Abstract
Nanosponges are nanosized drug carriers having three dimensional structures that can entrap both hydrophilic and hydrophobic drugs. Lovastatin, a hypolipidemic drug encounters the major problem of poor oral bioavailability. Hence, the main aim of the study was to formulate and evaluate them by Emulsion solvent evaporation method using ethyl cellulose, PVA and ?-Cyclodextrin and dichloromethane as a solvent. The FTIR test is conducted and it was concluded that the absence of drug polymer interactions. The nanosponges are evaluated for drug entrapment efficiency, particle size, surface morphology, Zeta potential. Average particle size of Lovastatin nanoparticles was found to be 84.29 to 191.58nm. The Nanosponge loaded capsules are tested for weight variation test, in vitro disintegration test, drug content, in vitro dissolution test and stability studies. In-vitro release of drug from capsule follows zero order and showed controlled release behaviour for a period of 24 h. The study indicates that nanosponges of Lovastatin are promising for controlled drug delivery.
Keywords
Lovastatin, Nanosponges, Dissolution, Controlled drug release.
Introduction
Nanosponges are made of microscopic particles with few nanometers in which a large variety of substances can be encapsulated. These particles possess the ability to carry both lipophilic and hydrophilic substances and thereby improving the solubility. Drug release occurs by diffusion, degradation, Swelling and affinity-based mechanisms. Newer development of pharmaceutical compounds in the form of targeted drug delivery system, in which the drug is only active in the target area of the body (for example, in cancerous tissues, brain, colon), sustained release formulations in which the drug is released over a period of time in a controlled manner from a formulation.1,2 Nanosponges have many applications in the field of drug delivery which includes the Solubility Enhancement because presence of cross-linking agent and cavities in the nanosponge structure helps interaction with active molecules. The hydrophilic hydroxyl groups on the external surface remain exposed to the environment, while the hydrophobic functionality of the complex hides in the interior cavity of the cyclodextrin the net effect is that a water-soluble complex is formed.3,4 Lovastatin is a lipid-lowering medication part of the statin drug class used to treat and prevent coronary heart disease, hypercholesterolemia, and adolescent patients with heterozygous familial hypercholesterolemia. This activity will review the mechanism of action, adverse effects, and other key factors (e.g., off-label uses, dosing, pharmacodynamics, pharmacokinetics, monitoring, relevant interactions) pertinent to the interprofessional team members in the treatment management of patients with hypercholesterolemia.5
MATERIALS AND METHODS
Lovastatin, Polyvinyl alcohol, Ethyl cellulose, ?-Cyclodextrin, Dichloromethane was received from Yarrow Chem Products, Mumbai.
Compatibility Studies using FTIR Spectroscopy:
FTIR spectral analysis of pure drug was carried out by using FTIR spectrometer, Jasco-FTIR- 4100 using the KBr disk method. The peak obtained in the spectrum was compared with the standard spectrum of Lovastatin.
METHOD OF PREPARATION OF LOVASTATIN LOADED NANOSPPONGES:6,7,8
Preparation of Nanosponges by Emulsion solvent diffusion method. The disperse phase consist of drug (lovastatin) and specified quantity of ethyl cellulose dissolved in required quantity of dichloromethane was slowly added to a definite amount of PVA in 100 ml of aqueous continuous phase. The mixture was stirred at 1000 rpm on a magnetic stirrer for 2 hrs. The formed lovastatin nanosponges were collected by vacuum filtration and dried in an oven at 40°C for 24 hrs.
Preparation lovastatin loaded Nanosponge capsule (Punch method or manual filling): Powder is placed on a sheet of clean paper or porcelain plate using spatula which is formed into a cake having depth approximately one-fourth to one-third the length of capsule body. the base of the capsule is held vertically and the open end is repeatedly pushed into the powder until the capsule is filled; the cap is then replaced to close the capsule.
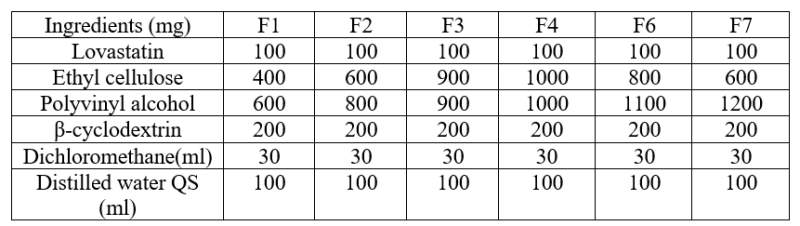
Table No. 1: Formulation of lovastatin loaded nanosponge.
