Abstract
The safety, efficacy and quality of a pharmaceutical product plays a crucial role in product development, stability study ensures the following about the product. Shelf life of a product is consider for its acceptance and approval. These stability studies are conducted by following guidelines issued by international regulatory agencies such as ICH, WHO etc. These guidelines provide a plan to conduct the stability study, various methods are involved in performing stability studies. The environment plays an important role in quality of a product, the stability studies helps to retain product specified limits throughout its period of storage and use. Which helps in determined its shelf life. In this overview the guidelines and trends of stability testing are briefly described.
Keywords
Stability, Stability studies, Shelf life, Regulatory agencies, ICH.
Introduction
Stability is an essential criterion for confirming quality and approval of the various manufactured preparations. Pharmaceutical industries depend upon the information on stability studies to assign shelf-life for the formulation manufactured and distributed for the purpose of marketing and also to make sure of the potency and safety of the drugs. Stability studies of drugs revolves around various details pertaining to the research and development process, such as preparation of formulation, performing analytical studies on it, and its quality check-and all of these have great influence on the regulatory aspects, starting from the synthesis of drug to formulation of the drug, its approval and marketing. Stability studies should be carried out on all the batches of a product and on various aspects. The data obtained should be satisfactory enough to fulfil all the parameters till the end of its shelf-life or expiry period, and thus becomes capable to be approved and registered by the regulatory bodies. In order to make certain that good products are prepared, which may be potent enough to last till their shelf life time, marketed well and reaches to the people on time, regulatory authorities in many countries have emphasize that the information regarding the potency or stability of drug or shelf-life period of the same should be made available by the manufacturers. The intention was to commission the similar testing methods by all pharmaceutical manufacturers. The guidance embody the simplest problems associated with potency of drugs/stability, the information on how to apply for manufacture of a product by providing the necessary information regarding the potency or stability or shelf-life of the product and the methods to bring them into action.
STABILITY STUDY: HISTORY IN BRIEF
FDA issued its first guidance in 1987. Food and Drug Association guidelines have stressed upon:
- Incorporating study designs on stability of drugs.
- Establishing accurate expiration date.
- The methods of storage and the care to be taken during storage of drugs.
- To submit the data on the stability study of investigational new drugs,
- Biological, new drug applications, and the biological product license application.
Along these lines, different administrative specialists of different nations adopted their own rules. These rules had different disparities and didn't adjust with one another, so a solid need was felt to fit the rules. Endeavors were made in 1990s to acquire consistency the solidness rehearses in the ICH areas (Joined together States, Europe and Japan). Sometime in the future they were made uniform inside the ICH, to advance and make enlistment of the items in better places. The Global Meeting on Harmonization, was an association where ideas were given routinely from administrative as well as assembling enterprises of ICH areas (i.e., the three nations like, Europe, Japan and US). ICH rules were additionally expanded later for veterinary items International Conference on Harmonization (ICH) was established in 1991 and different guidelines for drug substances came into existence regarding their quality, safety, efficacy and multi-disciplinary (also called as Q, S, E, M) guidelines.
Categorization of ICH guidelines
In November 2005, the Global meeting on harmonization Controlling Board of trustees distributed new codes to the ICH Direction's. The aim of designating new codes was to ensure; no disarray happens furthermore, it makes things simple for useful execution. In view of the number of times the amendments were made, codes like (R1), (R2), and (R3) were doled out. This was finished to make the ICH codification of rules more clearly to all. Numerous annexures have likewise presently been added to the fundamental direction and are named as modifications to the principal or center direction (e.g., R1) The ICH guidance is classified into four groups and codes have been allotted depending on these groups.
