Coccinia grandis (Linn) Voigt belonging to family Cucurbitaceae, commonly known as “Ivy gourd, and synonym Coccinia grandis var. wightiana M poem Greb scarlet gourd” (English), “Kanduri, kundru (Hindi) and “Bimbi, tundika” (Sanskrit) is distributed throughout the hotter parts of India. In the literature, Coccinia grandis is described to have wide range of medicinal applications. Cucurbitacin’s comprise of a group of tetracyclic triterpenoids found in the Cucurbitaceae family. Plants containing cucurbitacin’s are found to possess anti- inflammatory, antipyretic, analgesic, anti-tumor and anti-microbial, antiulcer, wound healing), antifungal, antidiabetic activities. Indian system of traditional knowledge i.e. Ayurveda is well-known for its effective herbal treatments. The main objective of present investigation to formulate and evaluate mucoadhesive buccal patches of Coccinia grandis using solvent casting method. HPMC K100 M were used as a mucoadhesive polymer and PEG 400 used as a plasticizer as well as penetration enhancers. The formulated patches of Coccinia grandis were evaluated for their appearance, weight variation, thickness, folding endurance, surface pH, swelling index, drug content, % elongation, mucoadhesive strength, in vitro drug release, kinetic release study and stability study. The Buccal patches are drug delivery systems that adhere to the mucosal lining of the buccal cavity and release the active ingredients gradually. These buccal patches are designed to provide controlled and sustained release of bioactive compounds from the plant extract, which may have various health benefits, such as anti-diabetic, anti-inflammatory, and antioxidant properties. The mucoadhesive property of these patches ensures prolonged contact with the buccal mucosa, enhancing drug absorption and bioavailability.
Herbal gel, antimicrobials, antibacterial activity, polyherbal formulations
Mouth ulcer is one of the common disorder caused due to variety of etiologycal factors. Two most common causes of oral ulceration are local trauma (e.g. rubbing from a sharp edge on a filling) and aphthous stomatitis ("canker sores"), a condition characterized by recurrent formation of oral ulcers for largely unknown reasons. Mouth ulcers often cause pain and discomfort, and may alter the person's choice of food while healing occurs. This review focusses on antiulcerogenic property of Coccinia grandis for the treatment of mouth ulcer.

Mucoadhesive Buccal Patches
Transdermal route is unsuitable for maintaining drug plasma level in systemic circulation because of skin the main barrier. In the parenteral administration drug directly enter into the systemic circulation and efficiently maintain plasma level of drug. However, parenteral route is not preferred because of the pain during the parenteral administration, can’t reverse a toxic dose, may be expensive and specialized trained person is required for administration. Therefore, the Oral mucosal drug delivery system is widely applicable as novel site for administration of drug for immediate and controlled release action in various body cavities, like the nasal, buccal, ocular, rectal and vaginal mucosae have the benefit of bypassing the hepatic first-pass elimination associated with oral administration. Because of the dual biophysical and biochemical nature of these mucosal membrane’s drugs with hydrophilic and lipophilic nature can be rapidly absorbed. The motivation behind this research lies in the pursuit of safer and more accessible solution to mouth ulcer common anti-inflammatory drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids, are associated with adverse effects that can limit their long-term use. Herbal remedies often derived from plants with a history of medicinal use. This project will follow a systematic approach in formulating mucoadhesive buccal patches, considering factors like the choice of polymers, plasticizers, and adhesives. These elements play a crucial role in achieving the desired properties of the patches, including sustained drug release and stability. Through this research, we aim to contribute to the development of a novel and effective alternative for anti- ulcer therapy, blending traditional herbal knowledge with contemporary pharmaceutical techniques. The findings of this study may not only enhance our understanding of herbal formulations but also provide practical solutions for individuals grappling with mouth ulcer.
Mouth Ulcer:
A mouth ulcer, also known as a canker sore, is a small, painful lesion that develops on the soft tissues inside the mouth, such as the inner cheeks, gums, tongue, or lips. They can be white, yellow, or gray in color, with a red border. Mouth ulcers can be caused by various factors such as injury, stress, hormonal changes, or certain medical conditions. They usually heal on their own within 1-2 weeks but can cause discomfort during that time.
