Abstract
COVID-19 is mainly a respiration ailment because of a newly observed SARS-CoV-2 virus and recognized in the town of Wuhan, China in December 2019. WHO has declared this ailment as a deadly disease, and warned different countries. Presently this has affected 216 countries, areas or territories worldwide, spreading of this ailment is very fast in United States, Brazil, and Russia than in the US of its starting place, China. Like other coronaviruses, this could develop respiratory tract infections inside the patient’s variety from slight to deadly infection like pneumonia and acute breathing distress syndrome (ARDS). As of now, no powerful drug, vaccine, or any technique is to be had and experiments are underway. But, empirical remedy is being observed to manipulate and save the lives of the patients. There’s a need for pharmacological alternatives to fight this lethal virus and its complications. Primarily based on the preceding experience with comparable coronavirus control and present preliminry information from uncontrolled research, drugs like chloroquine, hydroxychloroquine, remdesivir, lopinavir/ritonavir, and favipiravir were recommended with the aid of the researchers to manipulate COVID-19. This assessment had assessed the capacity mechanisms, safety profile, availability and fee of those tablets. This review concludes that the drugs referred to above are having one-of-a-kind houses and act otherwise in preventing the COVID-19 viruses. Rather than single drug, combination of antivirals with special mechanism of movement may be more effective and on the same time their adverse occasions need to not be underestimated.
Keywords
COVID-19, Coronavirus, Treatment, Hydroxychloroquine, Remdesivir
Introduction
COVID-19, formerly known as 2019 Novel Coronavirus (2019- nCoV) respiratory ailment due to a newly observed coronavirus, SARS-CoV-2 virus and recognized within the metropolis of Wuhan, Hubei province, China in December 2019. Global Fitness Enterprise (WHO) declared the reputable call as COVID-19 in February 2020 .Virus remoted from the COVID-19 sufferers belongs to the genus beta coronavirus, this institution of viruses can purpose simple/commonplace bloodless to excessive acute respiratory syndrome (SARS) due to SARS CoV become recognized in 2002, and every other syndrome Middle East respiratory syndrome (MERS), as a result of MERS-CoV recognized in 2012.3-6 in step with the document of the WHO and China Joint challenge on Coronavirus sickness 2019 (COVID-19), it's miles a zoonotic virus, based at the information to be had, bats seems to be the reservoir of COVID-19 virus.1
Epidemiology:
On 11 March 2020, WHO declared this ailment as a pandemic, based on its unfold to 118,000 instances in 114 countries, and 4291 deaths on that date and warned other countries approximately its seriousness. 8 consistent with the WHO COVID-19 dashboard globally, as of 9:20am CEST, 26 may additionally 2020, five, 370,375 showed instances of COVID-19, consisting of 3, 44,454 deaths, had been said to WHO from 216 international locations, areas or territories.2 Its unfold and mortality is more within the U.S.A of the use with 16,18,757 confirmed cases and 96,909 deaths, accompanied by using Brazil: 3,sixty three,211 instances and 22,666 deaths; Russian Federation: three, fifty three,427 cases and 3633 deaths; the United Kingdom: 2,59,563 instances and 36,793 deaths; pain: 235,772 instances and 28,752 deaths; Italy: 229,858 cases and 32,785 deaths; Germany: 178,570 cases and 8257 deaths; Turkey:156,827 instances and 4340 deaths; France: 142,204 instances and 28,315 deaths. In India as on, 26 might also 2020, 08:00 IST (GMTþ5:30), 141,213 had been infected, among them 60,490 were cured/discharged and 4167 were died. In India, the ordinarily affected states consists of Maharashtra with 52,667 cases (1695 deaths) observed through Tamil Nadu 17,082 (118); Gujarat 14,460 (888); and Delhi 14,053 (276)3
Virus characteristics and clinical manifestations:
Like other coronaviruses, these are spherical shaped containing genetic fabric inner and with spike proteins protruding from their floor, which allows to latch onto the human cellular observed by way of fusion and switch of genes to the host cell. The modern day records suggests that, just like the virus that triggered the 2002 SARS outbreak, SARS-CoV2 spikes bind to receptors at the human cell floor called angiotensin-changing enzyme 2 (ACE2).eleven This latest virus can increase respiratory tract infections within the sufferers variety from mild to fatal ailments like pneumonia and acute respiratory misery syndrome (ARDS). Most of the people of the patients will revel in moderate to moderate respiratory infection and get better with supportive remedy and do not want any unique care/remedy. Geriatric sufferers and sufferers with comorbidities like diabetes, cardiovascular, chronic respiration problems, most cancers, immune deficiency sufferers and different chronic issues are greater at risk of broaden critical pathological problems related to COVID-19.4
Pharmacological and clinical aspects of important SARS-1 and SARS-COV-2 therapeutic agents:
- FAVIPIRAVIR:
Favipiravir (T-705 or Avigan), an oral antiviral drug permitted in Japan for influenza contamination in 2014.26 It has also been used for remedy of Ebola virus contamination. It acts by means of direct inhibition of viral replication and transcription via misincorporation in nascent vRNA (viral ribonucleic acid), or with the aid of binding to conserved polymerase domains, preventing incorporation of nucleotides for vRNA replication and transcription.4,5
Preclinical evidence:
Consistent with the Madelain V et al non-human primate (NHP) version observe, favipiravir was discovered to have adaptive immune response in viral clearance, and might grow to be a treatment option for different emerging viral sicknesses. other research have also said that favipiravir acts by using inhibiting RNA based RNA polymerase (RdRp) by means of changing into its lively metabolite M. Venkatasubbaiah et al. / present day medicine research and practice 10 (2020) (favipiravirribofuranosyl-50 -triphosphate (RTP)) in cells and is identified as a substrate by means of viral RNA polymerase. As SARS-CoV-2, is an RNA virus, this drug is probably one of the alternatives for treating COVID-19.6
Clinical evidence:
National clinical merchandise management of China has included this drug in the capacity remedies for COVID-19. The medical proof at the efficacy of this drug changed into determined inside the scientific trial carried out with the aid of the 1/3 people's sanatorium of Shenzhen in Guangdong province, where favipiravir institution sufferers' (n ¼ 35) laboratory checks shown poor for COVID 19 after 4 days of remedy, while different group patients took eleven days for the equal.7 In an open-label non-randomized control examine conducted by using Q Caietal, favipiravir (FPV) 1600 mg two times day by day as a loading dose and 600 mg twice each day plus interferon (IFN)-a five million U two times every day by way of aerosol inhalation had been administered to 35 sufferers with a median age of 43 (35.5e59) years. In some other organization lopinavir 400 mg/ritonavir a hundred mg (RTV) two times day by day plus IFN-a 5 million U two times each day by using aerosol inhalation had been given in 45 sufferers (median age changed into 49 (36e61) years). They determined a shorter viral clearance time and extensive improvement in chest imaging in FPV organization with few ADRs. Initial consequences of any other comparative take a look at carried out in Wuhan, China, with one hundred twenty COVID-19 sufferers have also supported the efficacy of favipiravir and in addition they said that administration of favipiravir tablet is easier.8
Adverse drug reactions (ADR), cost and availability:
In step with the data obtained from the Uppsala tracking Centre (UMC)-global negative reactions reporting gadget as on 22.05.2020 best 24 ADRs were pronounced.34 the value of the drug is no longer observed with our good sized search and the worldwide industrial availability of this drug could be very less.
Current clinical research:
Currently, there are 17 scientific trials unique to COVID-19 are at various levels of improvement and researchers are looking forward to promising results in clearing the virus.35 currently, a reputed pharmaceutical enterprise has initiated. Segment-three clinical trials for COVID 19 in India with tablet favipiravir, and it's far looking ahead to the complete observe effects with the aid of August. 7,8
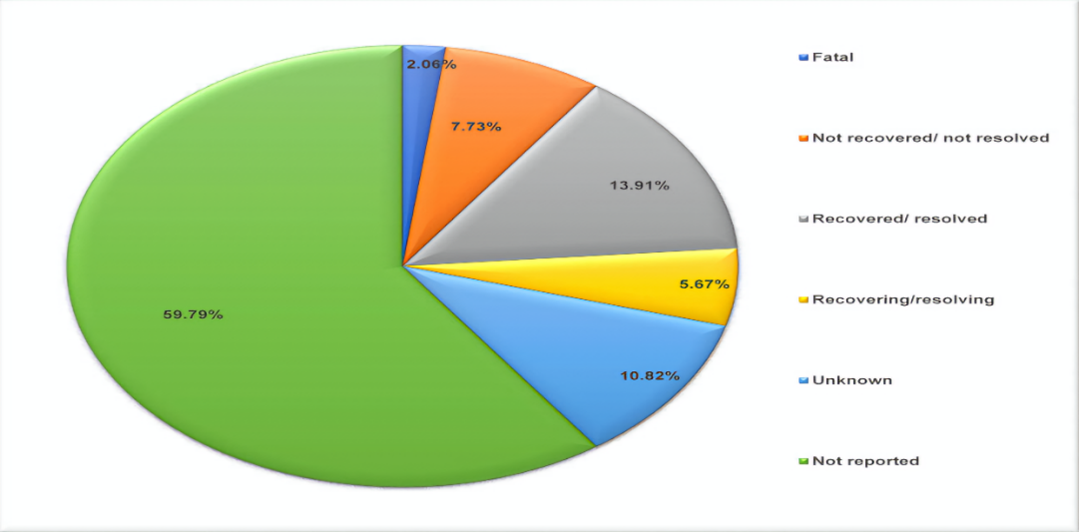
Fig.no.01:Adverse drug event outcomes of Favipiravir use in COVID-19
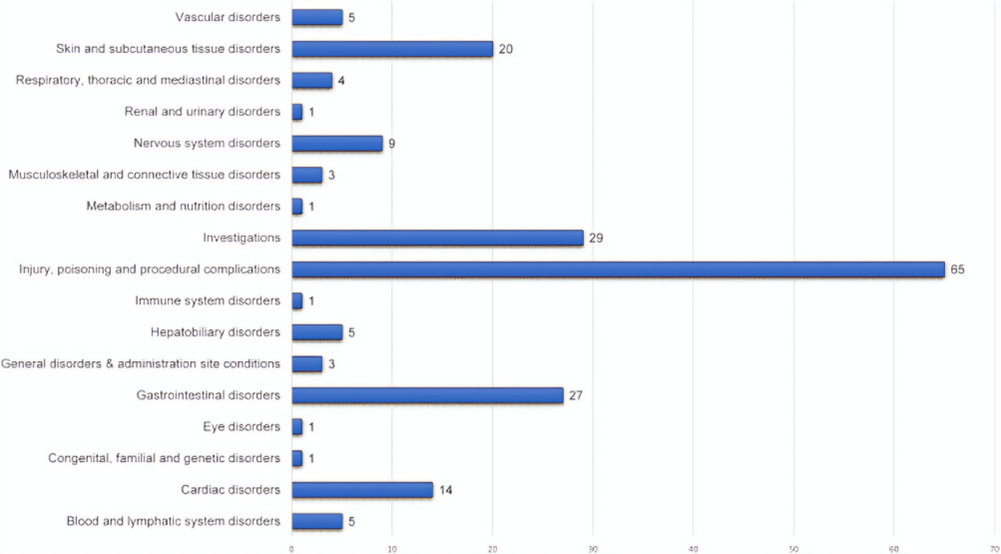
Fig.no.02:System-wise distribution of ADEs attributed to Favipiravir use in COVID-19.