Characterization of Nanosponge:9,10.
- Particle size and Malvern zeta sizer:
When, n = total no. of particles in that size range.
D = diameter of the particle of that size range. N = total no. of particle.
- Zeta potential:
Zeta sizer can be used to measure zeta potential, which is the measure of surface charge of nanosponges. In the present work, the nanosponges was diluted to 10 times with distilled water and analysed by zetasizer using laser Doppler micro electrophoresis. More than 30mv zeta potential value in water indicates good stability of nanosponges.
3. Percentage yield:
The prepared and dried nanosponges of all batches were accurately weighed. The measured weight of prepared nanosponges to the total amount of all the excipients and drug used in the preparation of nanosponges, gives the total percentage yield of nanosponges. It was calculated using the following equation.
4. Drug entrapment efficiency:
100 mg of omeprazole nanosponges of each batch were selected, powder in a mortar and placed in 100 ml of methanolic HCl. Omeprazole was extracted by centrifuging at 1000 rpm for 30 min, filtered and analysed by UV spectroscopy. Percentage entrapment was calculated as follows,
5. Shape and surface morphology:
Scanning electron microscopy (SEM) is used to determine the shape and morphology of nanosponges. Sample was deposited on a glass slide, and was kept under vacuum. Then nanosponges were coated with a thin gold/palladium layer using a spulter coater unit. Finally, the morphology and size of the nanosponges observed under the scanning electron microscopy at an accelerated voltage of 15Kv.
