Abstract
Since its introduction in 1999 Rabeprazole is widely used Proton pump inhibitor for the treatment of a number of disease and conditions that are related to the excessive secretion of the gastric acid but still there is a strong need of a dosage form of rabeprazole that meets requirement of all age group of patients. There is a requirement of a stable formulation whose dose can be easily adjusted according to patient. The aim of this review is to describe the discovery and development of Rabeprazole, discuss the issues that arise during formulation of dosage form with rabeprazole and their possible solution, present approaches and future prospectives to develop new dosage forms of rabeprazole. The review also outlines the physicochemical properties of Rabeprazole. The review is majorly focused on the presentation of the state of the art in the development of novel formulations with Rabeprazole, it covers different approaches involved employed in this like Nanoparticles, Nanosuspension, Microparticles, Suppositories etc. The review anticipates the future possibilities in the formulation of new dosage forms of rabeprazole.
Keywords
Rabeprazole, Enteric Coating, Mucoadhesive, Transdermal, Nanoparticles, Microparticles, Suppositories.
Introduction
Rabeprazole is a benzimidazole substitution proton pump inhibitor, it has several differences in comparison to other proton pump inhibitors. Rabeprazole was developed by Eisai Co., Ltd., a Japanese pharmaceutical company. The drug was discovered through efforts to improve upon existing PPI medications like omeprazole and lansoprazole. Rabeprazole received approval from the United States Food and Drug Administration (FDA) for medical use in February 1999. Rabeprazole covalently binds with and inactivates the gastric parietal cell proton pump (H+/K+-ATPase).[1] It works by inhibiting the proton pump in the stomach's cells responsible for producing acid. This in turn inhibits gastric acid secretion and raises gastric pH. Rabeprazole is indicated in treatment and management of several disorders related to gastric acid such as-
Gastroesophageal Reflux Disease (GERD):
Rabeprazole is commonly prescribed to relieve symptoms such as heartburn, regurgitation, and difficulty swallowing associated with GERD.
Erosive Esophagitis:
It is effective in healing erosions or ulcers in the lining of the oesophagus caused by excessive acid exposure.
Duodenal Ulcers:
Rabeprazole is used to treat and prevent duodenal ulcers (ulcers in the first part of the small intestine) caused by Helicobacter pylori infection or the use of nonsteroidal anti-inflammatory drugs (NSAIDs).
Gastric Ulcers:
It is also used to treat and prevent gastric ulcers (ulcers in the stomach), again often related to NSAID use or H. pylori infection.[2]
While generally considered safe and well-tolerated, rabeprazole may cause side effects in some individuals, including headache, diarrhoea, nausea, abdominal pain, and dizziness. Long-term use of PPIs like rabeprazole has been associated with potential risks such as an increased risk of bone fractures, vitamin B12 deficiency, and certain infections. The safety of rabeprazole during pregnancy and breastfeeding has not been fully established. Pregnant or breastfeeding individuals should consult their healthcare provider before using rabeprazole. Rabeprazole should not be used in individuals with a known hypersensitivity to PPIs or any of its components. It should also be used with caution in patients with severe liver impairment.[3]
FORMULA

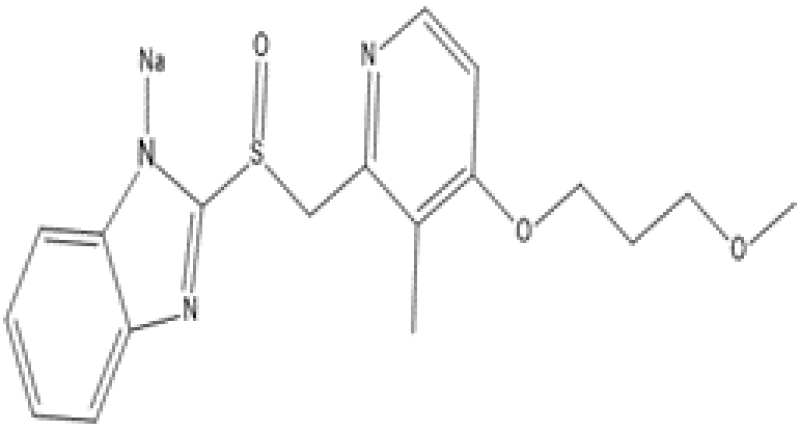
Figure 1: Rabeprazole Base Structure

Figure 2: Rabeprazole Salt Structure (Rabeprazole Sodium)
PHARMACOKINETICS AND PHARMACODYNAMICS
Mechanism of action of rabeprazole is same as other proton pump inhibitors. Proton pump inhibitors are benzimidazoles substitute, they have same basic structure. Being inactive compound Proton pump inhibitors require activation in the low pH of parietal cells to suppress the activity of the proton pump.[4,5,10] The formulation containing proton pump inhibitor are needed to be protected from gastric acid with enteric coating to avoid their premature activation in the stomach after oral administration. PPIs are absorbed from duodenum and transported to the canaliculi of parietal cells through blood stream. Once absorbed from the proximal small intestine these readily cross parietal cell membranes to enter the highly acidic (pH 1) parietal cell canaliculus. At low pH, proton pump inhibitors become protonated and converted to hydrophilic. This selectively hold them and leads to their activation to sulphenamide form within the canaliculus. The activated sulphenamide form binds covalently to the extra cytoplasmic cysteine residues of alpha subunit of H+, K+-ATPase leads to irreversibly inhibiting H+, K+-ATPase activity and gastric acid secretion.[6,7]