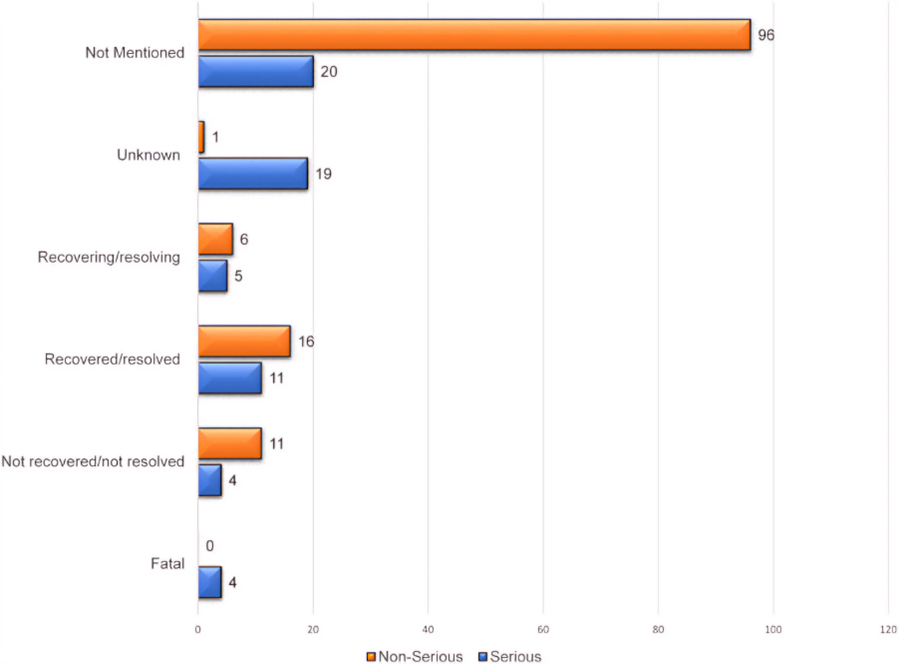
Fig.no.03: Comparison of serious and non-serious adverse drug events outcomes of Favipiravir use in COVID-19.
CHLOROQUINE:
Chloroquine is authorized for the prophylaxis and treatment of malaria, to deal with extra intestinal amebiasis and chloroquine is likewise used off label for the remedy of various rheumatic sicknesses, as nicely as remedy and prophylaxis of Zika virus. As in step with the Emergency Use Authorization (EUA) folks FDA for the unapproved use of this drug in COVID-19 patients and the dosing time table for this drug is 1g on day one, observed through 500mg once an afternoon for 4 to 7 days based on clinical assessment.9
Preclinical evidence:
Preclinical data from diverse research has showed the position of chloroquine in controlling the human coronavirus infection via exceptional mechanisms, which incorporates; through protease inhibition in SARSCoV; inhibition of salicylic acid biosynthesis; inhibition of HCoV-OC43 replication in HRT-18cells by chloroquine in newborn mice; the activation of p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK) in human coronavirus 229E infection in human epithelial lung cells; Inhibition of viral unfold in mobile subculture thru endosomal pH rise and interfering with terminal glycosylation of angiotensin converting enzyme 2 receptor; Inhibition of the replication of SARS-CoV in Vero E6 cells.10
Clinical evidence:
Chloroquine phosphate tablet 500mg 12 hourly for 10 days may have blessings in controlling the radical coronavirus pneumonia irrespective of their severity. In other research conducted on COVID-19 patients, researchers have located the prevalence of chloroquine over other remedy in phrases of both efficacy and protection in reducing the exacerbation of pneumonia. With the support of this records, Chinese authorities recommended chloroquine alongside with other cures for the prevention and remedy of COVID-19 pneumonia.10
Adverse drug reactions (ADRs), cost and availability:
The America Academy of Ophthalmology stated that hazard of toxicity with using chloroquine/hydroxychloroquine is dose and period established with the regular daily dose for much less than 5 years therapy has <1>
Current clinical research:
Currently, a total of 67 medical trials are underway with chloroquine for the control of COVID-19.
