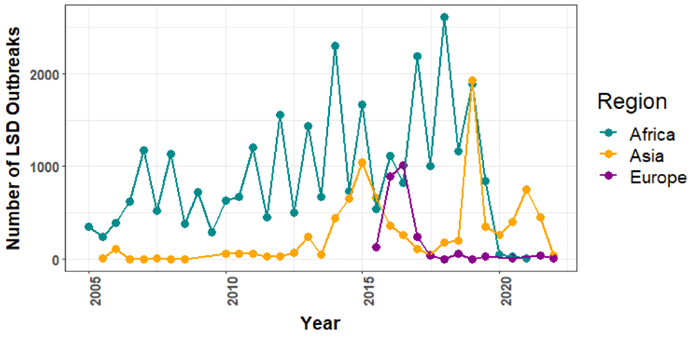
LSD pandemic was first reported in Zambia, 1929. An estimate of nearly 8.0 million cattle were affected during this decade. Previously it was suspected to exist because of poisoning or an excessive allergic response to bug stings. Between 1943 and 1945, there were additional cases in Zimbabwe, South Africa and Botswana. In 1949, South Africa was hit by a panzootic infection which decimated around 80 lakhs cattle and cost our economy an estimated R100 million. LSD then spread across Africa from the 1950s and the 1980s, impacting livestock within Sudan, Kenya, Tanzania, Cameroon and Somalia. Israel recorded its first LSD epidemic in 1989. It was the initial time that LSD was used ever documented outside of Africa and northern Sahara-desert. In this instance, it was recognized that winds dispersing contaminated Stomoxys calcitrans caused the outbreak to begin in Ismailiya, Egypt. LSD first appeared in 2013 in Turkey. From there, it spread to Azerbaijan (2014), Georgia (2016) and Armenia (2015) causing destruction across all of Russia and the Caucasian and Balkan countries. It was once limited to Africa, but since 2012, it has quickly expanded over Southeast Europe, the Near East, and West and Central Asia (Fig.2) (7). Lumpy skin disease has spread throughout Asia since In July 2019, it was initially discovered in Bangladesh and has already spread to 7 nations by the end of 2020. Right now, at least 23 countries in South, East, and Southeast Asia are in danger. India experienced its first instance of LSD in 5 districts of Odisha in 2019, with 182 out of 2,539 impacted animals showing no mortality and an 7.1% apparent morbidity rate (3). The illness has since spread to eight states and union territories, India had several breakouts, with the majority reported in Rajasthan and Gujarat. Punjab, Haryana, Uttarakhand, Himachal Pradesh, Uttar Pradesh, Jammu and Kashmir, Madhya Pradesh, and Delhi have also been affected. Regarding mortality and morbidity, the wave that stared in May or June 2022 was exceptional because initially the 2022 disease outbreak had reached 251 districts across 15 Indian states, infecting over 29.45 lakh cattle and resulting in about 1.55 lakh recorded deaths. Because of their anatomy, "stray cattle" are vulnerable to the illness because of impaired immunity. There are more reports of LSD-related deaths in Gujarati and Rajasthani districts that are prone to drought. LSD has also affected buffaloes, with exotic and high-breed animals more likely to succumb to the illness. As of June 2023, it has dispersed throughout the Koshi Province in Nepal, killing over 10,000 animals (Fig.3) (3)
Physiological Response Of LSD
There is limited research on the pathophysiology of LSD in cattle. In trials, the duration of incubation period for LSD can range from four to seven days, although naturally occurring infections can last up to five weeks. It takes seven to twenty-eight days to incubate in its natural host. There are three stages to the condition: acute, subacute, and habitual. Viralemia, vasculitis, and lymphangitis are brought on by intracellular reproduction of the infection within fibroblasts, pericytes, macrophages and endothelial cells (8). Clinical signs are classified into 4 stages: mending, ulceration, swelling of lymph nodules, and acute phase. Beasties have a high temperature of 41 degrees Celsius during the acute phase, which is followed by multinodular lesions, anorexia, depression, lacrimation, elevated discharge from the nose, and slaver hiding (9). It's a sickness that causes swelling in lymph nodes and a rise in nodules, which rupture after 1-2 days, spreading the infection to the environment. The last stage of the nodule develops into ulceration and necrosis, with extreme cases involving ulcerated sores, excessive salivation, respiratory discharge, and lacrimation (10). The fourth phase, lasting a minimum of one month, heals and the lesions' skin thickens. The LSD virus may be found in the animal's secretions and is easily detected in seminal fluid 42 days after infection (11). The infected animals may also experience bacterial infections, secondary pneumonia, and complications like anaemia, infertility, miscarriage, and reduced lactation (12).
