Abstract
The molecular docking and ADME screening of newer 4-[4-(dimethylamino)phenyl]-6-phenylpyrimidin-2(5H)-ones derivatives were carried out. One of the major subtypes of breast cancer has overexpression of HER2. So here, all the compounds are screened for HER2 inhibitor activity. The ADME studies are performed on the SWISSADME webserver. The docking studies are performed in Autodock Vina integrated PyRx. Results depict that all derivatives binding affinity is greater than standard trastuzumab at the active site of HER2 and have significant ADME properties.
Keywords
Pyrimidinone, Breast Cancer, Docking, ADME, HER2
Introduction
Breast cancer (BC) is a heterogenous disease and the most commonly diagnosed type of malignancy among women. It is the leading cause of global cancer incidence in 2020, with an estimated 2.3 million new cases and 6,85,000 deaths globally [1]. The global burden of breast cancer is anticipated to cross 2 million by the year 2023 [2]. Breast cancer is described based on the hormone receptor expression pattern, human epidermal growth factor receptor(HER2) and human receptors(ER/PR) [3]. Human epidermal growth factor receptor 2 (HER2) having tyrosine kinase activity is overexpressed in 15-30% of invasive breast cancer. HER2 gene amplification is associated with reduced survival and a higher recurrence rate in breast cancer [4]. The treatment of BC comprises chemotherapy, radiation therapy, and surgery. The long-term challenge of current chemotherapeutic drugs is the commencement of drug resistance [5] and cytotoxic effects at sites other than target sites. This prompts the development of novel molecules with high specificity, greater potency, minimal off-target effects, and drug resistance [6]. Pyrimidines are nitrogen-containing compounds that have anti-cancer potential due to their significant role in nucleic acid synthesis [7]. The synthesis of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) depends on several pyrimidine derivatives including adenine, guanine, cytosine, thymine, and uracil [8,9]. Here, 4-[4-(dimethylamino)phenyl]-6-phenylpyrimidin-2(5H)-ones derivatives were designed and docked for their anti-breast cancer HER2 inhibiting activities.
MATERIALS AND METHODS
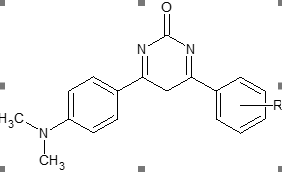
Figure 1 structure of designed compound
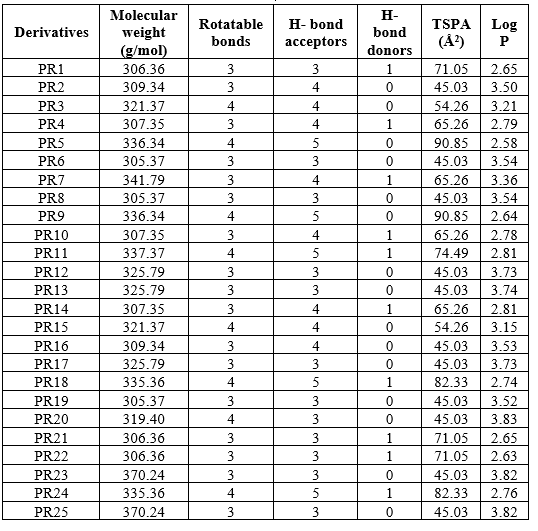
Table 1 different derivatives of designed compound