HYDROXYCHLOROQUINE (HCQ)
Hydroxychloroquine (HCQ) is an aminoquinoline. It is indicated for both the prophylaxis and treatment of uncomplicated malaria .It is also prescribed for the control of rheumatoid arthritis,
continual discoid lupus erythematous, and systemic lupus erythematous. In pediatric age group its miles used for the management of juvenile idiopathic arthritis (in aggregate with other healing procedures), discoid and systemic lupus erythematous.12 The advocated dosing schedule for each prophylactic and healing use in COVID-19: As per ICMR, the prophylactic dosing schedule of HCQ in COVID 19 is as follows For asymptomatic family contacts of laboratory-confirmed instances: 400mg two times a day on day 1, accompanied by 400mg as soon as weekly for the next 3 weeks; to be fascinated with meals).12 The dosing agenda of HCQ as per America FDA's EUA for hospitalized COVID-19 adults and adolescent patients who weigh >50kg is 800mg on the first day and then 400 mg daily for four to seven days, length of overall treatment is based totally on medical assessment.
Some U.S. clinicians have stated extraordinary dosing schedules 400mg 12hourly on day one, then day by day for five days; 400mg BID on day one, then 200mg 12th hourly for four days; 600mg 12th hourly on day one, then 400mg daily for 2-5 days.13
Preclinical evidence:
Literature confirms that both the CQ and HCQ have comparable residences and acts inside the same manner with minor modifications in their dosing schedule, researchers also stated that the HCQ is their first choice in treating the SARS-CoV-2 infection, as HCQ is displaying much less toxicity (~40%) in animals than chloroquine. An in-vitro look at performed by way of Yao X et al using SARS-CoV-2 inflamed Vero cells, concluded that the HCQ (EC50¼ 0.72mM) at an oral loading dose of 400mg twelfth hourly, followed by means of 200mg two times daily for four days is better than chloroquine (EC50¼5.47mM) 500mg twelfth hourly for 5 days for treating SARS-CoV 2 infection, additionally they quoted that, the immune modulatory effects of those two drugs can suppress the raised immune factors (cytokines IL-6 and IL-10) as an immune response to SARS-CoV 2 virus and forestalls the complications.14
Clinical evidence:
The revised advisory report of the Joint tracking Group under the Chairmanship of DGHS on the protection and efficacy of prophy lactic use of Hydroxychloroquine (HCQ) in India has drawn the following conclusions from the scientific research:
- A significant dose-reaction courting was discovered between the variety of prophylactic doses taken and frequency of occurrence of SARSCoV 2 contamination in symptomatic healthcare people.15
- The probability of SARSCoV-2 infection in healthcare workers who have taken the prophylactic HCQ was less while as compared to the ones who have no longer taken
- Any other examine conducted at AIIMS, New Delhi on prophylaxis HCQ (median 6 weeks of comply with up) had pronounced the decrease occurrence of this contamination in healthcare people.
Adverse drug reactions (ADRs), cost and availability:
The threat of toxicity with the use of HCQ is dose and length structured. With a regular daily dose for much less than 5 years remedy has a <1>
Current clinical research:
To date, 201 HCQ medical trials are being performed with or without different remedies in specific components of the world and are at extraordinary stages of improvement for prophylaxis and treatment of slight, mild, and excessive SARS-CoV-2 infections. Of 201 trials, 5 have been completed and waiting for described tips for the use of HCQ in COVID-19 sufferers with greater clinical evidence.16
Combination of HCQ with azithromycin:
Philippe Gautret et al have studied the efficacy of HCQ innaggregate with azithromycin, 22 showed COVID-19 sufferers received 600mg of hydroxychloroquine daily and relying on their scientific presentation, azithromycin changed into introduced to the deal withment. A great reduction of the viral load changed into found on day 6 when as compared to controls. They concluded that hydroxylchloroquine treatment had considerably reduced the viral load in COVID19 sufferers and its effect become synergized with azithromycin.17
Researchers are investigating the efficacy of this combination in diverse countries without or with different capsules.