LSD Communication
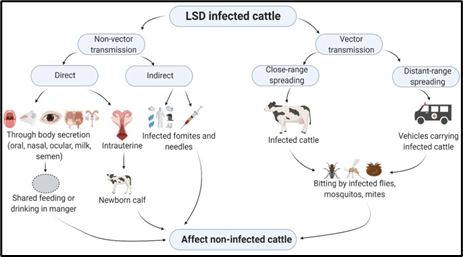
LSD contagion is primarily transmitted through skin lesions and scabs because of its long half-life, but it might be released as well through gashes, milk, nasal concealment, blood, and semen (11). DNA attenuation in the lesions on the skin is advanced, but blood, nasal concealment, slaver, milk, and coprolite samples do not contain positive LSD viral DNA. The contagion is detected in blood on days 18 and 29 and in nasal concealment on days 20 through 42 (13). Polymerase Chain response (PCR) was employed in studies on cattle in India to identify suspected LSD infections. Males infected with the contagion can naturally spread the contagion, and viral DNA can be found in semen up to 42 days after infection (3). LSD can Be either directly or indirectly, including non-vector and vector transmission (Fig.4). It is spreadable through animal secretions or contact with infected animals. In 2006, researchers found indirect contact as the primary mode due to difficulty in tracing the virus's spread within an infected population (14). Direct animal-to-animal contact, such as blood-sucking insects, is also useful. LSD disease spreads through communication, both direct and indirect, with contaminated food and aerosols from diseased animals being the main sources. Studies show this in countries like Iran, Azerbaijan, Georgia, Dagestan, and Russia (15). The contaminated feed can spread recombinant viruses during digestion, indicating indirect spawning channels (16). Preterm calves of mothers infected with LSD were positively identified by PCR containing viral DNA and developed pre-colostral serum antibodies. Arthropod/insect vectors like flies, ticks, and mosquitoes can also spread LSD. Blood-sucking arthropods are the most probable vectors for disseminating the virus (17).
Detection Of LSD Effects
Normal clinical signs and a number of laboratory techniques, including as viral isolation, serological testing, and PCR, are used to detect lumpy skin disease. However, detecting minor symptoms can be challenging. The most precise method is real-time PCR for identifying the LSD virus spotted on the skin (18). The circular ELISA system is more effective for detecting LSD contagion in cow serum samples than the IFAT system. RT-PCR is quick, sensitive, and accurate for detecting LSD contagion in skin nodes and blood necropsies. However, these techniques are limited to describing Capripoxvirus infections and are unable to distinguish between goat pox, lamb spell, and LSD. Contagion insulation is the best option for relating the LSD contagion, but it can take couple of weeks to insulate and culture the contagion from unembryonated funk eggs (19) (20). The WOAH endorses the VNT system as the gold standard for relating antibodies against contagion. The VNT test measures viral antibody titers following infection or vaccination but requires a position 3 biosafety laboratory and proficiency in using and interpreting results. ELISA is recommended as a cover serological fashion by WOAH but may affect in false cons due to non-specific commerce between Parapoxvirus and Capripoxvirus (10). Researchers have used various techniques to screen LSD, including Loop-mediated isothermal amplification (LAMP), Immunoperoxidase Monolayer Assay, and virological, molecular, and immunohistopathological techniques (21). For routine LSD diagnosis, LAMP has demonstrated good accuracy and precision in identifying the infectious agent in healthy animals. IPMA was found to be superior to VNT and ELISA in detecting LSD antibodies in animals that are contaminated, vaccinated, and vaccinated/infected. Amin et al. (2021) used immunohistochemistry to identify LSD viral agents in contaminated bovine skin using antibodies specific for the LSDV. These techniques help identify populations in danger of LSD exposure and provide a more accurate diagnosis (22).
Clinical Features Of LSD Intoxication
LSDV infects regions like the neck, head, and limbs leading to development of nodules on skin covering the most of the regions of the body. The lesions reduce production of milk and affect dairy operations. It is distinguished by lethargy, decreased appetite, fever, respiratory problems, swelling, and secondary infections. Lesions can cause a cough or nasal discharge. Swelling can aggravate limb pain accompanied by enlargement of the surrounding nodules. Generalized swelling occurring in various area of the body is termed as systemic edema. Secondary infection causes the development of abscesses and numerous other complications. Skin lesions can cause reproductive problems as the illness could be spread through ordinary breeding and even cause testicular enlargement or other reproductive problems. Nodules present in the eyes may cause irritation and conjunctivitis. There can be significant neurologic signs as well, which include incoordination or behavioural abnormalities, in very severe cases, while the intensity of the symptoms can cause serious effects onthe wellbeing and good health of bovine.