MEDICINAL USES
Rabeprazole can be used in the treatment and prevention of different gastrointestinal diseases, such as:
- Gastroesophageal Reflux Disease (GERD),
- Functional dyspepsia,
- Erosive/Non-erosive Esophagitis,
- Gastric and duodenal ulcers,
- Helicobacter pylori infections (combination therapy),
- Hypersecretory syndromes (e.g., Zollinger-Ellison syndrome), and
- In the prevention of NSAID-induced gastroduodenal ulcers.[8]
FACTORS TO BE CONSIDERED WHILE FORMULATING DOSAGE FORM WITH RABEPRAZOLE
The formulation of medicinal products that contain Rabeprazole, is a difficult process due to the low water solubility of Rabeprazole and stability problems. The main factors that effects the formulation of Rabeprazole are-
Physicochemical Properties:
The molecular structure of Rabeprazole is mainly based on the 2-pyridylmethylsulfinyl-benzimidazole moiety (Figure 2). Rabeprazole exists in several states of protonation which depends on the pH of the solution. Therefore, Rabeprazole exhibit two or three pKa values. The range of first value of pKa is 3.55 to 4.77 that is associated with the acceptance of protons in an acidic environment in the nitrogen atom of pyridine and second in presence of alkalis which is due to the dissociation of a proton from the benzimidazole ring. Chirality is another important aspect of the chemical structure in Rabeprazole. All PPIs contain an asymmetric sulfur molecule in the sulfinyl group, which helps in binding pyridine to the benzimidazole group. That’s why proton pump inhibitor can exists in both S- or R- enantiomers and also as racemates and so Rabeprazole. Both S- & R- enantiomeric forms of Rabeprazole are equally pharmacodynamically active.[9]
Stability:
Stability of Rabeprazole and other Proton pump inhibitors are affected by some physical and chemical factors such as-
- Acidic environment
- Light
- Temperature
- Oxidative conditions
- Presence of other salts.
- Interaction with another Drugs.[10]
Acidic Environment:
Rabeprazole is stable in basic environment (pH above 7.0) but highly unstable in acidic environment i.e. decompose very quickly. Rabeprazole and other PPI needed to be enteric coated for avoiding their pre-mature activation in gastric acid.[11]
Light:
Gracia et al observed that rabeprazole degrades and a new product formed when rabeprazole is exposed to UVC.[11] the experiment involves exposure of rabeprazole methanolic solution to UVC-254 nm for 1 hr.
Temperature:
Temperature had a direct effect on the stability of the Rabeprazole. Different research had shown that the PPI solution are stable on storage at low temperature but their stability decreases as the temperature increases.[12,13]
Presence of Salt:
Different studies had shown that the presence of other ion like phosphate, acetate etc has significant and different effect on different PPIs. The stability of Rabeprazole may increase or decrease depending upon the ion present.[14,15]
Interaction With Another Drugs:
Different studies were performed to understand the interaction of rabeprazole with other drug. The studies shows that rabeprazole interact significantly with several drugs like acetylsalicylic acid, clopidogrel, methotrexate etc.
DIFFERENT FORMULATION OF RABEPRAZOLE AVAILABLE ON MARKET
There are a number of formulations of rabeprazole that are available in market. The formulation presently available in market are mainly based on two basis and most important route of administration i.e. Oral and Parenteral.
The presently marketed products of rabeprazole are based on conventional dosage forms. The major part of rabeprazole market product includes-
- Enteric-Coated tablets
- Enteric-Coated Capsules
- Multiunit Pellets
- Suspension with microparticulate (To be prepared in specific vehicle just before administration)
- Powder for Injection
- Powder for Infusion & Other Parenteral Product, etc.
PAST WORK ON FORMULATION OF RABEPRAZOLE
In recent past a large number of studies had been carried out to develop a new formulation with Rabeprazole. These researches were mainly focused on to develop a formulation with Rabeprazole that shows enhancement in efficacy, safety, stability, quality and acceptability. The stability of rabeprazole in acidic environment is a major area of concern as all PPIs get pre activated in acidic pH before their absorption and become ineffective, this is the most important point which should be kept in mind while formulating dosage form with rabeprazole and other PPIs.