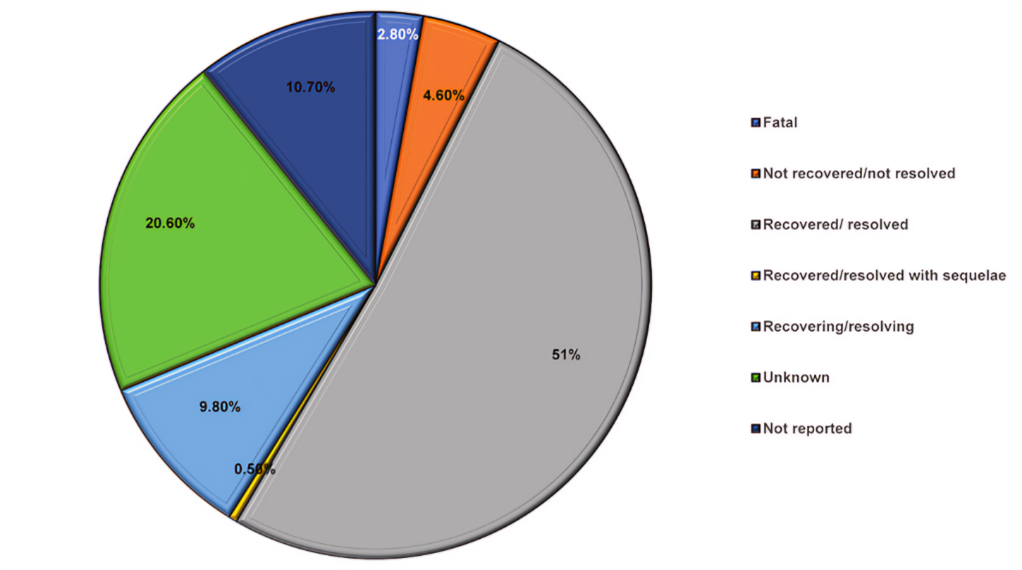
Fig.no.04:Adverse drug event outcomes of Hydroxychloroquine use in COVID-19
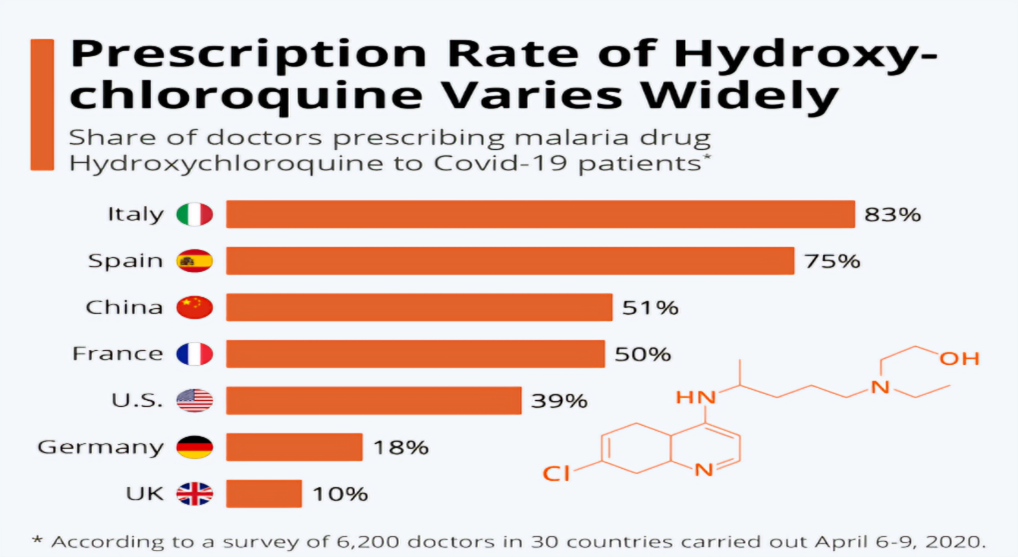
Fig.no.5: Prescription Rate of Hydroxychloroquine Varies Widely
REMDESIVIR
Remdesivir, or GS-5734, is an investigational adenosine triphosphate analog and used in the remedy for Ebola and coronavirus infections.18 Remdesivir shuts down viral replication by using inhibiting a key viral enzyme, the RNA-based RNA polymerase.19 Based at the preclinical data and initial proof on speedy recuperation in clinical trials, on 1st might also 2020, meals and Drug management (FDA) issued the EUA for its emergency use for the remedy of hospitalized COVID-19 patients.20,21
Preclinical evidence:
Preclinical studies performed by means of researchers have established the antiviral activity through numerous mechanisms towards corona viruses like MERS and SARS, which are structurally much like SARS CoV-2.22 Dong L et al stated that the remdesivir is a nucleoside analogue with capability and extensive-spectrum antiviral pastime. Wang M et al concluded that remdesivir acts via incorporation into nascent viral RNA chains and outcomes in untimely termination at a stage post virus access in RNA viruses along with SARS/MERSCoV.23 In 2020, Timothy P S et al found a strong inhibition in MERS-CoV replication with EC50 of 0.09mM, without a observable cytotoxicity up to 10mM in primary human lung epithelial cellular cultures. Tchesnokov E P et al have proven not on time chain termination within the Ebola virus, HIV-1, and the hepatitis B virus cellular cultures.24 Agostini M L et al has identified the efficacy of remdesivir in targeting the proofreading exoribo nuclease through the incorporation of the energetic triphosphate into viral RNA.25
Clinical evidence:
Holshue M L et al have stated the first case of a 35-12 months-vintage guy who was confirmed with novel COVID-19. Intravenous remdesivir become initiated from the night of day 7, because the patient's condition turned into worsen and persevered till the discharge day.26 0n 11th day the viral masses have been reduced in respiratory fluid specimens are at the twelfth day, specimen tested poor for 2019-nCoV and the patient's medical condition changed into progressed. And no adverse events were located in affiliation with the remdesivir infusion. Additionally they stated the need for full-size medical research on using remdesivir.27,28 A news file through Suryatapa Bhattacharya in the Wall avenue magazine discovered that the 14 American cruise passengers in Japan who contracted the novel coronavirus were treated efficaciously with the antiviral drug remdesivir.29,30 International Pharmaceutical Federation and American Society of fitness-device Pharmacists (ASHP) have said the significance of remdesivir in treating COVID-19 in their remedy guidelines for COVID-19 and they also cited that this drug is beneath evaluation in one of a kind medical trials.31
Current clinical research:
Currently, 24 clinical trials are being conducted to know the efficacy of remdesivir in COVID 19 sufferers, of them trial became completed. Some uncontrolled research are displaying some medical evidence and might count on the equal with those medical trials and this may become a capability remedy option for COVID-19 patients. Although it's miles effective in fighting COVID-19, its restrained availability and high value turns into task, so, governments and researchers must take vital movements to triumph over these troubles.32,33
LOPINAVIR-RITONAVIR (KALETRA):
Lopinavir and low dose of ritonavir are antiretroviral protease inhibitors; together they work efficaciously inside the treatment of HIV contamination, within the 12 months 2000 this mixture was launched by means of Abbott under the logo name Kaletra.34 The metabolism of lopinavir is inhibited by using ritonavir and complements the 1/2-existence and antiviral activity. American association for the have a look at of Liver sicknesses (AASLD) and Infectious sicknesses Society of the united states (IDSA) recommendations advise ritonavir-boosted mixture therapies as first-line remedy for HCV Genotype 1a/b and four treatment-naïve sufferers with or without cirrhosis.35,36