Prevention And Management Of LSDV
- Preventive action:
To stop future LSD incidents, the following precautions should also be taken right away, along with isolating the afflicted animal.
• Regulate of animal movement: Animal transportation to and from the infected area and from affected states should be strictly prohibited in order to deplete the financial effect of the epidemics and to regulate LSD. This will verify the distribution and LSD transmission.
• Restrictions for impacted animals as well as people who handle them: People traveling from and to the impacted region should be limited. Cattle that are in good health should be avoided by those handling the animals and caring for the afflicted ones. Hence, It is necessary to make sure that these safety precautions are in place.
• Vaccination: The afflicted villages are located such that ring vaccinations can be administered in villages up to a maximum of five kilometres from the afflicted village and preventive measures can be implemented in a designated area. The goat pox vaccine which is available ought to be given to the animals (those 4 months of age and older) by the subcutaneous route. The GTPV vaccine (Uttarkashi strain) has a 103.5 TCID50. Nonetheless, preventative vaccination or ring vaccination in the animals can be administered at a dosage of 103.0 TCID50, which is the same amount of the goat vaccine against goat pox.
- Biosecurity protocols:
- Sick animals have to be separated from healthy ones right away. Infected animals can get symptomatic therapy while taking all necessary safety and biosecurity precautions. It is advised to feed fodder, soft feed, and liquid feed.
- There should be more clinical monitoring for LSD in the impacted districts and the nearby communities.
- If the buffaloes were raised together, they should be inhabited apart until the afflicted animals have fully recovered.
- Frequent disinfection of the building.
- Those handling the diseased animal should always use hygienic and disinfecting practices and wear gloves and face masks.
Vector control Insecticides, repellents, and other chemical agents needed to be applied to regulate the quantity of vectors (flies, ticks, mosquitoes, fleas, and midges) on the property and on the animal carcass. Cleaning and disinfection procedures. Vehicles passing through the impacted animal holdings should be equipped with the proper chemicals and disinfectants, such as phenol (2%/15 minutes), chloroform, formalin (1%), iodine compounds (1:33 dilution), sodium hypochlorite (2–3%), ether (20%), and quaternary ammonium compounds (0.5%).
Advances In Vaccine Technology LSDV
A) Live Attenuated Vaccines
Live attenuated vaccines are the natural virulent forms of a microorganism, which are made non-pathogenic to its original host species by chemical, biological or physical process. The vaccines consist of strains having high immunogenicity or genetic characters. However, live vaccines have a few drawbacks-toxification, therapeutic adverse effects, and the acquiring of new diseases according to homologous recombination with other viruses of the genus (23) (24) (25). Therefore, using them in places where this illness is not present is not beneficial. The Neethling strain, which is the most prevalent LSDV variant in South Africa, was initially discovered to have several characteristics with the vaccinia virus. It had caused outbreaks in Botswana in 1943 and South Africa in 1945 (26). To assess the potential for use in LSD prophylaxis, a live vaccination derived from this kind of strain was put into clinical trials.
Weiss (1968) found that the strain's serial passage in funk embryos decreased the pathogenicity of the illness. The infection caused localized swelling in Just 50% of the animals who were injected, which was resolved within four to six weeks in the absence of signs of necrosis. Side effects under the name Neethling sickness were reported also for minor reactions of this vaccine; they appeared after vaccination of cows having Neethling strain (27). In cattle, live attenuated Neethling strain immunization has shown promise, with local reactions lasting up to almost three years (28). But just 30% of the cows immunized with the locally produced Neethling vaccine were capable of survive the virus's challenge(29). A survey conducted on the inoculated cows in Northern Greece indicated that 12% developed edema that later disappeared. Small skin nodules less than 0.5 cm diameter were evident in nine% of cows but not in calves. The herds that had been vaccinated may exhibit mildviremia, which is ephemeral (30). The virus discovered in the milk of cows immunized with the Ethiopian Neethling vaccine strain has proved that the immunization is ineffective against the illness (31). A more thorough assessment of the vaccine's efficacy and safety may be warranted if adverse events occur or if the intended immunological effects are not achieved. Haegeman et al. (2021) caried out clinical trials for colourful live downgraded LSDV vaccines, including LSDV, Lumpyvax, Kenyavac, Herbivac LS, and Vaccine LSD Neethling O vivant.They determined that although some vaccinations induce fever, they had no impact on feed intakes, daily progresses, and health in general. The lymph bumps of the Herbivac LS vaccination group were huge, and the other 3 South African vaccinations showed Neethling complaint (22). The specific genetic mutation that leadsto attenuation for live animal vaccines is not well understood. Because attenuating mutations are completely random, they are a ticking time bomb that could go off at any time (32). Some vaccination strains' effects have changed significantly over the past century, possibly because of base pair changes during manufacturing. Therefore, it suggests that attenuated immunizations pose a risk to human health. (Table 1).