In the researches performed in past the routes of administration that are most used are oral and parenteral although some research also involves alternative route of administration like rectal, topical etc. These formulations include –
Sublingual Tablet:
Abraham Sindhu et al 2010 had developed sublingual tablets of Rabeprazole by wet granulation method. For quantitative evaluation of interaction and main effect quadratic model was used. Crospovidone and Croscarmellose Sodium were used as super disintegrants and mannitol was used as diluent, for purpose of sweetening agent Sodium Saccharin was employed and for enhancing the flow property talc was used.[16]
Solanki Kinjal P. et al 2020 had developed Sublingual tablets for oromucosal delivery as oromucosal drug delivery shows faster pharmacological action by rapid absorption and high bioavailability. The Sublingual tablet was prepared with carrier particle covered with fine dry particle of rabeprazole sodium by direct compression method. Mucoadhesive polymer used was HPMC K4M.[17]
Floating Microspheres:
- Senthilkumar S. K. et al 2010 had developed floating microsphere of Rabeprazole sodium with the aim to increase the gastric retention time in stomach. Solvent evaporation method was used for the preparation of microsphere. Different polymers like Hydroxy Propyl Methyl Cellulose, Methyl Cellulose etc were employed. Optical microscope was used for determining the average diameter of microsphere and for other determining other surface morphology of the microsphere optical microscope was used.[18]
- K Swetha S Kamath et al 2012 had worked on the development of floating microsphere for the delivery of acid liable drug rabeprazole. Method employed for the formulation of microsphere was emulsion solvent evaporation method and polymer used were HPMC K15M and Ethyl Cellulose.[19]
Delayed Release Multiparticulates:
- Tirpude N. Rakesh et al 2011 had formulated delayed release multiparticulates (Pellets) with Rabeprazole sodium for anti-ulcer activity. Two different enteric coated polymers were employed in study i.e. Acrylic & Cellulosic. Four different formulations were developed using different coating polymer i.e., two with single coating and two with double coating using different ratios of polymers.[20]
Buccal Tablet:
- N Rajput et al 2011 had developed a tablet containing Rabeprazole sodium with buccoadhesive property to avoid first pass metabolism. For preparation of tablet the method employed was direct compression method. The study employed different polymers with mucoadhesive property like Carbopol-934, Xanthan Gum and Olibanum Gum. For permeability enhancement Sodium deoxycholate was employed.[21]
- A. Jignyasha Raval et al 2012 had prepared bucco-adhesive tablet containing Rabeprazole sodium to avoid gastric degradation of drug. The aim was to increase drug bioavailability and one set of action. In the study different ratio of Bio-adhesive polymers like Gantrez MS 955 and HPMC K4M were used.[22]
Mucoadhesive Enteric Coated Tablet:
- A. Badoni et al 2012 had developed an enteric coated tablet that contain mucoadhesive property. For preparation of enteric coated tablet wet granulation method was employed. Different polymers like HPMC, HPMC-P, Xanthan Gum and Carbapol were employed. Other excipient includes Avicel PH 102 (MCC) which was used as filler and starch was used as binder. pH 1.2 acidic buffer and pH 7.4 phosphate buffer was used for in-vitro drug release test, the formulation also shows a protection index of 100% at a dose of 10mg/kg.[23]
Mucoadhesive Microcapsules:
- Swain Suryakanta et al 2013 had developed mucoadhesive microcapsules containing rabeprazole sodium for anti-ulcer activity. For the formulation of microcapsules solvent evaporation method was employed. For mucoadhesive property Ethyl cellulose based polymers were used and for enteric coating Eudragit L-100 & HPMC K100M polymer were used. Different In Vitro & In Vivo studies were performed for evaluation of formulation.[24]
Sustained Release matrix tablets:
- Khan R. et al 2014 had developed sustained release matrix tablets of Rabeprazole for this they employed wet granulation method. Polymer employed for development of tablet include HPMC-E15, Carbopol 934, Sodium Carboxymethyl Cellulose etc.[25
- Swathi P. et al 2017 had formulated delayed release pellets with rabeprazole sodium and domperidone. The aim of the study is to develop a formulation with modified release time which is capable of releasing drug when and where required. The method employed for preparing modified release Rabeprazole pellets was drug layering. Pellets were coated using seal coating and enteric coating. For enteric coating polymer used was Eudragit L-100 & for seal Coating Polymer used was Eudragit L-55. For preparation of Domperidone pellets sustained release coating was employed using Ethyl cellulose 7 cps as sustained release polymer.[26]
Pulsatile Drug Delivery System:
- Garg Ashish Kumar et al 2017 had developed a Pulsatile Drug Delivery System for the delivery of Rabeprazole sodium. Pulsatile drug delivery system is the system that deliver drug repeatedly over a defined period of time to maintain drug concentration in body for a longer time period. The aim of the study is to develop a Pulsatile release tablet containing Rabeprazole sodium for anti-ulcer activity. The tablet is coated with polymers like HPMC K4M, Ethyl Cellulose, Xanthan Gum etc.[27]
Buffered Tablets :
- Kumari Mamta et al 12019 had formulated buffered tablet with rabeprazole sodium that can be administered orally. The study employed buffering agent to protect Rabeprazole (an acid labile drug) from gastric acid. For the formulation the buffer employed were Sodium bicarbonate, trisodium phosphate, magnesium oxide, Magnesium Hydroxide and calcium carbonate. Crospovidone was used as disintegrant.[28]
Enteric Coated Tablet:
- B.Rama et al (2020) had developed enteric coated tablet containing rabeprazole in which mannitol was used as diluent and croscarmellose was used as super disintegrant. For Coating the formulation different ratios of sub coating and enteric coating was used.[29]
- Gautam D. Mehetre et al (2020) had developed enteric coated rabeprazole sodium tablet for anti-acidity activity. For formulation of tablet different quantity of HPMC, Xanthan Gum, Mannitol, Crosspovidone etc is used.[30]
The enteric coating is done to prevent the disintegration of tablet in stomach this is done to avoid pre-activation of rabeprazole in acidic environment of stomach. If PPIs get released in stomach after pre-activation the become inactive so enteric coting prevents their release in stomach leads to drug release in intestine at basic pH where drug gets absorbed.