RESULT AND DISCUSSION
Drug-Excipients compatibility studies
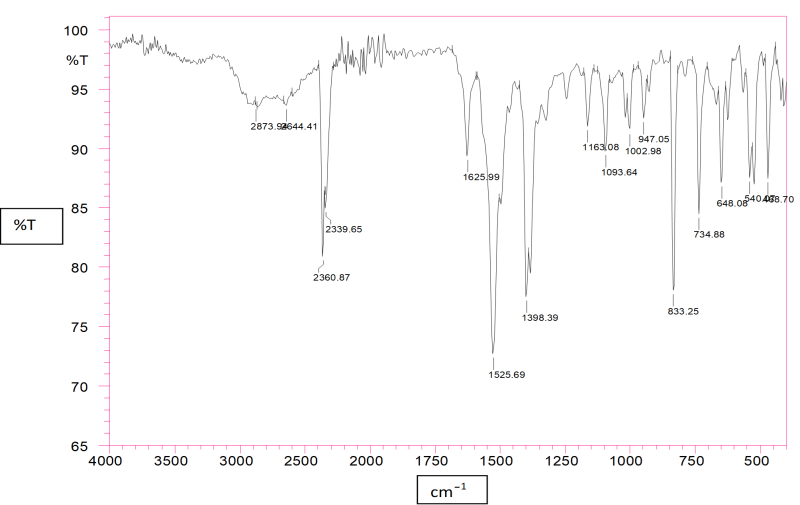
Figure No.1: FTIR Spectra of Lovastatin
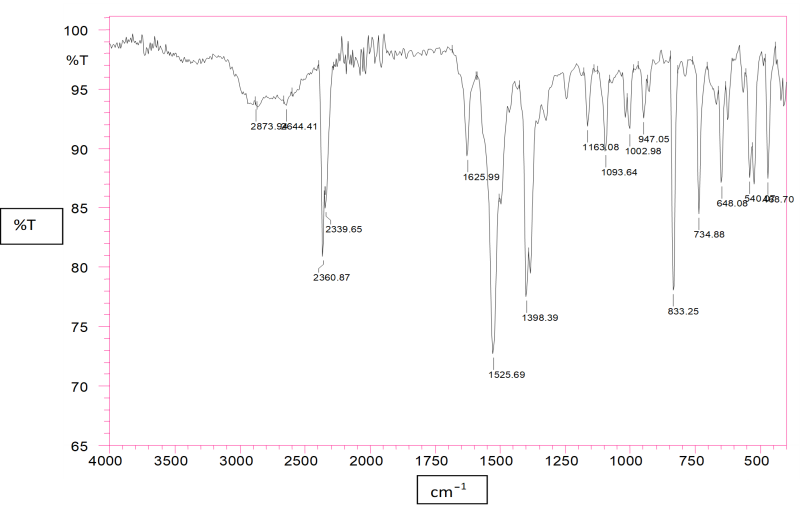
Figure No.2: FTIR Spectra of Lovastatin loaded Nanosponges
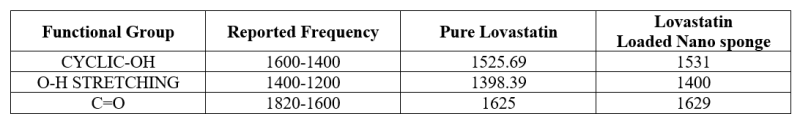
Table No. 2: Interpretation of IR Spectra
The characteristic absorption peaks of Pure Lovastatin and Lovastatin loaded nanosponge were obtained at 1525.69cm?1, 1398.39 cm?1, and 1625 cm?1 . Compatibility studies were conducted to check possible interactions between drug and polymers and drug and all excipients. Compatibility study for drug –polymer and drug excipients compatibility by FTIR gave confirmation about the purity of the drug and showed no interaction between drug – polymers and drug-excipients.
EVALUATION PARAMETER
Evaluation of lovastatin nanosponges:
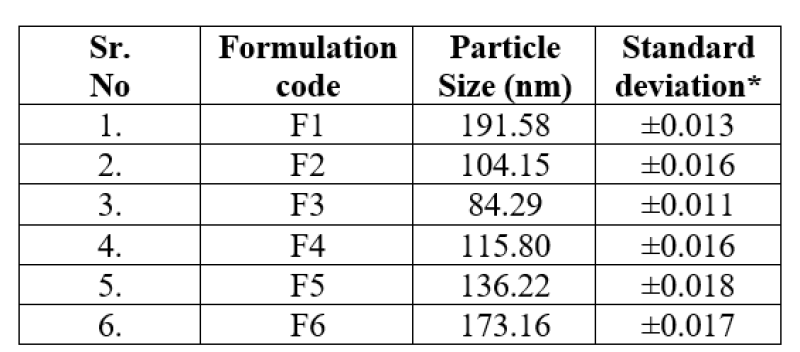
Table No. 3: Average particle size of the lovastatin nanosponges.
*Each value is an average of three determinations.
Particle size of the lovastatin nanosponges ranges from 84.29nm to 191.58nm.
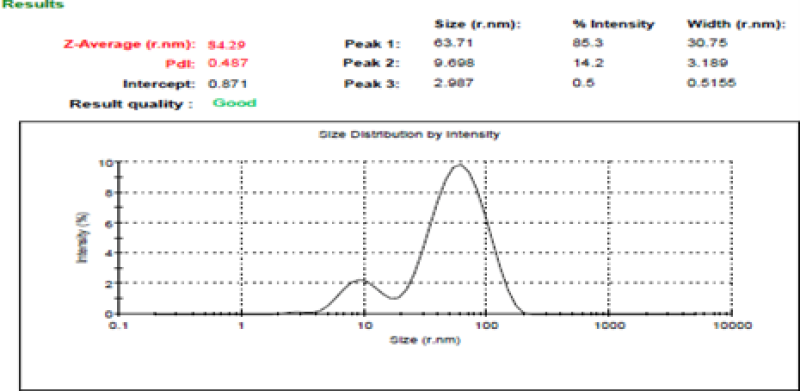
Figure No.3: Particle size of lovastatin loaded nanosponge
Percentage yield
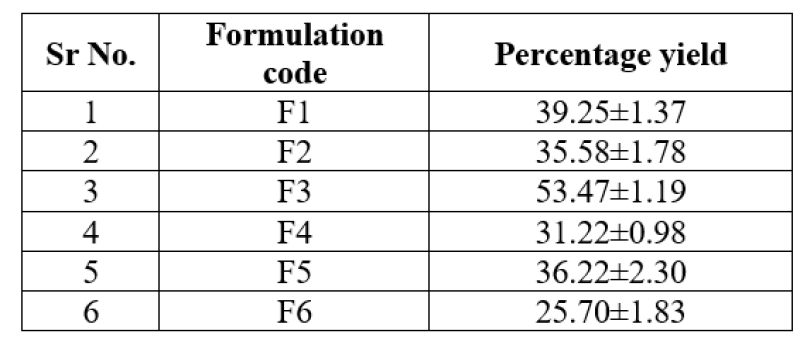
Table No. 4: Percentage yield of lovastatin nanosponges
The result of percentage yield is shown in Table No: 12 The percentage yield value of nanosponges was found to be best for F3. Further increasing the concentration of polymer the % yield was found to be decreased due to the sticky nature of the product. This cannot be filtered

Figure No.4: Photograph of the prepared lovastatin nanosponges.