B) Inactivated Vaccine
The chemical, biological, and physical processes used to destroy whole viruses or bacteria, remove their pathogenicity, and maintain their immunogenicity are known as inactivated vaccines. They feature strong utilization impacts, minimal production costs, and a brief cycle of development. Unlike live attenuated vaccines, they frequently need booster shots to stop virus invasion (33). Inactivated LSD vaccinations are not yet available, but inactivating the attenuated Neethling strain with bi-ethylimine bromure provides effective protection. Compared to the live attenuated vaccine, the inactivated vaccination has a 37% greater rate of antibody reaction and more antibodies (34). However, the precise clinical effect must be verified through fresh exploration taking animal welfare and ethics into consideration, Es-sadeqy and associates created an inactivated bivalent vaccination with oil painting adjuvants against LSDV and Bluetongue infection in 2020 (5). As stated by Wolff et al. (2020), certain vaccination adjuvants can increase the inactivated vaccines' efficacy. Adjuvants A and B, which contain cholesterol, amphotericin, and Quil A, were employed in the Neethling and Serbia vaccines without any negative effects. The inactivated vaccine's components make the body respond, disrupting the host's immunological reaction and triggering unintended immunological responses (35). The creation of a more secure and more effective vaccination against LSDV that is inactivated is necessary since inactivated vaccines have a limited immunological spectrum and are ineffective in cell-mediated and immunological responses of the mucosa (Table 2).
B) Recombinant Vaccines
The most efficient technique is live attenuated immunization for humoral and cellular protection against pathogens. However, standard methods cannot reduce all infections, as pathogenicity may reappear even after attenuation. Researchers have tried to find the genes that cause a disease's virulence, alter the disease's virulence by deleting or changing it, and recombinate to create attenuated strains. For ex., the herpes virus's normal in vitro replication was unaffected by deletion of its glycoprotein genes thymidine kinase and glycoprotein genes (36). By deleting the thymidine kinase gene from the pox virus and recombining it with the fusion protein gene from the rinderpest virus, a recombinant capripox virus vaccine was created using homologous recombination that successfully protects vaccinated cows from both rinderpest and lumpy skin disease. Ngichabe et al. (2002) claim that the recombinant vaccine's effectiveness for goat pox and rinderpest 10 yrs. later. Cattle were shielded for a maximum of one or three years (37). Poxviruses have the ability to encode an interferon gamma homologue that prevents the host's receptor from binding it (4). The gene of the aphthous virus can also produce interleukin-10-like proteins, which have immune-restrictive impacts on the host cells. Using homologous recombination, D. Kara et al. approached to create recombinant vaccines like LSDV-WB005KO and LSDV-WB008KO. In research studies, the two together enhance vaccinated cattle's neutralizing antibodies, allowing them to resist the LSDV invasion (6)However, modest clinical responses may occur during the initial stages of immunization. LSDV-WB005KO also protects against GTPV and SPPV, making it an excellent decision-making in clinical settings.
Researchers are exploring non-replicating or suicidal recombinant poxviruses to address environmental concerns and animal safety threats. Graham and Prevec produced adenoviruses with reduced replication in 1992, which can grow in cells with the E1 region in vitro, making it feasible to introduce healthy animals to the virus. To replicate in cell lines that contain glycoproteins but not in normal cells, many herpes viruses require the deletion of glycoprotein genes. This trend toward safe use also introduces a new notion for production of vaccine subsequent LSDV recombination with additional pathogens (Table 3) (38).