Delayed Release Tablet:
- Gupta A. et al 2020 had developed a stable delayed release formulation that avoid acid degradation in stomach. Other than rabeprazole Ingredient used to develop tablet includes Mannitol which is used as diluent, magnesium stearate and talc was used as lubricant and gladient. For enteric coating of tablet Eudragit L-30, HPMCP was used and for seal coating Ethyl-cellulose was used. They also conducted stability studies for which parameters includes 40ºC Temp at 75% RH for 3 months.[31]
Mouth Dissolving Tablet:
- Vali C. Sadak et al 2020 had developed mouth dissolving tablet containing Rabeprazole sodium. HPMC K4M was used as mucoadhesive polymer and sodium starch glycolate was used as Super disintegrant. Different In-vivo and In-Vitro Studies were performed for the evaluation of formulation.[32]
Raft Forming System:
- Saha Shahid et al 2020 had developed a raft forming system that elevates the stomach fluid pH to above 3.5. The aim of the study was to increase the pH of stomach fluid for a desired time period to enhance rabeprazole bioavailability. FTIR studies were performed to check drug and excipient compatibility.[33]
Transdermal Patches:
- Soral Manisha et al 2021 had formulated transdermal patches containing Rabeprazole sodium. The polymer employed for the development of transdermal patches were Hydroxy Propyl cellulose, PVP K-30, PVP K-90 (for formation of film) and PEG 400 (plasticizer). Tween 80 was used as permeation enhancer.[34]
Immediate Release Tablet:
- Lee Sang-Ho et al 2021 had formulated Immediate release oral tablet containing Rabeprazole with increased gastric stability and rapid onset of action. The aim of the study is developing a stable Immediate release dry coated tablet containing rabeprazole in which the API drug forms the inner core and the outer is made with sodium bi carbonate that provide stability to the inner drug from acidic environment of stomach. The stability study shows that the formulation is stable for about 12 months.[35]
Buccal Film:
- Panchal Priya et al 2023 had formulated buccal film with the aim to localize the delivery of rabeprazole from the tissue of oral cavity for the treatment of bacterial and fungal disease. For the formulation of buccal film Hibiscus rosasinensis mucilage and basil leaves extract were used. Method used for preparation of buccal film was solvent casting method.[36]
Different route of administration and polymer used in different formulations of rabeprazole are given in the table below

Table 2: Route of administration and Polymer used in different formulation
DEVELOPMENT OF NEW FORMULATION WITH RABEPRAZOLE
Rabeprazole was discovered in 1986 and was approved for medical use in 1997. Sence then many research were performed on rabeprazole and many formulations are proposed to enhance its stability, safety and efficacy. There is a lack of dosage form of the rabeprazole that may be suitable for all paediatric groups. There are many conventional dosage forms of rabeprazole but in recent years newer dosage forms like nanoparticles, microparticles etc have gained the interest of researchers. Some Formulation which gains interest in recent years and could be a great area of interest as rabeprazole formulation in future are-
Nanoparticles:
Nanoparticles had gained the attention of researchers a lot in field of pharmacy due to their unique properties that can be tailored for various applications. Nanoparticles are biodegradable and bio compatible. One of the most prominent applications of nanoparticles in pharmacy is drug delivery. Nanoparticles can encapsulate drugs, protecting them from degradation and improving their solubility and bioavailability. Additionally, nanoparticles can be engineered to target specific cells or tissues, allowing for targeted drug delivery and reducing systemic side effects. Nanoparticles can be designed to release drugs in a controlled manner over an extended period. This controlled release can improve the efficacy and safety of drugs by maintaining therapeutic levels in the body and reducing the frequency of dosing. Theragnostic nanoparticles combine diagnostic and therapeutic functions in a single platform. They enable simultaneous imaging and drug delivery, allowing for personalized medicine approaches and real-time monitoring of treatment response. Nanoparticles can serve as carriers for vaccines, enhancing their stability, immunogenicity, and targeted delivery to immune cells. This can lead to improved vaccine efficacy and the development of novel vaccination strategies. Overall, nanoparticles offer versatile and promising solutions for various challenges in pharmacy, ranging from improving drug delivery and efficacy to advancing diagnostic and therapeutic approaches. Ongoing research in nanoparticle-based drug delivery systems continues to expand the possibilities for innovative pharmaceutical interventions.[37]
- Ahmed Mohammed Nasef et al 2017 had developed Nanoparticle containing pantoprazole. The aim of the research was to develop nanoparticle which were pH sensitive and protect pantoprazole from acidic environment of stomach. The polymer used was Eudragit S100 & HPMC Phthalate HP55. Different physicochemical property of nanoparticles like Particle size, entrapment efficacy, loading capacity ant in vitro drug release were evaluated.[38]
- Yasin Turanli et al 2022 had formulated pH & time dependent Colon-specific nanoparticle with the aim to treat chronic inflammatory bowel disease like Ulcerative Colitis, Crohn’s Disease etc. Polymer used for formulation of nanoparticle were anionic and cationic polymethacrylate polymer.[39]
- Milind Alai et al 2014 had formulated enteric coated sustained release nano particle containing lansoprazole. The aim of the study was to develop Lansoprazole loaded nanoparticle for acid reflux disease. The polymer used were Eudragit RS100 and PLGA.[40]
Nano Suspension:
Nanosuspensions are a type of colloidal dispersion consisting of submicron-sized drug particles suspended in an aqueous or non-aqueous liquid medium, typically stabilized by surfactants or polymers. These nanoparticles are typically in the size range of 10 to 1000 nanometres. Nanosuspensions have gained significant attention in the field of pharmacy due to their potential to overcome challenges associated with poorly water-soluble drugs, such as low bioavailability and variable therapeutic efficacy. Nanosuspensions increase the surface area of drug particles, leading to improved dissolution rates and enhanced solubility of poorly water-soluble drugs. This can result in better bioavailability and therapeutic outcomes. By reducing particle size, nanosuspensions can enhance the physical and chemical stability of drugs that are prone to degradation, aggregation, or crystallization.[41]