Q Guidelines
Table 1: Q - Guidelines & codes
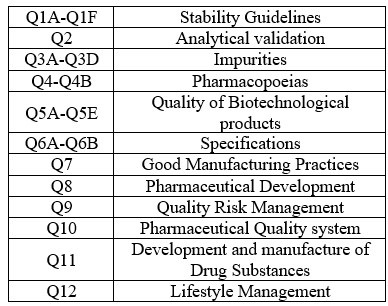
Table 2: S - Guidelines
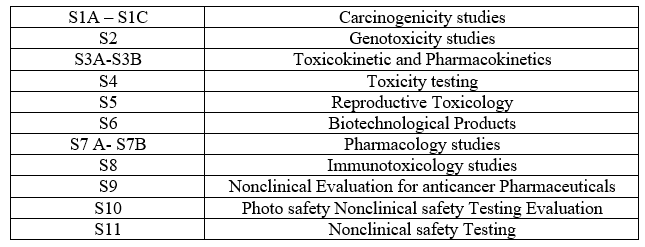
Table 3 – E Guidelines
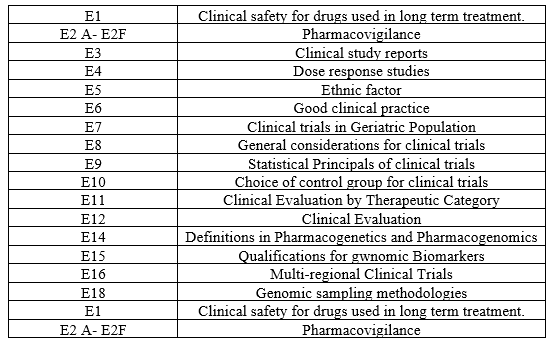
Table 4 – M Guidelines
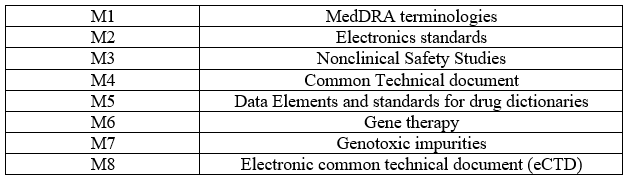
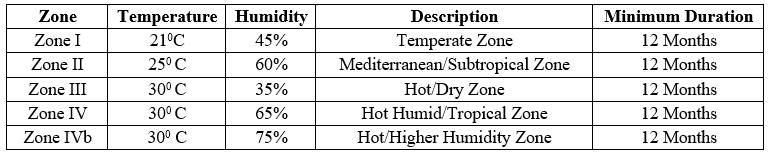
CLIMATIC ZONES
The International Council for Harmonization (ICH) has established four distinct stability zones to guide the pharmaceutical industry in the stability testing of drugs. These zones represent different climatic conditions worldwide to ensure that pharmaceutical companies test products under environmental conditions similar to where they will be stored and used. Each zone has specific temperature and humidity conditions that simulate the climatic environment of various geographic regions.
Table 5 – ICH Stability Zones & Testing conditions
The WHO Guidelines for stability
Guidance for stability studies of manufacturing products made up of drug substances in the conventional dosage forms were issued as annexure 5 to the World Health Organization Expert Committee on Specifications for Pharmaceutical Preparations Technical Export Series, No: 863, 1996 The World Health Organization brought about certain modifications in the international conference on harmonization in the year, 1996. This guidance was revised in 2003 and 2006 because of changes in the long-term storage conditions to support climate zone IV regions. Guidance on stability testing in global environment were also released by the World Health Organization in the year, 2004. The first draft of the new World Health Organization, stability guidance’s were provided for comments and suggestions in the year, April 2007. The second draft was made available in October in the year, 2007 based on the WHO eastern Mediterranean region stability guidelines.
CONCLUSION
Steadiness testing is presently the vital procedural part in the drug improvement program for another medication as well as new definition. Steadiness tests are done so that suggested capacity conditions and time span of usability can be remembered for the mark to guarantee that the medication is protected and successful all through its rack life. Over a timeframe and with expanding experience and attention, the administrative prerequisites have been made progressively severe to accomplish the above objective in all potential circumstances to which the item may be oppressed during its rack life. Therefore, the dependability tests ought to be completed following legitimate logical standards and after understandings of the ongoing administrative necessities and according to the climatic zones. Present review summarizes the significant land marks in the improvement of the rules for steadiness studies. It is trusted that a prepared to begin reference is produced by the review. FDA, ICH, CPMP, and WHO rules of explicit circumstances for dependability studies and explicitly, ICH Q1A (R2) are required to have been considered for solidness study.
REFERENCES
- Pharmaceutical Stability Testing to Support Global Market: Pharm asp, Edited by: Huynh Ba K, (2010) Published by: Springer Science+ Business Media, LLC, 2333 Spring Street, NY 10013, USA
- www.ICH.org
- Kaur M, Kaur H, Overview on Stability Studies. International Journal Pharmaceutical, Chemical and Biological Sciences. 2013; 3(4):1231-1241.