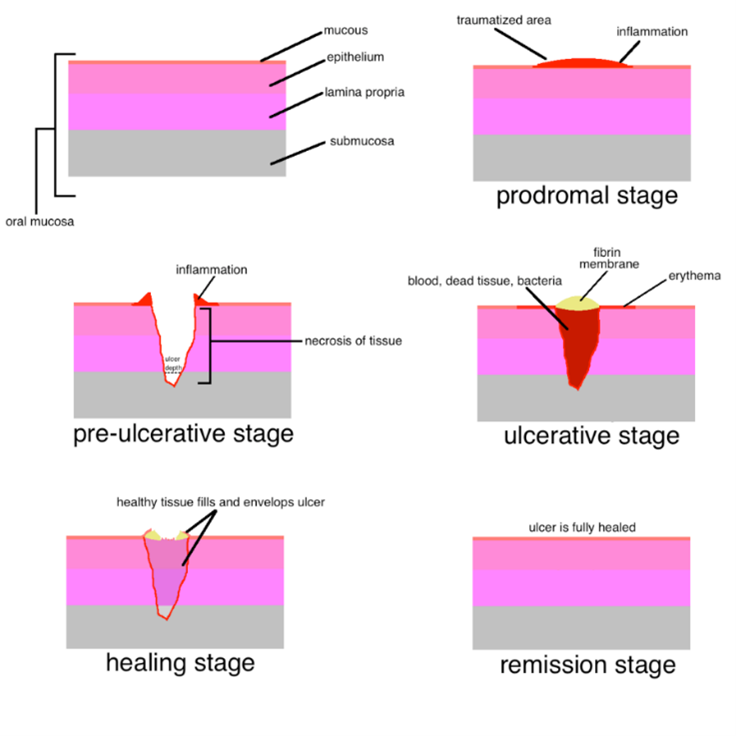
Types of mouth ulcer:
- Minor ulcers:
These are small, round or oval ulcers with a white or yellow center and a red border. They are the most common type and usually heal within one to two weeks without scarring.
- Major ulcers:
Also known as Sutton's disease or periadenitis mucosa necrotica recurrens, these ulcers are larger and deeper than minor ulcers. They often have irregular borders and can take several weeks to heal. Scarring may occur.
- Herpetiform ulcers:
These ulcers consist of clusters of multiple small sores that merge together to form a larger ulcer. Despite the name, they are not caused by the herpes virus.
- Traumatic ulcers:
These ulcers are caused by physical trauma, such as accidentally biting the inside of the cheek or lip, or irritation from dental appliances.
- Aphthous stomatitis:
Also known as canker sores, these ulcers are recurring and can be triggered by factors like stress, hormonal changes, or certain foods.
- Drug-induced ulcers:
Some medications, particularly those that affect the immune system, can cause mouth ulcers as a side effect.
- Mechanism of action of mouth ulcers:
The mechanism of action of mouth ulcer involves a complex interplay of factor including inflammation, tissue damage and impaired healing.
- Initiating Factors:
Mouth ulcers can be triggered by multiple factors, including mechanical trauma (such as accidental biting), chemical irritants (like certain foods or dental products), hormonal changes, stress, and microbial infections.
- Immune Response:
In susceptible individuals, these triggering factors can lead to an abnormal immune response in the oral mucosa. Immune cells, such as T lymphocytes, become activated and initiate an inflammatory cascade.
- Inflammation:
The activation of immune cells and the release of inflammatory mediators, such as cytokines and chemokines, cause localized inflammation in the mucosal tissue. This inflammation contributes to pain, swelling, and redness at the site of the ulcer.
- Tissue Damage
The inflammatory process can also lead to damage of the epithelial cells lining the oral mucosa. This damage may result in the formation of a shallow crater-like ulcer, exposing underlying nerve endings and causing pain.
- Healing Process:
Once the triggering factor is removed or resolved, the body initiates the healing process. This involves the recruitment of various cells, such as fibroblasts and epithelial cells, to repair the damaged tissue.
Buccal drug delivery system:
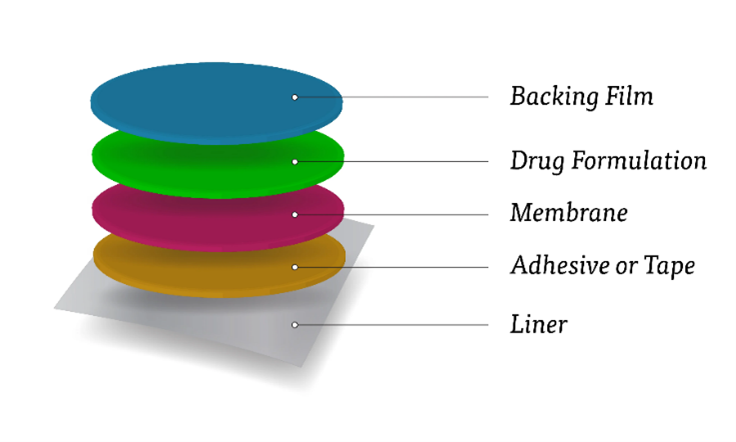
Buccal drug delivery is a method of administering drugs through the mucous membrane lining the cheek. It offers several advantages over traditional oral administration, such as by passing the digestive system and avoiding first-pass metabolism in the liver, resulting in improved bioavailability and faster onset of action for certain drugs. This route of administration is particularly useful for drugs that are poorly absorbed in the gastrointestinal tract, drugs that are unstable in the acidic environment of the stomach, or drugs that undergo extensive metabolism in the liver.