Important Distinctions Between Vaccine-Resistant And Wild-Type LSDV Viruses
Menasherow et al. discovered three techniques (2014) to distinguish between vaccination and wild-type strains of the encapsulated virions gene (39). The initial tactic is through inheritable sequencing by identifying the Israeli malign strain, the second tactic occurs by using manuals in enhancing the contagion's genome and the third, through the breakdown of amplicon of PCR response to ascertain the contagion's composition. These are more rapid although not in virulent strains, and extensively utilized in clinical settings. A dual real-time PCR method was reported by Agianniotaki et al. in 2017 to assess the efficacy of vaccination and wild-type virus amplification. (40). Evaluation of data for this kind of virus is part of this technique. Möller et al. (2019) used TaqMan probe-based qPCR to differentiate between vaccination and virulent strains with 100% sensitivity and specificity (41). In order to identify wild-type LSDV in high titer immunization samples, Agianniotaki et al. (2021) employed a duplex real-time PCR approach. With and without the LSDV vaccine virus, they obtained amplified frequencies of 99.0 and 98.6%, respectively (42).
Post-Vaccination Effects
Live homologous LSD vaccinations in countries banned by LSD are often questioned due to safety concerns. About two to three weeks following vaccination, protective immunity begins to build, but wild-type LSD-infected animals may still show symptoms. Negative responses, like local responses at the immunization site or widespread skin lesions called a "Neethling response," typically occur a week or two after vaccination (22). There might be a short decrease in milk production in animals getting immunizations (30). The prevalence of sickness and local or broad skin reactions are strongly impacted by the vaccination product's level of attenuation. GTPV and SPPV vaccinations in cattle typically do not cause negative side effects, but a few minor negative consequences were observed when administering high dosage of the strain RM65 SPPV (43). Homologous vaccines only produce adverse effects when given in previously disease-free nations (28). An investigation in Israel set up that a homologous LSDV- predicated vaccine had a low rate of mild side good (0.4%). The cohort of cattle had pre-vaccinated with the SPP RM65 vaccine strain, potentially reducing side goods 30 days following vaccination, shaped milk products were in contrast to the previous month in follow-up research that gathered milk product information from 21,844 cattle throughout 77 dairy ranches. There wasn't discernible variation in routine or critical culling, mortality, or milk quality between the times before and after vaccination, according to survival analysis. Vaccines must not contain undesirable contagions, such as pestiviruses, bluetongue, bottom, rabies, oral complaints, and others, in order to guarantee safety (44). Gershon et al. (1989) demonstrated the recombining of the Yemen goatpox-1 isolate's genome with the KS-1 strain's genome, illustrating spread of viruses naturally. This recombination may occur when an animal Vaccinated against an infection field strain inadequately attenuated live vaccine(45). Sequencing results of two transgenic LSDV strains recently showed an underlying structure in the vaccination virus and fragments that resemble Neethling of the wild-type virus (46) (47) (48). Russian scientists discovered the original recombinant LSDV Russia/Saratov/2017 and the latter LSDV Russia/Udmurtiya/2019 from a cow in Saratov, near the Kazakhstani border (49). The study highlights the possible function of illicit LSDV vaccine usage, which might result in recombination. The two vaccination viruses coming into contact with one another is greater due to manufacturers producing both the Neethling vaccines and KS1 in LSDV-endemic nations (50). Recombination is possible in vitro under controlled laboratory conditions, as demonstrated by the Dry-vax vaccination. The results emphasize the danger of administering cattle vaccines that are either heterologous or homologous but have been attenuated in the in the middle of an outbreak (51).
CONCLUSION
LSD or lumpy skin disease, is a fatal and economically disastrous virus impacting livestock with a great influence on the production, health, and livelihoods of farmers. It has evolved into an international concern with its distribution across the many continents, which was by the insect vectors and increased livestock migration. Although the clinical course of the illness varies, reduced milk output, infertility, poor hide quality, and even demise can cause significant losses in financial terms. This has made LSD difficult to manage and control due to the varied symptoms and transmission methods. However, diagnostics like PCR and serological testing greatly enhanced thechances of detection and diagnosis compared to similar diseases. Even though controlling vector’s populations and strong biosecurity measures are both equally important in stopping the disease's spread, vaccination remains the best preventive measure. Among these promising treatments under investigation for more effective vaccinations and measures for control are required. Ultimately, It is predicated on early detection, appropriate management, and continuous research into the pathophysiology of the illness to lessen its impact and save the financial security of farmers and cattle.
REFERENCES
- ?evik M, Avci O, Do?an M, ?nce ÖB. Serum Biochemistry of Lumpy Skin Disease Virus-Infected Cattle. Biomed Res Int. 2016;2016:1–6.