Nanosuspensions can be engineered to target specific sites within the body, such as tumours or inflamed tissues, by modifying their surface properties or incorporating targeting ligands. This enables localized drug delivery and minimizes systemic side effects. Nanosuspensions can be formulated using various manufacturing techniques, including high-pressure homogenization, media milling, and precipitation methods. This flexibility allows for the development of nanosuspensions for a wide range of drugs and therapeutic applications. Nanosuspensions can be administered via different routes, including oral, intravenous, intramuscular, and subcutaneous routes, depending on the specific drug and therapeutic indication. Despite their potential benefits, scaling up the production of nanosuspensions for commercialization can pose challenges related to reproducibility, stability, and manufacturing costs. Addressing these challenges requires careful optimization of formulation and processing parameters. Nanosuspensions represent a promising approach for improving the delivery of poorly water-soluble drugs in pharmacy, offering opportunities for enhanced therapeutic outcomes and patient care. Ongoing research and development efforts continue to explore new applications and address the challenges associated with the formulation and commercialization of nanosuspensions.[42]
- Gupta Manish Kumar et al 2022 had formulated Nanosuspension with Omeprazole. The aim of the research was betterment of the solubility and bioavailability of omeprazole. To ensure the quality and efficacy of the developed formulation different in vitro and in vivo test were performed Scanning Electron microscopy, stability study etc.[43]
- Helissara S. D. et al 2020 had developed a nanosuspension of Omeprazole to treat hyperacidity linked disorder in children’s. The of the study is to develop a stable liquid oral formulation of omeprazole for paediatric administration. The polymer used for the preparation of nanosuspension were Eudragit L-100 & Eudragit RS-100. Different In vitro and In vivo evaluation parameters were performed on the prepared nanosuspension of omeprazole like Mean Diameter, Particle Size, Zeta potential, Entrapment efficacy & in vivo study on mice.[44]
Microparticles:
Microparticles play a significant role in drug delivery systems. These are small particles with diameters typically ranging from 1 to 1000 micrometres. They can be made from various materials such as polymers, lipids, proteins, or inorganic materials like metals or ceramics. Microparticles offer several advantages in pharmaceutical applications Microparticles can be designed to release drugs in a controlled manner, either through diffusion, degradation, or a combination of both. This allows for sustained release formulations, minimizing dosing frequency and reducing side effects. Encapsulation of drugs within microparticles can protect them from degradation in the body, ensuring stability and maintaining their therapeutic efficacy. Functionalization of microparticles with ligands or antibodies allows for targeted delivery to specific tissues or cells, increasing drug concentration at the site of action while minimizing systemic exposure.[45] Microparticles can enhance the bioavailability of poorly soluble drugs by increasing their solubility and dissolution rate, thereby improving absorption and therapeutic outcomes. Microparticles serve as scaffolds for tissue regeneration and engineering by providing a substrate for cell attachment, proliferation, and differentiation. They can be loaded with growth factors or other bioactive molecules to enhance tissue regeneration. Microparticles can be functionalized with probes or markers for diagnostic purposes, such as imaging or detection of biomarkers, pathogens, or specific cell types. Microparticles are used as carriers for vaccine antigens, providing protection and controlled release to enhance immune response and efficacy.
Microparticles offer versatile platforms for drug delivery and various other pharmaceutical applications, contributing to advancements in therapy, diagnostics, and tissue engineering.[46]
Renata Platcheck Raffin et al 2007 had developed microparticle of pantoprazole with aim to develop a formulation that provide controlled release to the drug. He technique used to prepare microparticle of pantoprazole was Emulsion/solvent evaporation method. Polymer used for the preparation of microparticle was Eudragit S 100.[47]
- Bindu Madhavi Boddupalli et al 2014 had developed microsphere of Omeprazole. The aim of the study was to prepare Gastoretentive formulation of omeprazole which is a acid liable drug.[48]
Transdermal Drug Delivery:
Transdermal drug delivery involves the administration of drugs through the skin for systemic distribution. This method offers several advantages over traditional oral or injectable routes. Transdermal delivery avoids the pain and discomfort associated with injections and the inconvenience of swallowing pills, making it more patient-friendly. Transdermal patches can provide controlled and sustained release of drugs over an extended period, maintaining constant plasma drug levels and reducing the need for frequent dosing. By bypassing the gastrointestinal tract and liver, transdermal delivery can avoid first-pass metabolism, leading to improved bioavailability of certain drugs. Transdermal delivery can minimize gastrointestinal side effects and fluctuations in drug levels compared to oral administration, leading to better tolerability and compliance. Transdermal patches are easy to apply and remove, requiring minimal effort on the part of the patient. This convenience can improve adherence to treatment regimens. Transdermal patches can be designed to deliver drugs locally to specific sites, such as for pain relief or skin conditions, while minimizing systemic exposure and side effects. Transdermal patches can incorporate multiple drugs, allowing for combination therapy and improved treatment outcomes for conditions requiring complex medication regimens. Despite these advantages, transdermal drug delivery also has limitations, including limited drug permeability through the skin, the need for drugs with suitable physicochemical properties, and potential skin irritation or sensitization. However, ongoing research and development efforts aim to overcome these challenges and expand the utility of transdermal delivery systems in clinical practice.[49] Soral Manisha et al 2021 had formulated transdermal patches containing Rabeprazole sodium. The polymer employed for the development of transdermal patches were Hydroxy Propyl cellulose, PVP K-30, PVP K-90 (for formation of film) and PEG 400 (plasticizer). Tween 80 was used as permeation enhancer.[36]
Suppositories:
Suppositories are solid dosage forms intended for insertion into body orifices, usually the rectum, vagina, or urethra. They are designed to deliver medications locally or systemically, depending on the drug and its intended therapeutic effect. Suppositories can be composed of various bases, including cocoa butter, glycerinated gelatine, polyethylene glycol, or a combination of these. The choice of base depends on factors such as drug compatibility, melting point, stability, and patient preferences. The drug release from suppositories depends on factors such as the physicochemical properties of the drug, the composition of the suppository base, and the physiological conditions at the site of insertion. Suppositories can release drugs through dissolution, diffusion, or a combination of both. Suppositories offer several advantages, including avoidance of first-pass metabolism, rapid onset of action, and improved patient compliance, especially in cases where oral administration is not feasible or practical. Despite their advantages, suppositories may cause local irritation or discomfort upon insertion, and they may require specialized training for proper administration. Additionally, some patients may find them socially or culturally unacceptable.