Basic component of buccal drug delivery.
- Drug substance:
Before formulating buccal drug delivery system, one has to decide whether the intend action is for rapid release/prolonged release and for local systemic effect. The selection of suitable drug for the design of Bucco adhesive drug delivery systems should be based on pharmacokinetic properties.
- Bioadhesive polymers:
Bioadhesive polymer play a major role in bucoadhesive drug delivery systems of drugs. A Bioadhesive polymer which adhere to the mucin /epithelial surface is effective and lead to significant improvement in the oral drug delivery.
- Backing membrane:
Backing membrane plays a major role in the attachment of Bioadhesive devices to the mucus membrane. the material used as backing membrane should be inert and impermeable to the drug and penetration enhancer.
- Penetration Enhancer:
Penetration enhancers are used in buccal formulations to improve the release of the drug. They aid in the systemic delivery of the drug by allowing the drug to penetrate more readily into viable tissues.
Applications of buccal drug delivery system:
- Pain Management:
Pain management: Buccal tablets or films can deliver pain-relieving medications, such as fentanyl or buprenorphine, directly into the bloodstream through the cheek mucosa
- Allergic reactions:
Buccal epinephrine tablets can treat severe allergic reactions.
- Insulin delivery:
Researchers are exploring buccal insulin delivery systems for diabetes management.
- cardiovascular diseases:
Buccal administration of nitroglycerin can quickly relieve angina pectoris symptoms.
- Smoking cessation:
Buccal nicotine replacement therapy helps manage nicotine cravings.
Advantages of buccal drug delivery system:
- Rapid absorption:
Drug are absorbed quickly through the buccal mucosa. Resulting in fast onset of action
- Easy administration:
Buccal tablets or film are simple to use, especially for patient with swallowing difficulties.
- High bioavailability:
Buccal delivery can achieve higher bioavailability compared to oral administration.
- By passing first pass metabolism:
Drugs avoid liver metabolism, reducing degradation and increasing bioavailability.
- Potential for local drug delivery:
Buccal delivery can local areas, reducing systemic exposure side effects.
- Improved patient compliance:
Easy and painless administration enhance patient adherence to treatment.
AIM AND OBJECTIVES
AIM-:
To develop mucoadhesive buccal patches utilizing Coccinia grandis leaves extract as a Natural bioactive ingredient for controlled and targeted drug delivery.
OBJECTIVES:
- Extraction optimization.
- Formulation development.
- Characterization.
- In vitro drug release.
- Mucoadhesive properties.
- Biocompatibility.
- In vivo studies.
- Stability assessment.
- Comparative analysis.
- Optimization and scale up.
PLANT PROFILE :
- Kingdom: Plantae
- Order: Cucurbitales
- Family: Cucurbitaceae
- Sub family: Cucurbitoideae
- Tribe: Benincaseae
- Sub-tribe: Benincasinae
- Genus: Coccinia Wight & Arn.
- Species: Coccinia indica

Botanical Classification of Coccinia grandis
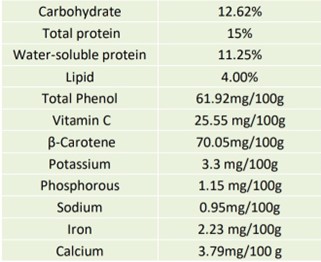
Nutrient composition of Coccinia grandis
Chemical constituents:
The plant contains resins, alkaloids, fatty acids, flavonoids and proteins as chief chemical constituents. Aspartic acid, Glutamic Acid, Asparagine, Tyrosine, Histidine, Phenylalanine, Threonine, Valine, and Arginine are also found. The methanolic extract of fruit contains alkaloids, steroids, tannins, saponins, ellagic acid, phenols, glycosides, lignans, and triterpenoids. Roots contain Triterpenoid, saponin coccinioside, Flavonoid glycoside ombuin 3-o- arabino furanoside, Lupeol, ?-amyrin, and ?- sitosterol and Stigmast -7- en-3-one.