Drug Entrapment Efficiency:
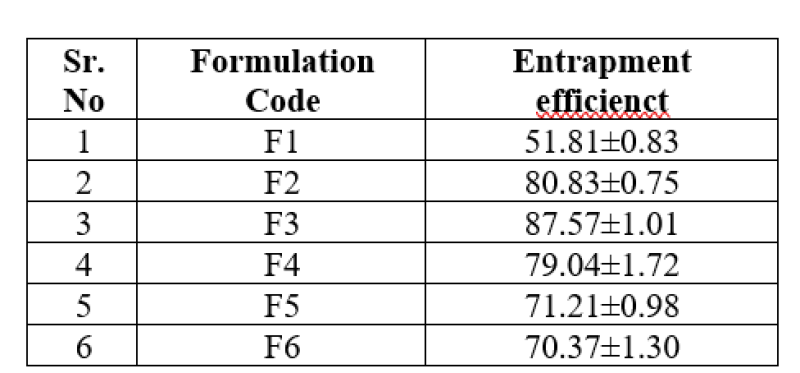
Table No. 5: Drug Entrapment Efficiency
The drug entrapment efficiency was found to be in the range of 51.81±0.83 to 87.83±0.75.
Shape and Surface morphology:
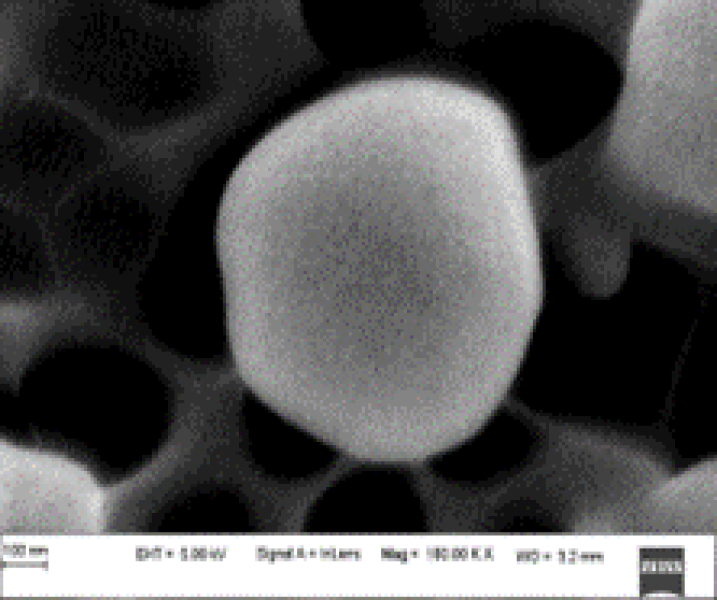

Figure No.5: SEM images of lovastatin nanosponge F3 formulation
The shape and surface morphology of the prepared nanosponges were observed by scanning electron microscopy. SEM photograph of the formulations F3 revealed that nanosponges were spherical, discrete with smooth surface.