Petra Bestebreurtje et al 2020 had developed Suppositories of omeprazole with the aim of to provide a treatment for hyperacidity in infants. The base used for formulation of suppository were witepsol H15 and arginine.[50]
Hydrogel:
Hydrogels are three-dimensional network structures composed of hydrophilic polymers that can absorb and retain large amounts of water or biological fluids. Hydrogels are widely employed as drug delivery systems due to their ability to encapsulate and release drugs in a controlled manner. Drugs can be incorporated into the hydrogel matrix either through physical entrapment or chemical conjugation. The swelling and dissolution properties of hydrogels can be tailored to control the release rate of drugs, offering sustained or targeted delivery to specific tissues or organs. Hydrogels can also prolong the residence time of drugs in the eye, enhancing their therapeutic efficacy. Hydrogels are explored for oral drug delivery to improve the bioavailability and controlled release of drugs. They can protect drugs from degradation in the gastrointestinal tract, enhance their solubility and absorption, and provide targeted delivery to specific regions of the gastrointestinal tract. Hydrogels are used in topical formulations such as creams, gels, or patches for the delivery of drugs to the skin. They provide hydration and occlusion, enhancing drug penetration into the skin and improving the therapeutic outcomes of dermatological treatments. Hydrogels offer versatile platforms for drug delivery, wound healing, tissue engineering, and diagnostic applications in pharmacy and biomedicine. Their tunable properties make them attractive candidates for a wide range of pharmaceutical and biomedical applications.
- Saruchi et al 2014 had formulated Hydrogel system for delivery of Pantoprazole with the use of natural polymers like Gum Tragacanth and Acrylic acid for anti-ulcer activity. In the formulation potassium persulphate and glutaraldehyde were used as initiator and crosslinker respectively.[51]
- Gupta N.V. et al 2009 had developed a pH sensitive hydrogel system containing Pantoprazole. Hydrogel was prepared by polymerization of methacrylic acid and acrylamide with te help of N,N-methylene-bis-acrylamide as a crosslinking agent. [52]
CONCLUSION
Despite being discovered more than 20 years ago and its efficacy in treatment and prevention of several gastrointestinal disorder like Peptic ulcer, Crohn’s Disease, Acid reflux disease etc there are certain issues that are not solved yet. As mentioned, in the review there are several approaches that can be useful in eliminating problems like stability, etc associated with the rabeprazole but there still remains room for improvement. Availability of a formulation of rabeprazole for children and all medical conditions is the most important issue that needed to be resolved. There is a lack of dosage form of the rabeprazole that may be suitable for all paediatric groups due to this, practices like opening capsules to withdraw their content, preparing dispersion of capsule content in some random media etc leads to the degradation of the drug or optimum quantity of drug did not reach the systemic circulation. Hence there is a strong need to develop a formulation that is suitable for paediatric administration. The route of administration on which most researches were performed is oral route of administration but in recent year other novel routes of administration like Transdermal drug delivery, Buccal Drug delivery, etc had gained interest of researchers. The polymer which are most used in the formulation of rabeprazole sodium dosage form are HPMC and Eudragit based polymers.
REFERENCES
- Prakash, Amitabh, and Diana Faulds. "Rabeprazole." Drugs 55 (1998): 261-267.
- Fabio Pace, Stefano Pallotta, Stefania Casalini & Gabriele Bianchi Porro (2007) A review of rabeprazole in the treatment of acid-related diseases, Therapeutics and Clinical Risk Management, 3:3, 363-379
- Pallotta Stefano, Pace Fabio, Marelli Silvia, Rabeprazole: a second generation proton pump inhibitor in treatment of acid related disease. Expert Rev. Gastroenterol. Hepatol. (2008)2(4), 509-522
- Dadabhai, Alia, and Frank K. Friedenberg. "Rabeprazole: a pharmacologic and clinical review for acid-related disorders." Expert Opinion on Drug Safety 8.1 (2009): 119-126.
- Swansk, H., A. M. Hoyummpa, and G. J. Merritt. "Review article: the pharmacokinetics of rabeprazole in health and diseases." Aliment Pharmacol Ther 13.11 (1999).
- Pounder. "The pharmacology of rabeprazole." Alimentary Pharmacology & Therapeutics 13 (1999): 3-10.