- Yeruham I, Nir O, Braverman Y, Davidson M, Grinstein H, Haymovitch M, et al. Spread of lumpy skin disease in Israeli dairy herds. Vet Rec. 1995 Jul 22;137(4):91–3.
- Sudhakar SB, Mishra N, Kalaiyarasu S, Jhade SK, Hemadri D, Sood R, et al. Lumpy skin disease (LSD) outbreaks in cattle in Odisha state, India in August 2019: Epidemiological features and molecular studies. Transbound Emerg Dis. 2020 Nov;67(6):2408–22.
- Johnston JB, McFadden G. Poxvirus immunomodulatory strategies: current perspectives. J Virol. 2003 Jun;77(11):6093–100.
- Es-Sadeqy Y, Bamouh Z, Ennahli A, Safini N, El Mejdoub S, Omari Tadlaoui K, et al. Development of an inactivated combined vaccine for protection of cattle against lumpy skin disease and bluetongue viruses. Vet Microbiol. 2021 May;256:109046.
- Boshra H, Truong T, Nfon C, Bowden TR, Gerdts V, Tikoo S, et al. A lumpy skin disease virus deficient of an IL-10 gene homologue provides protective immunity against virulent capripoxvirus challenge in sheep and goats. Antiviral Res. 2015 Nov;123:39–49.
- Al-Salihi KA, Hassan IQ. Lumpy Skin Disease in Iraq: Study of the Disease Emergence. Transbound Emerg Dis. 2015 Oct;62(5):457–62.
- Tuppurainen ESM, Antoniou SE, Tsiamadis E, Topkaridou M, Labus T, Debeljak Z, et al. Field observations and experiences gained from the implementation of control measures against lumpy skin disease in South-East Europe between 2015 and 2017. Prev Vet Med. 2020 Aug;181:104600.
- Kumar N, Barua S, Kumar R, Khandelwal N, Kumar A, Verma A, et al. Evaluation of the safety, immunogenicity and efficacy of a new live-attenuated lumpy skin disease vaccine in India. Virulence. 2023 Dec;14(1):2190647.
- Ratyotha K, Prakobwong S, Piratae S. Lumpy skin disease: A newly emerging disease in Southeast Asia. Vet World. 2022 Dec;15(12):2764–71.
- Namazi F, Khodakaram Tafti A. Lumpy skin disease, an emerging transboundary viral disease: A review. Vet Med Sci. 2021 May;7(3):888–96.
- Sendow I, Assadah NS, Ratnawati A, Dharmayanti NI, Saepulloh M. Lumpy Skin Disease: Ancaman Penyakit Emerging Bagi Kesehatan Ternak Sapi Di Indonesia. Indonesian Bulletin of Animal and Veterinary Sciences. 2021 Jun 30;31(2):85.
- Parvin R, Chowdhury EH, Islam MT, Begum JA, Nooruzzaman M, Globig A, et al. Clinical Epidemiology, Pathology, and Molecular Investigation of Lumpy Skin Disease Outbreaks in Bangladesh during 2020-2021 Indicate the Re-Emergence of an Old African Strain. Viruses. 2022 Nov 15;14(11).
- Magori-Cohen R, Louzoun Y, Herziger Y, Oron E, Arazi A, Tuppurainen E, et al. Mathematical modelling and evaluation of the different routes of transmission of lumpy skin disease virus. Vet Res. 2012 Jan 11;43(1):1.
- Issimov A, Rametov N, Zhugunissov K, Kutumbetov L, Zhanabayev A, Kazhgaliyev N, et al. Emergence of the First Lumpy Skin Disease Outbreak Among Livestock in the Republic of Kazakhstan in 2016. 2020.
- Shumilova I, Nesterov A, Byadovskaya O, Prutnikov P, Wallace DB, Mokeeva M, et al. A Recombinant Vaccine-like Strain of Lumpy Skin Disease Virus Causes Low-Level Infection of Cattle through Virus-Inoculated Feed. Pathogens. 2022 Aug 16;11(8).
- Seerintra T, Saraphol B, Wankaew S, Piratae S. Molecular identification and characterization of Lumpy skin disease virus emergence from cattle in the northeastern part of Thailand. J Vet Sci. 2022 Sep;23(5):e73.
- Zeedan GSG, Mahmoud AH, Abdalhamed AM, El-Razik KAEHA, Khafagi MH, Zeina HAAA. Detection of lumpy skin disease virus in cattle using real-time polymerase chain reaction and serological diagnostic assays in different governorates in Egypt in 2017. Vet World. 2019 Jul;12(7):1093–100.