- Somvanshi Y and Satbhai P. ICH Guidelines and Main Focus on Stability Guidelines for New Formulation and Dosage Forms. World Journal of Pharmaceutical Research. 2015; Vol 4, Issue 10: 561-578.
- Chinchole AS, Paul BN, Panchal CV, and Chavan DV. A Review on Stability Guidelines by ICH and USFDA Guidelines for New Formulation and Dosage form. Pharma Tutor. 2014; 2(8):32-53.
- Eggert J & Flower C. Conducting Stability Studies during Development to Ensure Successful Regulatory Approval. In: Pharmaceutical Pre-Approval Inspections, a Guide to Regulatory Success. (2008) 2nd Edition. Edited by: Hynes III M D. Eli Lilly and Company, Indianapolis, Indiana, USA. Published by: Informa Healthcare USA, Inc., 52 Vanderbilt Avenue, New York, NY 10017.
- ICH. Harmonized tripartite guideline. Stability testing of new drug substances and Products Q1A (R2). February 2003.
- ICH. Harmonized Guideline: Stability Testing: Photo Stability Testing of New Drug Substances and Products Q1B, November 1996.
- ICH. Harmonized Guideline: Stability Testing: Requirements for New Dosage Forms QIC, November 1996.
- ICH. Harmonized Guideline: Bracketing and Matrixing Designs for Stability Testing of New Drug Substances and Products Q1D. February 2002.
- ICH. Harmonized Guideline: Evaluation of Stability Data Q1E, February 2003.
- ICH. Harmonized Guideline: Quality of Biotechnological Products: Stability Testing of Biotechnological/Biological Products Q5C, November 1995.
- FDA. Guidance for Industry: Guideline for Submitting Documentation for the Stability of Human Drugs and Biologics, 1987.
- FDA. Draft Guidance for Industry: stability testing of drug substances and drug Products, 1998 (draft).
- FDA. Pharmaceutical current good manufacturing practices (cGMP) for the 21st Century: a risk-based approach. Available at: www.fda.gov/cder/gmp.
- FDA. Guidance for industry: quality systems approach to pharmaceutical CGMP Regulations, September 2006.
- EMEA Committee for Proprietary Medicinal Products (CPMP), Guideline on Stability Testing: Stability Testing of Existing Active Substances and Related Finished Products CPMP/QWP/122/02, March 2004.
- EMEA Committee for Medicinal Products for human Use (CHMP). Guideline on Stability Testing for Applications for Variations to a Marketing Authorization CPMP/QWP/576/96, December 2005.
- EMEA CPMP. Note for Guidance on In-Use Stability Testing of Human Medicinal Products CPMP/QWP/2934/99, September2001.
- EMEA CPMP. Note for Guidance on Maximum shelf-life for sterile products for human use after first opening or following reconstitution. CPMP/QWP/159/96, July 1998.
- EMEA CPMP. Note for Guidance on declaration of storage conditions: A: In the Product Information of Medicinal Products, B: For Active Substances. CPMP/QWP/609/96, October 2003.
- EMEA CPMP. Note for Guidance on start of shelf-life of the finished dosage form CPMP/QWP/072/96, December 2001.
- WHO. WHO Technical Report Series, No, 863, Annex 5 Guidelines for stability testing of pharmaceutical products containing well established drug substances in conventional dosage forms, 1996. Available at: www.who.int/medicines/areas/quality_safety/quality_assurance/regulator y_standards/en.
- WHO. WHO Technical Report Series, No. 908. Item 11.1 WHO Guidelines for stability testing of pharmaceutical products containing well established drug substances in conventional dosage forms, 2003.
- WHO. WHO Technical Report Series, No.937. Item 10.1 Stability Testing Conditions, 2006.
- Yasmeen A, Sofi G. A Review of Regulatory Guidelines on Stability Studies. J Phytopharmacol 2019; 8(3):147-151


 Parag Mangilal Lalwani *
Parag Mangilal Lalwani *
 R. B. Darade
R. B. Darade





 10.5281/zenodo.11109892
10.5281/zenodo.11109892