- Gardner, J. D., Perdomo, C., Sloan, S., Hahne, W. F., Barth, J. A., Rodriguez?Stanley, S., & Robinson, M. (2002). Integrated acidity and rabeprazole pharmacology. Alimentary pharmacology & therapeutics, 16(3), 455-464.
- Bakheit, Ahmed H., Hamad M. Al-Kahtani, and Salem Albraiki. "Rabeprazole: A comprehensive profile." Profiles of Drug Substances, Excipients and Related Methodology 46 (2021): 137-183.
- Marelli, Silvia, and Fabio Pace. "Rabeprazole for the treatment of acid-related disorders." Expert Review of Gastroenterology & Hepatology 6.4 (2012): 423-435.
- Srebro, Justyna, Witold Brniak, and Aleksander Mendyk. "Formulation of dosage forms with proton pump inhibitors: State of the art, challenges and future perspectives." Pharmaceutics 14.10 (2022): 2043.
- Garcia, Cássia V., et al. "Structural elucidation of rabeprazole sodium photodegradation products." Journal of pharmaceutical and biomedical analysis 46.1 (2008): 88-93.
- Quercia, R.A.; Fan, C.; Liu, X.; Chow, M.S.S. Stability of omeprazole in an extemporaneously prepared oral liquid. Am. J. Health-Syst. Pharm. 1997, 54, 1833–1836.
- El-Badry, M.; Taha, E.I.; Alanazi, F.K.; Alsarra, I.A. Study of omeprazole stability in aqueous solution: Influence of cyclodextrins. J. Drug Deliv. Sci. Technol. 2009, 19, 347–351.
- Ekpe, A.; Jacobsen, T. Effect of various salts on the stability of lansoprazole, omeprazole, and pantoprazole as determined by high-performance liquid chromatography. Drug. Dev. Ind. Pharm. 1999, 25, 1057–1065.
- Raffin, R.P.; Colomé, L.M.; Guterres, S.S.; Pohlmann, A.R. Validação de metodologia analítica por cromatografia líquida para doseamento e estudo da estabilidade de pantoprazol sódico. Quim. Nova 2007, 30, 1001–1005.
- Abraham, Sindhu, et al. Formulation and optimization of sublingual tablets of rabeprazole sodium. Int J Pharm Sci Res 5.2 (2010): 50-54.
- Solanki, Kinjal P., et al. Formulation and Evaluation of Sublingual Tablets of Rabeprazole Sodium using Bioadhesive Polymers." International Journal of Pharmaceutical Sciences and Nanotechnology (IJPSN) 13.3 (2020): 4932-4941.
- Senthilkumar, S. K., B. Jaykar, and S. Kavimani. "Formulation, characterization and in vitro evaluation of floating microsphere containing rabeprazole sodium." Jitps 1.6 (2010): 274-282.
- Shwetha, S., K. Kamath, and S. K. Kumar. "Design and evaluation of floating microspheres of Rabeprazole sodium." International Journal of Pharmacy and Pharmaceutical Sciences 4.3 (2012): 104-120.
- Tirpude, Rakesh N., and Prashant K. Puranik. "Rabeprazole sodium delayed-release multiparticulates: Effect of enteric coating layers on product performance." Journal of advanced pharmaceutical technology & research 2.3 (2011): 184-191.
- Rajput, N., et al. "Formulation and Evaluation of Olibanum Gum Based Rabeprazole Buccal Tablets as Permeation Enhancing System." International Journal of Pharmaceutical & Biological Archive 2 (2011).
- Raval, A., V. Modi, and N. P. Shah. "Formulation and process optimization of buccoadhesive tablet of Rabeprazole." Int J Pharma Chem Sci 123 (2012): 41-49.
- Badoni, A., G. Gnanarajan, and P. Kothiyal. A Research Article on Formulation And Evaluation of Enteric Coated Tablet Loaded With Rabeprazole for Mucoadhesive Drug Delivery. The Pharma Innovation 1.8, Part A (2012): 50.
- Swain, Suryakanta, et al. "Design and characterization of enteric-coated controlled release mucoadhesive microcapsules of rabeprazole sodium." Drug Development and Industrial Pharmacy 39.4 (2013): 548-560.
- Khan, Ruqaiyah, et al. Formulation and evaluation of sustained release matrix tablet of rabeprazole using wet granulation technique. Journal of Pharmacy and Bioallied Sciences 6.3 (2014): 180-184.
- Swathi, P. "Formulation and evaluation of rabeprazole sodium and domperidone pellets." Indo Am. J. Pharm. Res 7 (2017): 235-241.
- Garg, Ashish Kumar, et al. "Formulation and evaluation of chronotherapeutic pulsatile drug delivery system containing rabeprazole sodium." Journal of Applied Pharmaceutical Science 7.2 (2017): 093-100.
- Kumari, Mamta, and Nishi Prakash Jain. "Formulation Development & Evaluation of Buffered Tablet of Proton Pump Inhibitors Drug Rabeprazole Sodium." Journal of Drug Delivery and Therapeutics 9.4-s (2019): 315-321.
- Rama, B., S. Raju Talluri, and Grace Rathnam. Formulation development and evaluation of enteric coated tablets of rabeprazole sodium. IOSR J. Pharm. Biol. Sci 9 (2014): 14-20.
- Mehetre, Gautam D., Rameshwar S. Cheke, and Vinayak N. Shrikhande. Formulation and in-vitro evaluation of enteric coated tablet incorporating rabeprazole. Journal of Drug Delivery and Therapeutics 10.2-s (2020): 50-57.