- Amin DM, Shehab G, Emran R, Hassanien RT, Alagmy GN, Hagag NM, et al. Diagnosis of naturally occurring lumpy skin disease virus infection in cattle using virological, molecular, and immunohistopathological assays. Vet World. 2021 Aug;14(8):2230–7.
- Sthitmatee N, Tankaew P, Modethed W, Rittipornlertrak A, Muenthaisong A, Apinda N, et al. Development of in-house ELISA for detection of antibodies against lumpy skin disease virus in cattle and assessment of its performance using a bayesian approach. Heliyon. 2023 Feb;9(2):e13499.
- Mwanandota JJ, Macharia M, Ngeleja CM, Sallu RS, Yongolo MG, Mayenga C, et al. Validation of a diagnostic tool for the diagnosis of lumpy skin disease. Vet Dermatol. 2018 Dec;29(6):532-e178.
- Haegeman A, De Leeuw I, Saduakassova M, Van Campe W, Aerts L, Philips W, et al. The Importance of Quality Control of LSDV Live Attenuated Vaccines for Its Safe Application in the Field. Vaccines (Basel). 2021 Sep 13;9(9):1019.
- Lee SW, Markham PF, Coppo MJC, Legione AR, Markham JF, Noormohammadi AH, et al. Attenuated Vaccines Can Recombine to Form Virulent Field Viruses. Science (1979). 2012 Jul 13;337(6091):188–188.
- Sprygin A, Babin Y, Pestova Y, Kononova S, Wallace DB, Van Schalkwyk A, et al. Analysis and insights into recombination signals in lumpy skin disease virus recovered in the field. PLoS One. 2018;13(12):e0207480.
- Krotova A, Byadovskaya O, Shumilova I, van Schalkwyk A, Sprygin A. An in-depth bioinformatic analysis of the novel recombinant lumpy skin disease virus strains: from unique patterns to established lineage. BMC Genomics. 2022 Dec 24;23(1):396.
- Hunter P, Wallace D. Lumpy skin disease in southern Africa?: a review of the disease and aspects of control. J S Afr Vet Assoc. 2001 Jul 9;72(2):68–71.
- Yeruham I, Nir O, Braverman Y, Davidson M, Grinstein H, Haymovitch M, et al. Spread of lumpy skin disease in Israeli dairy herds. Vet Rec. 1995 Jul 22;137(4):91–3.
- Ben-Gera J, Klement E, Khinich E, Stram Y, Shpigel NY. Comparison of the efficacy of Neethling lumpy skin disease virus and x10RM65 sheep-pox live attenuated vaccines for the prevention of lumpy skin disease - The results of a randomized controlled field study. Vaccine. 2015 Sep 11;33(38):4837–42.
- Gari G, Bonnet P, Roger F, Waret-Szkuta A. Epidemiological aspects and financial impact of lumpy skin disease in Ethiopia. Prev Vet Med. 2011 Dec 15;102(4):274–83.
- Katsoulos PD, Chaintoutis SC, Dovas CI, Polizopoulou ZS, Brellou GD, Agianniotaki EI, et al. Investigation on the incidence of adverse reactions, viraemia and haematological changes following field immunization of cattle using a live attenuated vaccine against lumpy skin disease. Transbound Emerg Dis. 2018 Feb;65(1):174–85.
- Bedekovi? T, Šimi? I, Kreši? N, Lojki? I. Detection of lumpy skin disease virus in skin lesions, blood, nasal swabs and milk following preventive vaccination. Transbound Emerg Dis. 2018 Apr;65(2):491–6.
- Minor PD, John A, Ferguson M, Icenogle JP. Antigenic and Molecular Evolution of the Vaccine Strain of Type 3 Poliovirus during the Period of Excretion by a Primary Vaccinee. Journal of General Virology. 1986 Apr 1;67(4):693–706.
- Bhanuprakash V, Indrani BK, Hegde R, Kumar MM, Moorthy ARS. A Classical Live Attenuated Vaccine for Sheep Pox. Trop Anim Health Prod. 2004 May;36(4):307–20.
- Hamdi J, Boumart Z, Daouam S, El Arkam A, Bamouh Z, Jazouli M, et al. Development and Evaluation of an Inactivated Lumpy Skin Disease Vaccine for Cattle. Vet Microbiol. 2020 Jun;245:108689.
- Stephens LR, Little PB, Wilkie BN, Barnum DA. Isolation of Haemophilus somnus antigens and their use as vaccines for prevention of bovine thromboembolic meningoencephalitis. Am J Vet Res. 1984 Feb;45(2):234–9.