The government of India Ministry of fitness & circle of relatives Welfare Directorate popular of fitness services (EMR department) has recommended this drug aggregate for the medical control of COVID -19 inside the following dosing agenda .
- Lopinavir 200 mg/ritonavir/50mg 2 drugs twice every day.37
- Lopinavir400 mg/ritonavir 100mg/5ml, 5ml suspension twice day by day, (if the patient is unable to take orally).38
- The duration: for 14 days or for 7 days after becoming asymptomatic.39
Preclinical evidence:
Chymotrypsin-like protease (3CLpro) and a papain-like protease (PLpro) are critical for the replication of SARS-CoV. Those proteases are important targets for the improvement of antiviral pills.40 In-vitro/animal research performed in 2020 have revealed the lack of ability of this drug to bind to major goals like 3CLpro, PLpro, RdRp, and a comparative examine between the remdesivir and lopinavir/ritonavir (LPV/RTV), and interferon-beta (IFN-b) in opposition to MERS-CoV in mice discovered mild reduction in viral loads and progressed the pulmonary characteristic but no longer reduced the viral replication or intense lung pathology.41,42,43
Clinical evidence:
Consistent with the case document published by means of Han W et al this mixture has shown efficacy in a 47 years antique male SARSCoV-2 affected person in 800/200mg dose in conjunction with, methylprednisolone 40mg (for 2 days handiest), recombinant human interferon alfa-2b 10 million IU in step with day, affected person changed into recovered at the 10th day and examined bad for the virus and discharged.44
In Chen Qet al case collection, lopinavir and ritonavir capsules (800/200mgdaily) were given to nine sufferers together with different remedy based totally on the severity, all of the sufferers come to be poor for SARS CoV-2 (range four-eleven remedy days). The average days of health facility stay had been 14.2 (range from 9 to twenty) for the restoration from the lung lesions, as well as from the clinical signs and symptoms. Fortuitously, no deaths had been reported all through the treatment duration and authors concluded that effective remedy have to consist of the aggregate of traditional Chinese language and western remedy.45
Adverse drug reactions (ADRs), cost and availability:
A complete 10,786 ADRs mentioned to UMC Sweden of which, most of the people had been related to injury, gastro intestinal issues(3117),observed via standard disorders and administration site situations (1913), damage, poisoning and procedural complications (1544), investigations (1483) metabolism and nutrition disorders (1267), skin and subcutaneous tissue problems (1189), anxious machine disorders (1083).34 The value of this drug aggregate is around100-150 INR for one tablet of 400mg/200mg composition and the supply isn't always plenty.46,47
Current clinical research:
Currently, sixty four scientific trials are underway on this aggregate in conjunction with different drug interventions and most people at a preliminary stage of the development. Based at the effectiveness inside the medical conditions in preference to preclinical research and while compared to other anti-viral pills' fee and availability, this drug has the possibility of becoming a powerful remedy alternative for COVID-1948,49,50
CONCLUSION
This evaluate concludes that the drugs noted above are having special residences and act in a different way in fighting the COVID-19 viruses. No drug can be advanced or inferior, however, the usage of unmarried drug may not be powerful enough to govern this lethal virus, so use of mixture of antivirals with one of a kind mechanism of motion may be greater effective and on the same time their destructive occasions ought to not be underestimated.