- Gupta, Aditya, and Gurdeep Singh. Formulation and evaluation of rabeprazole sodium delayed release tablets. Am. J. PharmTech Res 10 (2020): 114-127.
- Vali, C. Sadak, et al., Formulation development and characterization of mouth dissolving tablet of rabeprazole containing sodium starch glycolate as super disintegrant, (2020) 2(3), 44-49.
- Shah, Shahid, et al. "Prompt drug delivery of rabeprazole through raft formation: In vitro and in vivo evaluation." Journal of Drug Delivery Science and Technology 60 (2020): 101932.
- Soral, Manisha, Shivakumar H. Nanjappa, and Prajila Alayadan. "Formulation and evaluation of transdermal patch of rabeprazole sodium." Journal of Reports in Pharmaceutical Sciences 10.2 (2021): 240-246.
- Lee, Sang-Ho, and Joo-Eun Kim. "Quality by design applied development of immediate-release rabeprazole sodium dry-coated tablet." Pharmaceutics 13.2 (2021): 259.
- Panchal, Priya, et al. "Formulation and Evaluation of Buccal film of Rabeprazole sodium." Research Journal of Pharmacy and Technology 16.5 (2023): 2297-2300.
- Sheikh, Atiya, and Sandesh Asati. "Preparation, evaluation and optimization of solid lipid nanoparticles composed of pantoprazole." Journal of Drug Delivery and Therapeutics 12.1 (2022): 12-18.
- Nasef, Ahmed Mohammed, Ahmed Rifaat Gardouh, and Mamdouh Moustafa Ghorab. "Formulation and in-vitro evaluation of pantoprazole loaded pH-sensitive polymeric nanoparticles." Future Journal of Pharmaceutical Sciences 3.2 (2017): 103-117.
- Turanl?, Yasin, and Füsun Acartürk. "Preparation and characterization of colon-targeted pH/Time-dependent nanoparticles using anionic and cationic polymethacrylate polymers." European Journal of Pharmaceutical Sciences 171 (2022): 106122.
- Alai, Milind, and Wen Jen Lin. "Novel lansoprazole-loaded nanoparticles for the treatment of gastric acid secretion-related ulcers: in vitro and in vivo pharmacokinetic pharmacodynamic evaluation." The AAPS journal 16 (2014): 361-372.
- Patel, Vishal R., and Y. K. Agrawal. "Nanosuspension: An approach to enhance solubility of drugs." Journal of advanced pharmaceutical technology & research 2.2 (2011): 81-87.
- Lakshmi, Prasanna, and Giddam Ashwini Kumar. "Nanosuspension technology: A review." Int J Pharm Pharm Sci 2.4 (2010): 35-40.
- Gupta, Manish Kumar, Sreethu K. Sreedharan, and C. I. Sajeeth. "Design development and characterization of omeprazole loaded nanosuspension." Journal of Pharmaceutical Negative Results (2022): 1195-1208.
- Diefenthaeler, Helissara Silveira, et al. "Omeprazole nanoparticles suspension: Development of a stable liquid formulation with a view to pediatric administration." International Journal of Pharmaceutics 589 (2020): 119818.
- Dhurke, R.; Kushwaha, I.; Desai, B.G. Improvement in photostability of pantoprazole sodium by microencapsulation. PDA J. Pharm. Sci. Technol. 2013, 67, 43–52. [CrossRef]
- Raffin, R.P.; Colomé, L.M.; Schapoval, E.E.S.; Pohlmann, A.R.; Guterres, S.S. Increasing sodium pantoprazole photostability by microencapsulation: Effect of the polymer and the preparation technique. Eur. J. Pharm. Biopharm. 2008, 69, 1014–1018.
- Raffin, R.P.; Colomé, L.M.; Guterres, S.S.; Pohlmann, A.R. Enteric controlled-release pantoprazole-loaded microparticles prepared by using Eudragit S100 and Poly(-Caprolactone) blend. Pharm. Dev. Technol. 2007, 12, 463–471.
- Boddupalli, B.M.; Anisetti, R.N.; Ramani, R.; Malothu, N. Enhanced pharmacokinetics of omeprazole when formulated as gastroretentive microspheres along with piperine. Asian Pac. J. Trop. Dis. Suppl. 2014, 4, S129–S133.
- Lin, W.J.; Duh, Y.S. Nanostructured lipid carriers for transdermal delivery of acid labile lansoprazole. Eur. J. Pharm. Biopharm. 2016, 108, 297–303.
- Bestebreurtje, Petra, et al. "Development and stability study of an omeprazole suppository for infants." European journal of drug metabolism and pharmacokinetics 45 (2020): 627-633.
- Saruchi; Kaith, B.S.; Jindal, R.; Kapur, G.S. Synthesis of gum tragacanth and acrylic acid based hydrogel: Its evaluation for controlled release of antiulcerative drug pantoprazole sodium. J. Chin. Adv. Mater. Soc. 2014, 2, 110–117.
- Gupta, N.V.; Shivakumar, H.G. Preparation and characterization of superporous hydrogels as pH- sensitive drug delivery system for pantoprazole sodium. Curr. Drug Deliv. 2009, 6, 505–510.


 Aman Kumar Singh* 1
Aman Kumar Singh* 1
 Alka Verma 2
Alka Verma 2
 Ram Sevak Verma 3
Ram Sevak Verma 3
 Ramnivas 4
Ramnivas 4
 Pranjul Verma 6
Pranjul Verma 6




 10.5281/zenodo.10868683
10.5281/zenodo.10868683