Drug Profile: -
- Chemical Structure: -
Lupeol, a triterpene
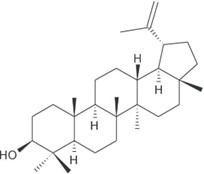
Lupeol
- Chemical formula:
C30H50O and a pentacyclic structure.
3. Natural Source: -
Lupeol is found in various plants, including fruits, vegetables, and medicinal herbs. Common sources include mangoes, olives, white cabbage, and various species of the Diospyros and Crataegus genera.
4.Biological Activity: -
Lupeol exhibits various biological activities, such as:
Lupeol has anti-inflammatory properties, which can help in reducing inflammation. Antioxidant: It acts as an antioxidant, protecting cells from oxidative stress.
Some studies suggest that lupeol may have anti-cancer properties by inhibiting the growth of cancer cells.
Lupeol has shown activity against certain bacteria and fungi, indicating potential antimicrobial properties.
There is research suggesting lupeol's role in improving insulin sensitivity and potentially aiding in diabetes management. It's important to note that while lupeol shows promise in these areas, further research is ongoing to fully understand its mechanisms and potential therapeutic applications. Always consult with a healthcare professional before considering any use for medicinal purposes.
MATERIAL AND METHOD:
- Drying:
Lay the leaves on newspaper and let them dry. The leaves are Dry when they have a crinkly Texture.
2. Grinding:
Start by grinding the dried Coccinia grandis into fine powder.
Preparation of Plant Extract: (Soxhlet extraction method)
-
-
- A portion of dried leaves (100 g) of Coccinia grandis procumbens was placed in a Soxhlet apparatus. Extraction was performed with 250 ml of methanol for 36 h at 64 °C.
- The extract was filtered through a Whatmann filter paper.
- The resulting solution was concentrated in vacuum to give dryness to the methanol extract.
- The extract was stored in a refrigerator at 4 °C for further use.


Identification and Screening phytochemical constituents
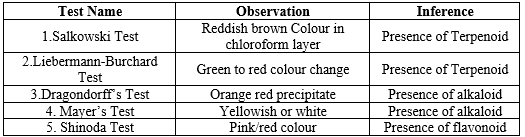
I. Test of terpenoids
1. Salkowski Test:
Procedure:
Dissolve the sample in chloroform and add concentrated sulfuric acid carefully along the sides of the test tube fest
Observation:
A reddish-brown color in the lower chloroform layer indicates the presence of terpenoids
2. Liebermann-Burchard Test:
Procedure:
Dissolve the sample in chloroform, add acetic anhydride followed by
concentrated sulfuric acid
Observation:
A green color initially, which changes to blue, violet, and finally red, indicates the presence of terpenoids.
II. Test of Alkaloids
1. Dragendorff"s Reagent
Procedure:
By adding 1 mL of Dragendorff"s reagent to 2 mL of extract, an orange red precipitate was formed, indicating the presence of alkaloids
Observation:
As orange red Precipitate was formed.
2. Mayer's Test:
Few drops of Mayer's reagent were added to 1 mL of extract.
Observation:
A yellowish or white precipitate was formed, indicating the presence of alkaloids.
III. Test Flavonoids
1. Shinoda Test
Prepare a solution of the plant extract in methanol. Add a few drops of concentrated hydrochloric acid (HCl) to the solution.
Observation: Observe the appearance of a red color, indicating the presence of flavonoids.

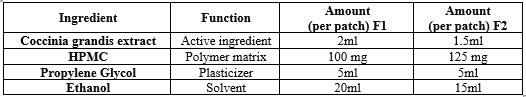
- FORMULATION OF BUCCAL PATCHES
Material:
- Coccinia grandis extract (ethanolic or other suitable solvent extract)
- Polymer(s) for patch matrix (e.g., polyvinyl alcohol, ethyl cellulose)
- Plasticizer (e.g., glycerin, propylene glycol)
- Backing membrane (e.g., polyethylene or polypropylene film)
- Solvent (e.g., ethanol, methanol)
- Glassware (beakers, stirring rod)
- Weighing balance
- Petri dish or mold
Instruments:
Weighing balance, Heating mantle, Beaker, Measuring cylinder, RBF, Stirrer.


 Kirti Nandkishor Raut *
Kirti Nandkishor Raut *
 Pankaj vishnu Vyawhare
Pankaj vishnu Vyawhare












 10.5281/zenodo.11623311
10.5281/zenodo.11623311