Zeta Potential:
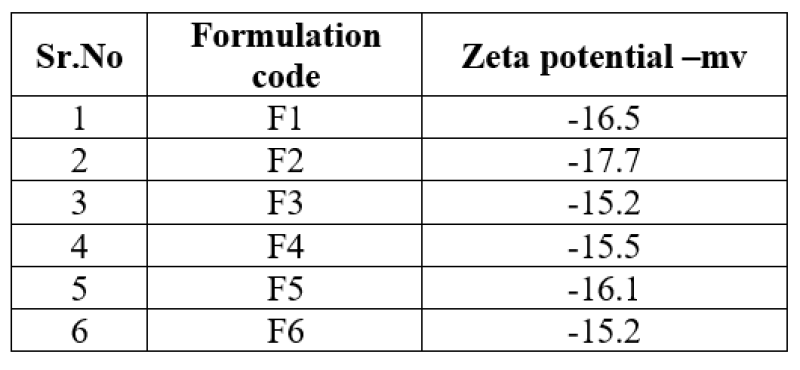
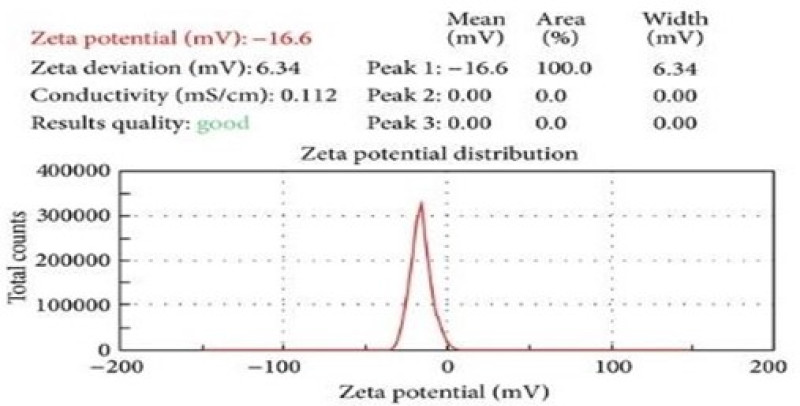
Figure No.6: Zeta potential of lovastatin nanosponges F3.
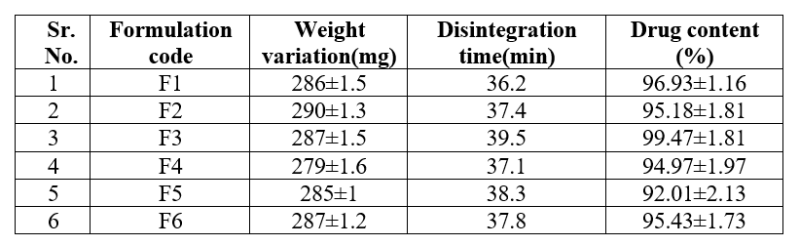
Table No. 7: weight variation test, In vitro disintegration test, drug content of Lovastatin loaded nanosponge capsule
Table no.7 showed the weight variation, disintegration time and percentage drug content, in each formulation of Lovastatin loaded capsules.
- Weight variation test
The formulation F4 showed lowest weight variation value 279±1.6mg,formulation F2 showed highest weight variation value 290± 1.3mg.The weight variation test of Lovastatin loaded capsule were found within the limits.
- Disintegration test
The formulation F1 showed lowest disintegration time 36.2 mins and the formulation F3 showed highest disintegration time 39.5 mins. The disintegration time of Lovastatin loaded capsule were found within the limits.
- Drug content test
Formulation F5 showed lowest drug content value 92.01±2.13%, F3 showed highest drug content 99.47±1.81%. Percentage drug content of lovastatin in all the formulated capsules were found within the limits. The results within the range 99.47±1.81% indicate uniform of mixing.
- In-vitro dissolution study
In vitro drug release study was performed using USP dissolution- ? test apparatus at 100 rpm using 900 ml of 6.8 pH phosphate buffer maintained at 37±0.5 ºC as the dissolution medium.