REFERENCES:
- World Health Organization Coronavirus. https://www.who.int/healthtopics/ coronavirus#tab¼tab_1 Accessed 23 March 2020.
- Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269e271. https://doi.org/10.1038/s41422-020-0282-0.
- COVID-19: Prevention & Investigational Treatments. https://www.drugs.com/ condition/covid-19.html Accessed 23 March 2020.
- Peeri Noah C, Shrestha Nistha, Rahman Md Siddikur, et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Inter J of Epid. 2020:1e10. https://doi.org/10.1093/ije/dyaa033.
- Berger A, Preiser W. SARS. Encyclopedia of Environmental Health. second ed. vol. 5. 2011. https://doi.org/10.1016/B978-0-444-63951-6.00624-0.
- Six questions and answers about the coronavirus (COVID-19) (News). https:// www.wur.nl/en/Research-Results/Research-Institutes/Bioveterinary Research/show-bvr/Six-questions-and-answers-about-the-Wuhan coronavirus.htm. Accessed 30 March 2020.
- Report on the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID 19). https://www.who.int/publications-detail/report-of-the-who-china-joint mission-oncoronavirus-disease-2019-(covid-19). Accessed 30 March 2020.
- WHO Director-General's opening remarks at the media briefing on COVID-19- 11 March 2020. https://www.who.int/dg/speeches/detail/who-director general-s-openingremarks-at-the-media-briefing-on-covid-19—11-march 2020 Accessed 30 March 2020.
- Coronavirus disease (COVID-19) Situation Dashboard. https://experience. arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd Accessed 26 May 2020.
- Epidemiology in India: https://www.mohfw.gov.in Accessed 26 May 2020.
- National Institute of Health (NIH) Novel coronavirus structure reveals targets for vaccines and treatments. https://www.nih.gov/news-events/nih-research matters/novelcoronavirus-structure-reveals-targets-vaccines-treatments Accessed 30 March 2020.
- Centers for disease control and prevention. Coronavirus Disease 2019 (COVID 19) Symptoms of Coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/ symptoms testing/symptoms.html Accessed 30 March 2020.
- Interim Clinical Guidance for Management of Patients with Confirmed Coro navirus Disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/ hcp/clinical-guidance-management-patients.html Accessed 20 May 2020.
- Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020:1e7. https://doi.org/10.1111/ liv.14435.
- Hu Hongde, Ma Fenglian, XinWei, Yuan Fang. Coronavirus fulminantmyo carditis saved with glucocorticoid and human Immunoglobulin. Eur Heart J. 2020. https://doi.org/10.1093/eurheartj/ehaa190. ehaa190.
- Acute Kidney Injury in Patients Hospitalized with COVID-19 study. https:// clinicaltrials.gov/ct2/show/NCT04316299? recrs¼e&cond¼COVID19&draw¼2&rank¼2 Accessed 01 April 2020.
- WHO: Severe Acute Respiratory Infections Treatment Centre: Practical manual to set up and manage a SARI treatment centre and a SARI screening facility in health care facilities. https://apps.who.int/iris/rest/bitstreams/ 1273270/retrieve Accessed 30 March 2020.
- National Health Commission of the People’s Republic of China. Potential Treatments for COVID-19; 2020. http://en.nhc.gov.cn/2020-03/19/c_77977. html. Accessed March 18, 2020.
- ICMR Recommendation for empiric use of hydroxy-chloroquine for prophy laxis SARS-CoV-2 infection https://www.google.com/url?sa¼t&rct¼j&q¼&esrc¼s&source¼web&cd¼1&cad¼rja&uact ¼8&ved¼2ah UKEwjcvMqzpMToAhXEfH0KHS9tCJcQFjAAegQIAhAB&url¼https://icmr.nic.in/sites/default/files/upload_documents/HCQ_Recommendation_22March_final_ MM.pdf&usg¼AOvVaw2x2JJ3yMp21njj9Opfir-q; Accessed 31 March 2020.
- Government of India Ministry of Health & Family Welfare Directorate General of Health Services (EMR Division). Guidelines on Clinical Management of COVID-19 dated 17th March 2020.https://www.aiims.edu/images/pdf/notice/Guidelines Clinical Management C OVID19-23-3-20.pdf Accessed 25 March 2020.
- Spanish Society of Hospital Pharmacy (SEFH)- Hospital pharmacy procedures for the management of antiviral treatment in the new coronavirus SARS-CoV 2 disease (COVID-19). https://www.eahp.eu/sites/default/files/hospital_ pharmacy_procedures_covid-19_march19th.pdf Accessed 23 March 2020.
- EAHP: COVID-19 Resource Centre. https://www.eahp.eu/hp-practice/ hospital pharmacy/eahp-covid-19-resource-centre Accessed 23 March 2020.
- Centers for disease control and prevention: Information for Clinicians on Therapeutic Options for COVID-19 Patients. https://www.cdc.gov/coronavirus/ 2019ncov/hcp/therapeutic-options.html Accessed 30 March 2020.
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond¼covid&Search¼Apply&recrs¼e&age_ v¼&gndr¼&type¼Intr&rslt¼ Accessed 23 May 2020.