Receptor Protein preparation
The crystal structure of the kinase domain of human HER2 along with cocrystallized ligand (PDB ID: 3PP0, 2.25 Å) were downloaded from RCSB protein data bank in pdb format. It is viewed and water molecules, ligand, and Chain-B were removed using Biovia Discovery Studio 2024 software while retaining chain A for docking study [10].
Screening for compounds
SWISS-ADME web server was used for the screening of compounds. The compounds were filtered Lipinski’s rule of five (RO5). Then drug-likeliness, pharmacokinetics, medicinal chemistry and physicochemical properties were analyzed for the prediction of absorption, distribution, metabolism and excretion (ADME) of compounds [11].
Ligand preparation
The two dimensional structures of pyrimidinone derivatives were drawn with the help of ChemSketch and then, converted to 3D structure using Chem3D pro 12.0 software. The ligands molecular geometry optimization was achieved with energy minimization applying molecular mechanics (MM2) force field and saved in PDB format [12].
Molecular docking using PyRx
AutoDock Vina integrated into PyRx was used for the molecular docking. The optimization was done with Open Babel and PyRx to prepare them for docking. The grid was arranged to cover only the active site of the HER2 protein. Visualization of docking results was done using Biovia Discovery Studio 2024. Docking result analysis mostly focused on binding affinity and binding site on HER2 [13,14].
Protein validation
In the validation of docking protocols, the ligand such as positive control trastuzumab was docked against the active site of 3PP0. The amino acid residues Leu755, Arg756, Phe731, Ala763, Gly865 And Glu766 were found in common interaction for these selected control against the target [11].
RESULT AND DISCUSSION
Insilico ADME studies
The insilico ADME studies are carried out in SWISSADME web server There are five different types of rule-based filters, namely Lipinski filter, Ghose filter, Veber filter, Egan and Muegge filter. Lipinski filter includes Molecular weight 500, MLOGP (lipophilicity) 4.15, hydrogen bond acceptors 10, and hydrogen bond donors 5 [15]. Ghose's filter has a molecular weight of 480, a lipophilicity of 0.4 WLOGP (5.6), a molar refractivity of 40, and a number of atoms of 20 [16]. The number of rotatable bonds is 10 and the total polar surface area is 140 in Veber's filter [17].WLOGP (Lipophilicity) 5.88 and total polar surface area 131.6 are included in Egan's filter [18]. Muegge's filter contains the following parameters: 200 molecular weight 600, 2 XLOGP3 (lipophilicity) 5, total polar surface area 150, number of rings 7, number of carbon > 4, number of heteroatoms > 1, number of rotatable bonds 15, hydrogen bond acceptors 10, and hydrogen bond donors 5 [19]. All the compounds obey all these filter and show drug likeness. Different physicochemical properties are tabulated in table 2. These shows that all the compounds are promising drug candidates, having improved ADME properties than the standard drug.
Table 2 different physicochemical properties of drug
Molecular docking results
All compounds has greater binding affinity than the standard trastuzumab. The different binding energy of derivatives are tabulated in table 3.
Table 3 binding energy of derivatives

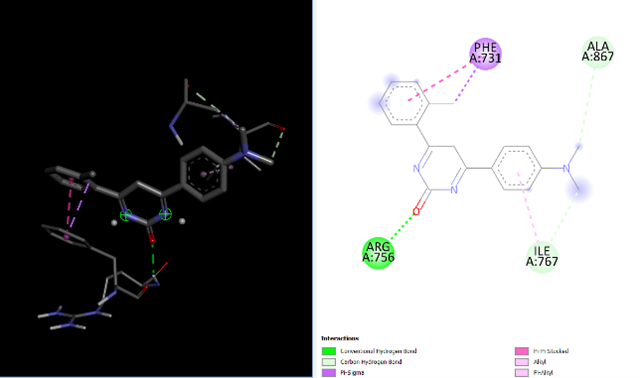
The compounds PR9 and PR19 shows maximum binding affinity than the trastuzumab. PR9 interact with Ala867, Phe731, Ile767, Leu755, Arg756, Arg868 through hydrogen and hydrophobic bonds. PR19 interact with Phe731, Ile767, Ala867, Arg756 through hydrogen and hydrophobic bonds [20]. The 2d and 3d interaction at the active site of HER2 are shown in figure 2 and 3.