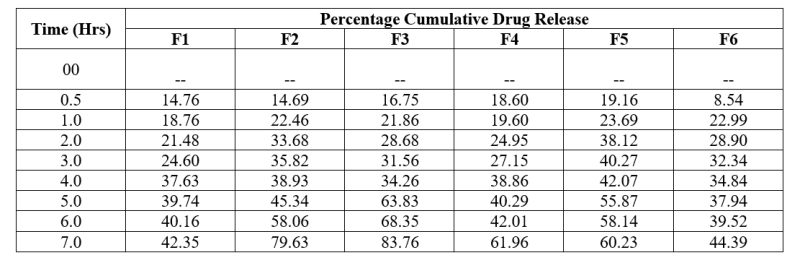
Table No. 8: In-vitro drug release study of preliminary formulations of Lovastatin
Nanosponge
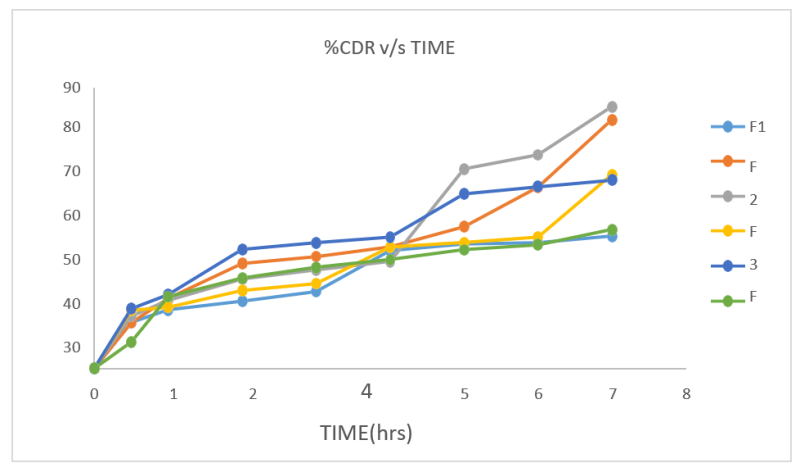
Figure No. 7: In-vitro dissolution study of preliminary formulations
Table no. 17: Dissolution studies for preliminary formulations were done keeping 7 hrs limits. Formulation F1 showed least percentage cumulative drug release value 42.35% at 7 hrs. and
formulation F3 showed highest percentage of drug release value 83.76% in 7 hrs. Percentage cumulative drug release value of all formulations ranges from 42.35% to 83.76% at 7h.
In-Vitro Dissolution Study

Table No. 9: in vitro drug release profile of lovastatin loaded nanosponge capsules
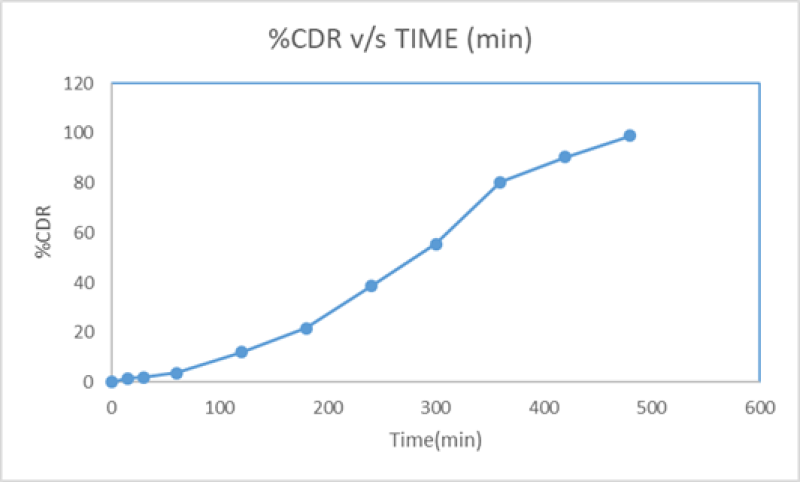
Figure No. 8: In-vitro dissolution study of lovastatin loaded nanosponge capsule
In vitro drug release study was performed using USP Type ? dissolution test apparatus at 100rpm using 900 ml of 6.8 pH phosphate buffer maintained at 37±0.5 ºC as the dissolution medium.
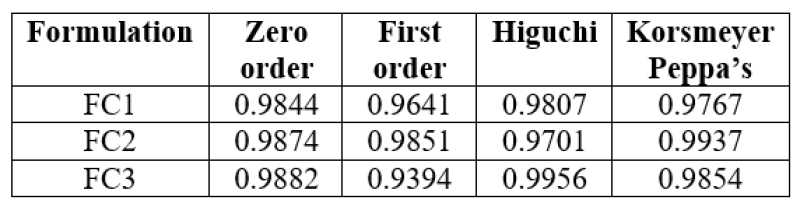
Table No. 10: Release kinetics of optimized formulation.