- FDA Emergency Use Authorization (EUA) information and list of all current EUAs. https://www.fda.gov/emergency-preparedness-and-response/mcm legal-regulatory-andpolicy-framework/emergency-use-authorization Accessed 23 May 2020.
- Favipiravir. https://www.drugbank.ca/drugs/DB12466 Accessed 26 March 2020.
- Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir Res. 2013;100: 446-454. https://doi.org/10.1016/j.antiviral.2013.09.015
- Madelain V, Mentre F, Baize S, et al. Modeling favipiravir antiviral efficacy against emerging viruses: from animal studies to clinical trials. CPT Pharm Syst Pharmacol. 2020;9:258e271. https://doi.org/10.1002/psp4.12510.
- Dong L, Hu Shasha, Gao Jianjun. Discovering drugs to treat coronavirus dis ease 2019 (COVID-19). Drug Dis & Ther. 2020;14:58-60.
- Delang L, Rana Abdelnabi, Neyts Johan. Favipiravir as a potential counter measure against neglected and emerging RNA viruses. Antivir Res. 2018;153: 85e94. https://doi.org/10.1016/j.antiviral.2018.03.003.
- Potential treatments for COVID-19. https://covid-19. chinadaily.com.cn/a/ 202003/19/WS5e72e741a3101282172806a4.html Accessed 26 March 2020.
- Cai Q, Yang M,Liu D, et al. Experimental treatment with favipiravir for COVID 19: an open-label control study. Engineering. 2020. https://doi.org/10.1016/ j.eng.2020.03.007.
- News: Zhejiang Hisun shows positive effect of Favipiravir on COVID-19. https://www.biospectrumasia.com/news/25/15639/zhejiang-hisun-shows positive-effectof-favipiravir-on-covid-19.html Accessed 26 March 2020.
- Vigi Access: Uppsala Monitoring Centre. http://www.vigiaccess.org/Accessed 02 April 2020.
- NIH US National Library of Medicine clinical trial database. https:// clinicaltrials.gov/ct2/results? cond¼COVID&term¼favipiravir&cntry¼&state¼&city¼&dist¼ Accessed 23 May 2020.
- News: Glenmark begins Phase-3 clinical trials on antiviral drug Favipiravir for COVID-19 patients in India. https://www.thehindu.com/news/national/ glenmark-begins-phase-3-clinical-trials-on-antiviral-drug-favipiravir-for covid-19-patients-in-india/article31563198.ece Accessed 25 May 2020.
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-Chloroquine. [Updated 2017 Feb 2]. https://www.ncbi. nlm.nih.gov/books/NBK548224/.
- Li C, Zhu X, Ji X, et al. Chloroquine, a FDA-approved drug, prevents Zika virus infection and its associated congenital microcephaly in mice. EBioMed. 2017;24:189-94. https://doi.org/10.1016/j.ebiom.2017.09.034.
- Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Inf Dis. 2006;6:67-69. https://doi.org/ 10.1016/S1473-3099(06)70361-9.
- Keyaerts E, Li S, Vijgen L, et al. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimic Agents Chemo. 2009;53: 3416e3421. https://doi.org/10.1128/AAC.01509-08.
- Kono M, Tatsumi K, Imai AM, Saito K, Kuriyama T, Shirasawa H. Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK. Antivir Res. 2008;77: 150e152. https://doi.org/10.1016/j.antiviral.2007.10.011. 42.
- Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. https://doi.org/ 10.1186/1743-422X-2-69.
- Keyaerts E, Vijgen L, Maes P, Neyts J, Ranst MV. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264e268. https://doi.org/10.1016/j.bbrc.2004.08.085.
- Wu C, Liu Y, Yang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Phar Sinica B. 2020. https://doi.org/10.1016/j.apsb.2020.02.008.
- Zhang S, Yi C, Li C, et al. Chloroquine inhibits endosomal viral RNA release and autophagy-dependent viral replication and effectively prevents maternal to fetal transmission of Zika virus. Antivir Res. 2019;169:104547. https://doi.org/ 10.1016/j.antiviral.2019.104547.
- Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydrox ychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020. https://doi.org/10.1016/j.ijantimicag.2020.105932
- Multicenter collaboration group of department of science and technology of Guangdong province and health commission of Guangdong province for chloroquine in the treatment of novel coronavirus pneumonia [Abstract only] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E019. https://doi.org/10.3760/ cma.j.issn.1001-0939.2020.0019.
- Gao J, Tian Z, Breakthrough Yang X. Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci bst.2020.01047. Trends. 2020;14(1):72-73. https://doi.org/10.5582/
- Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF, American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123: 1386-1394. https://doi.org/10.1016/j.ophtha.2016.01.058.
- Cost of Chloroquine. https://www.medindia.net/drug-price/chloroquine.htm Accessed 28 March 2


 Tushar P Patil* 1
Tushar P Patil* 1
 Mayur B Mane 2
Mayur B Mane 2
 Rishikesh S Bachhav 3
Rishikesh S Bachhav 3





 10.5281/zenodo.1131787
10.5281/zenodo.1131787