- Kitching RP, Hammond JM, Taylor WP. A single vaccine for the control of capripox infection in sheep and goats. Res Vet Sci. 1987 Jan;42(1):53–60.
- Ngichabe CK, Wamwayi HM, Ndungu EK, Mirangi PK, Bostock CJ, Black DN, et al. Long term immunity in African cattle vaccinated with a recombinant capripox-rinderpest virus vaccine. Epidemiol Infect. 2002 Apr;128(2):343–9.
- Heffner S, Kovács F, Klupp BG, Mettenleiter TC. Glycoprotein gp50-negative pseudorabies virus: a novel approach toward a nonspreading live herpesvirus vaccine. J Virol. 1993 Mar;67(3):1529–37.
- Menasherow S, Rubinstein-Giuni M, Kovtunenko A, Eyngor Y, Fridgut O, Rotenberg D, et al. Development of an assay to differentiate between virulent and vaccine strains of lumpy skin disease virus (LSDV). J Virol Methods. 2014 Apr;199:95–101.
- Agianniotaki EI, Chaintoutis SC, Haegeman A, Tasioudi KE, De Leeuw I, Katsoulos PD, et al. Development and validation of a TaqMan probe-based real-time PCR method for the differentiation of wild type lumpy skin disease virus from vaccine virus strains. J Virol Methods. 2017 Nov;249:48–57.
- Möller J, Moritz T, Schlottau K, Krstevski K, Hoffmann D, Beer M, et al. Experimental lumpy skin disease virus infection of cattle: comparison of a field strain and a vaccine strain. Arch Virol. 2019 Dec;164(12):2931–41.
- Agianniotaki EI, Chaintoutis SC, Haegeman A, Tasioudi KE, De Leeuw I, Katsoulos PD, et al. Development and validation of a TaqMan probe-based real-time PCR method for the differentiation of wild type lumpy skin disease virus from vaccine virus strains. J Virol Methods. 2017 Nov;249:48–57.
- Abutarbush SM, Tuppurainen ESM. Serological and clinical evaluation of the Yugoslavian RM65 sheep pox strain vaccine use in cattle against lumpy skin disease. Transbound Emerg Dis. 2018 Dec;65(6):1657–63.
- Morgenstern M, Klement E. The Effect of Vaccination with Live Attenuated Neethling Lumpy Skin Disease Vaccine on Milk Production and Mortality-An Analysis of 77 Dairy Farms in Israel. Vaccines (Basel). 2020 Jun 19;8(2).
- Gershon AA, Steinberg SP, Gelb L, Galasso G, Borkowsky W, LaRussa P, et al. Live attenuated varicella vaccine. Efficacy for children with leukemia in remission. JAMA. 1984 Jul 20;252(3):355–62.
- Sprygin A, Pestova Y, Bjadovskaya O, Prutnikov P, Zinyakov N, Kononova S, et al. Evidence of recombination of vaccine strains of lumpy skin disease virus with field strains, causing disease. PLoS One. 2020;15(5):e0232584.
- Sprygin A, Artyuchova E, Babin Y, Prutnikov P, Kostrova E, Byadovskaya O, et al. Epidemiological characterization of lumpy skin disease outbreaks in Russia in 2016. Transbound Emerg Dis. 2018 Dec;65(6):1514–21.
- Sprygin A, Babin Y, Pestova Y, Kononova S, Wallace DB, Van Schalkwyk A, et al. Analysis and insights into recombination signals in lumpy skin disease virus recovered in the field. PLoS One. 2018;13(12):e0207480.
- Aleksandr K, Olga B, David WB, Pavel P, Yana P, Svetlana K, et al. Non-vector-borne transmission of lumpy skin disease virus. Sci Rep. 2020 May 4;10(1):7436.
- Qin L, Favis N, Famulski J, Evans DH. Evolution of and evolutionary relationships between extant vaccinia virus strains. J Virol. 2015 Feb;89(3):1809–24.
- Qin L, Upton C, Hazes B, Evans DH. Genomic analysis of the vaccinia virus strain variants found in Dryvax vaccine. J Virol. 2011 Dec;85(24):13049–60.

Fig.1: Clinical signs and gross lesions of cattle affected by LSD


 N. K. Gupta*
N. K. Gupta*
 S. Shrivastav
S. Shrivastav

 10.5281/zenodo.14690391
10.5281/zenodo.14690391