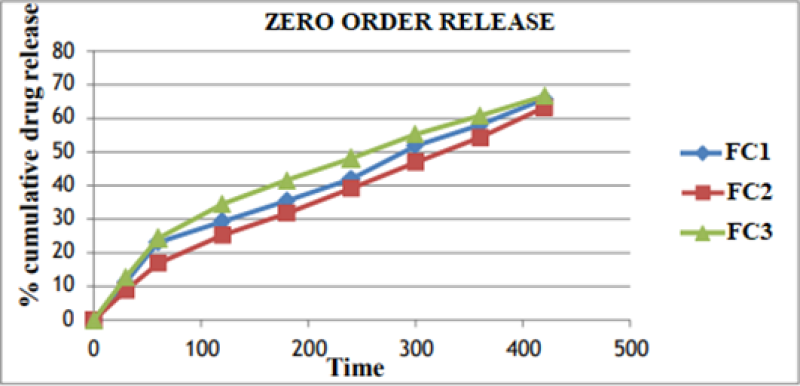
Figure No. 9: Zero Order Plot for Optimized Formulation
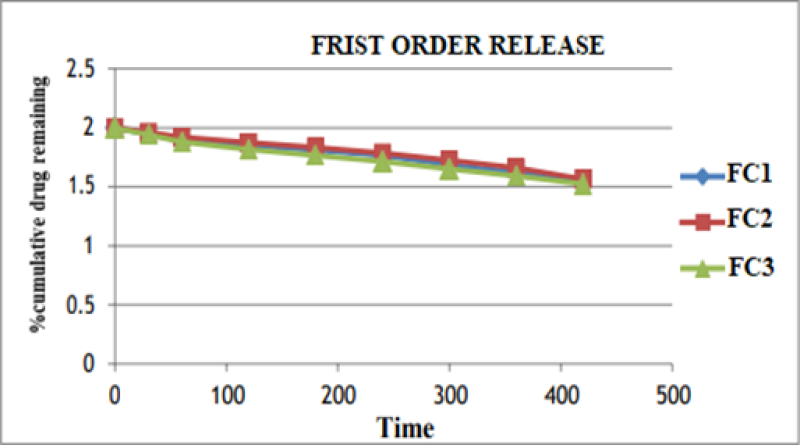
Figure No. 10: First order plot for optimized formulation.
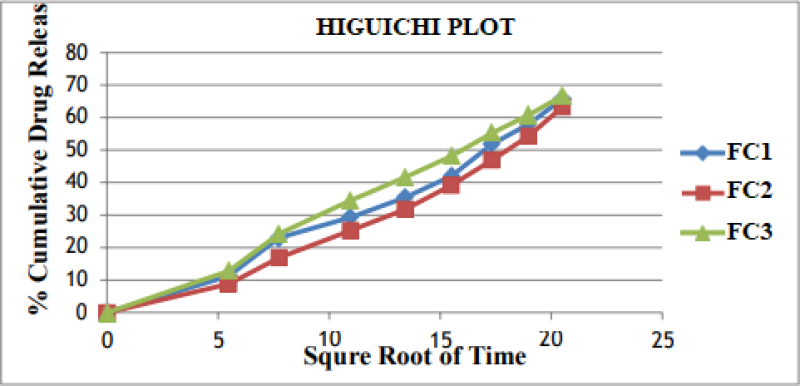
Figure No. 11: Higuchi Plot of Optimized Formulation.
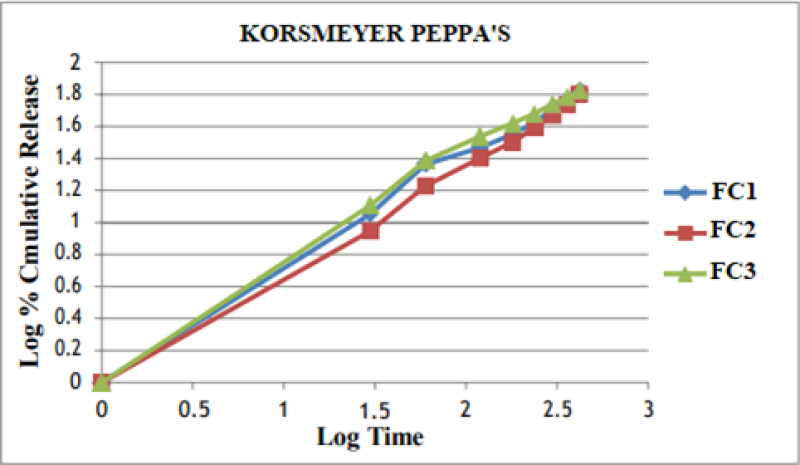
Figure No. 12: Korsmeyer Peppa’s Plot of Optimized Formulations.