CONCLUSION
Newer pyrimidionone derivatives were designed and docked. Results show that all the compounds have greater affinity than the standard compound. The compounds are screened for ADME properties. It also depicts the derivatives as promising candidates as HER2 inhibitors. Further investigation is needed for its anticancer activity. For that, the selected derivatives are synthesized using suitable methods, and their invitro anticancer activities are studied in the future.
REFERENCE
- Mehrotra R, Yadav K. Breast cancer in India: Present scenario and the challenges ahead. World Journal of Clinical Oncology. 2022 Mar 3;13(3):209.
- DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin 2011; 61: 409-418.
- 3.Ghose A, Stanway S, Sirohi B, Mutebi M, Adomah S. Advanced breast cancer care: the current situation and global disparities. InSeminars in Oncology Nursing 2024 Feb 1 (Vol. 40, No. 1, p. 151551). WB Saunders.
- Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Molecular biology international. 2014;2014(1):852748.
- Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S. Drug resistance in cancer: an overview. Cancers. 2014 Sep 5;6(3):1769-92.
- Gao F, Sun Z, Kong F, Xiao J. Artemisinin-derived hybrids and their anticancer activity. European journal of medicinal chemistry. 2020 Feb 15;188:112044.
- Sultana F, Bonam SR, Reddy VG, Nayak VL, Akunuri R, Routhu SR, Alarifi A, Halmuthur MS, Kamal A. Synthesis of benzo [d] imidazo [2, 1-b] thiazole-chalcone conjugates as microtubule targeting and apoptosis inducing agents. Bioorganic chemistry. 2018 Feb 1;76:1-2.
- Malik YA, Awad TA, Abdalla M, Yagi S, Alhazmi HA, Ahsan W, Albratty M, Najmi A, Muhammad S, Khalid A. Chalcone Scaffolds Exhibiting Acetylcholinesterase Enzyme Inhibition: Mechanistic and Computational Investigations. Molecules. 2022 May 16;27(10):3181.
- Parker WB. Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer. Chemical reviews. 2009 Jul 8;109(7):2880-93.
- Dubey K, Dubey R, Gupta R, Gupta A. In-silico Reverse Docking Studies for the identification of potential of Betanin on some enzymes involved in diabetes and its complications. Journal of Drug Delivery and Therapeutics. 2019 Apr 24;9(2-A):72-4.
- Sohrab SS, Kamal MA. Screening, docking, and molecular dynamics study of natural compounds as an anti-HER2 for the management of breast cancer. Life. 2022 Oct 28;12(11):1729.
- Katari NK, Gundla R, Reddy PK, Vanam A, Talatam A, Motohashi N, Gollapudi R. Molecular Docking Studies of Glabrene and Human Epidermal Growth Factor Receptor Kinase.
- Widyananda MH, Muhammad Ansori AN, Kharisma VD, Rizky WC, Antonius Dings TG, Rebezov M, Maksimiuk N, Denisenko A, Nugraha AP. Investigating The Potential Of Curcumin, Demethoxycurcumin And Bisdemethoxycurcumin As Wildtype And Mutant Her2 Inhibitors Against Various Cancer Types Using Bioinformatics Analysis. Biochemical & Cellular Archives. 2021 Oct 1;21(2).
- Ukonu CU, Alagamba EA. In silico Anticancer Assessment of the Bioactive Compounds of Afromomium Melengueta on HER2 Protein Associated with Breast Cancer. International Journal of Women in Technical Education and Employment. 2023;4(2):1-5.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001 March. 46 (1–3): 3–26.
- Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Comb Chem. 1999 January. 1 (1): 55–68.
- Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002 June.45 (12): 2615–23.
- Egan WJ, Merz KM Jr, Baldwin JJ. Prediction of drug absorption using multivariate statistics. J Med Chem. 2000 Oct 19;43(21):3867-77.
- Muegge I, Heald SL, Brittelli D. Simple selection criteria for drug-like chemical matter. J Med Chem. 2001 Jun 7;44(12):1841-6. [doi: 10.1021/jm015507e] PMID: 11384230
- Bandgar SA, Gaikwad DT, Shah VV, Jadhav NR. Simvastatin and Alendronate sodium repurposing for cancer as HER2, EGFR kinase and AR potential inhibitors: In silico approach. Annals of the Romanian Society for Cell Biology. 2021 Jul 1:19128-38.


 A Nazrin Fathima*
A Nazrin Fathima*
 A Sumathy
A Sumathy






 10.5281/zenodo.13895931
10.5281/zenodo.13895931