The In vitro drug release data was subjected to goodness of fit test by linear regression analysis according to zero order, first order kinetic equation, Higuchi’s and Nordmeyer’s and Peppa’s models in order to determine the mechanism of drug release. When the regression coefficient values of zero order and first order plots were compared, it was observed that ‘r’ values of zero order plots were in range of 0.9844 to 0.9882 indicating drug release from most of the formulations was found to follows zero order kinetics. It is not able that the ‘r’ values of the linear regression for Higuchi’s plot were found to be in range of 0.97 to 0.99 indicating that the data fits the Higuchi’s model well and the drug release was found to be predominantly controlled by diffusion process. When the In-vitro dissolution data was fitted to Korsmeyer’s and Peppa’s model, the ‘r’ values were found to be in range of 0.97 to 0.99 in most of the formulations, indicating the data fits the Korsmeyer’s and Peppa’s model well. The slopes (n) values of Korsmeyer’s and Peppa’s equation were found to be > 0.45 and < 0>

Table No. 11: In-Vitro drug release of formulated F3 lovastatin loaded nanosponge capsule
A *:25±2 ?C and 60±5%RH, B**:40±2 ?C and 75±5%RH Short-term accelerated stability
study was performed on the optimized formulations by storing the samples at 60 °C±20 ?RH with 38 ?±2%/5% RH for 60 days. The samples were tested for any changes in drug content and in vitro drug release studies at monthly intervals. The results of stability studies did not show any significant change in the drug content and in-vitro dissolution studies of above three formulations.
REFERENCES
- Panda S, Vijayalakshmi SV, Pattnaik S, Swain RP. Nanosponges; A novel carrier for targeted drug delivery. Int.J.PharmTech Res.2015; 8(7):213-24.
- Subramanian S, singreddy A, Krishnamoorthy K, Rajappan M. Nanosponges: A Novel Class of Drug Delivery System-review. J pharm pharmaceut sci.2012; 15(1):103-111.
- Selvamuthukumar S, Anandam S, Krishnamoorthy K, Rajappan M. Nanosponges: A novel class of drug delivery system-review. J Pharm Pharm Sci. 2012 Jan 17;15(1):103- 11.
- Shringirishi M, Prajapati SK, Mahor A, Alok S, Yadav P, Verma A. Nanosponges: a potential nanocarrier for novel drug delivery-a review. Asian pacific journal of tropical disease. 2014 Sep 1;4: S519-26.
- Al-Musawi JF. Evaluation of CPK (Creatinine Phosphokinase) in Hypertensive Patients used either Statin and/or Ezetimibe. Journal of Population Therapeutics and Clinical Pharmacology. 2023 Mar 26;30(4):301-8..
- Riventi P, Gupta Nv. Development and Evaluation of Nanosponge Loaded Topical Herbal Gel of Wrightia Tinctoria. International Journal of Applied Pharmaceutics. 2020 Jan 15:89-95.
- Patil TS, Nalawade NA, Kakade VK, Kale SN. Nanosponges: A novel targeted drug delivery for cancer treatment. Int. J. adv. Res. Dev. 2017;2(4).
- Jilsha G, Viswanad V. Nanosponges: A novel approach of drug delivery system. Int J Pharm Sci Rev Res. 2013;19(2):119-23.
- Upadhye SS, Balkundhi S, Ghorpade VS, Ambavade SD, Abhang SV, Mulla SI, Patil PA. Nanosponges: An Innovative Approach for Targeted Drug Delivery System. Research Journal of Pharmacy and Technology. 2021 Mar 1;14(3):1797-804.
- Tamkhane V, Sharma PH. Nanosponge-A Novel Drug Delivery System. Journal of Current Pharma Research. 2014 Apr 1;4(3):1186.


 Anusha*
Anusha*
 G. Sumana
G. Sumana
 F. C. Miranda
F. C. Miranda
























 10.5281/zenodo.10776063
10.5281/zenodo.10776063