Abstract
We wanted to make asthma treatment better by creating quick-dissolving oral films with Montelukast Sodium. So, we tried different ways to make these films, using special materials like HPMC E5, HPMC 15M, and HPMC K100. We tested each film to see how thick it was, how well it held up, and how fast it broke down. Out of all the films we made, one called F4 was the best. It dissolved in just 17 seconds and released almost all of the drug within 30 minutes. We picked F4 as the optimized batch. Tests showed that the drug and the materials in F4 worked well together. In the end, these fast-dissolving films could be a great help for people with asthma, giving them relief quickly and easily.
Keywords
Mouth Dissolving Films, Montelukast Sodium, Asthma, Oral Route.
Introduction
The oral route remains the perfect route for the administration of therapeutic agents because the low cost of therapy and ease of administration lead to high levels of patient compliance. Oral dosage forms are more popular than other dosage forms because of ease of administration, accurate dosage, self- medication, pain avoidance, patient compliance, etc. Despite of tremendous advancement in drug delivery the oral route of drug administration is the most important method of administration of drug for systemic effect.1 Oral route is most preferred route by medical practitioners and manufacturer due to highest acceptability by patients. About 60% of all dosage forms available are the oral solid dosage form. The lower bioavailability, long onset time and dysphagia patients turned the manufacturer to the parenteral and liquid orals. But the liquid orals (syrup, suspension, emulsion etc.) have the problem of accurate dosing mainly and parenteral are painful drug delivery, which may cause patient non- compliance. Each pharmaceutical company wants to formulate the novel oral dosage form which has the higher bioavailability, quick action and most patient compliance. The most popular solid dosage forms are tablet and capsules. Many patients find it difficult to swallow tablets and hard gelatin capsules particularly geriatric and pediatric patients and do not take their medicine as prescribed. Difficulty in swallowing or dysphagia is seen to afflict nearly 35% of general population. In some cases, such as motion sickness, sudden episode of allergic attack or coughing, fear of chocking and an unavailability of water, the swallowing of tablet and capsule become difficult.2 The delivery system is simply placed on a patient's tongue or any Oro-mucosal tissue. Instantly wet by saliva due to presence of hydrophilic polymer and other excipients, the film rapidly hydrates and adheres on to the sight of application and dissolves to release the medication for Oro-mucosal absorption. It rapidly disintegrates or dissolves or disintegrates to release the medicine for mucosal absorption or with modification, allows for oral GIT absorption with quick dissolving properties. Mouth dissolving Film is prepared using hydrophilic polymers that rapidly dissolves on the tongue or buccal cavity, delivering the drug to the systemic circulation via dissolution when contact with liquid is made. Water- soluble polymers are used as film formers for mouth dissolving films. The water-soluble polymers achieve rapid disintegration, good mouth feel and mechanical properties to the films.1

Definition of FDF: Orally mouth dissolving film is the type of drug delivery system which “A dosage for that employs a water dissolving polymer which allows the dosage form to quickly hydrate by saliva, adhere to mucosa, and disintegrates within a few seconds, dissolves and releases medication for oro-mucosal absorption when placed on tongue or oral cavity.3
Drug delivery via the oral mucosa is a promising route, when one wishes to achieve a rapid onset of action or improved bioavailability for drugs with high first- pass metabolism. The oral mucosa is composed of an outermost layer of stratified squamous epithelium. Below this lies a basement membrane, a lamina propria followed by the submucosa as the innermost layer. The salivary glands secrete mucin as part of saliva. The pH of saliva ranges from 6.8 to 7. The permeability of buccal mucosa is found to be 4000 times greater than skin. The drug administered via the oral mucosa gain access to the systemic circulation through a network of arteries and capillaries. The major artery supplying the blood to the oral cavity is external carotid artery. The venous backflow goes through branches of capillaries and veins and finally taken up by the jugular vein. Fast dissolving buccal film is new oral drug delivery system. This delivery system consists of a very thin oral strip, which is simply placed on the patient’s tongue or any oral mucosal tissue (buccal/sublingual), instantly wet by saliva the film rapidly hydrates and adheres onto the site of application. It then rapidly disintegrates and dissolves to release the medication for oro-mucosal absorption. This fast-dissolving action is primarily due to the large surface area of the film, which wets quickly when exposed to the moist oral environment.4,7
Manufacturing methods of oro-dispersible films
One or more of the following processes can be used to manufacture the mouth dissolving films,
- Solvent casting
- Semisolid casting
- Hot melt extrusion
- Solid dispersion extrusion
- Rolling methods 5-6
- Solvent casting method
Solvent casting is the most commonly used method for the preparation of ODFs using water soluble excipients, polymers and drug which are dissolved in de-ionized water, consequently, a homogenous mixture is obtained by applying high shear forces generated by a shear processor. Solvent casting is the century old film making process. It is a commonly implemented technique for preparing oro- dispersible films. The type of API, which has to be incorporated in ODF, governs the selection of a suitable solvent depending on critical physicochemical properties of API such as melting point, shear sensitivity and polymorphic form. Compatibility of drug with solvent and other excipients is also brought under consideration before finalizing a formulation. During formulation, entrapment of air bubbles can hinder the uniformity of prepared films. Thus, deaeration of the mixture is carried out with the help of a vacuum pump or by sonication method. This technique is employed to manufacture films of size 2x2 cm and 3x2 cm?2;. Water soluble ingredients are dissolved to form a clear viscous solution. The API and other agents are dissolved in smaller amounts of the solution and combined with the bulk. This mixture is then added to the aqueous viscous solution. Polymers that solubilize in solvents are dissolved in suitable vehicle and the drug along with other required additive are dissolved either in aqueous or organic solvent and finally both are mixed and stirred. The entrapped air is removed by vacuum. It is then carefully casted on petri dish or plate made up of glass, Teflon or suitable material and dried. Specific types of equipment (Figure-XIV) which is used at large scale production with the appropriate rollers are utilized for pouring the solution on an inert base. Entrapped air is eliminated utilizing vacuum. The final step concludes by drying the films and remove the trace of solvent to obtain the finished product. After the films are dried, the cutting, stripping and packaging is done 8-14
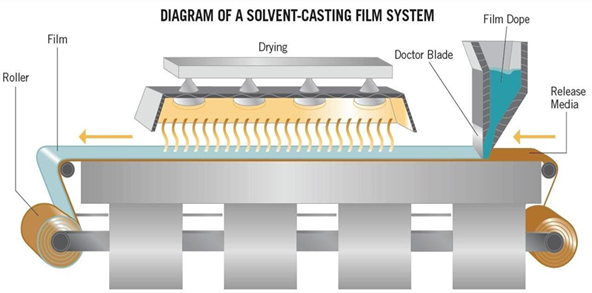
Figure : Equipment for solvent casting film production.
Materials:
Montelukast Sodium is obtained from Concept Pharma Aurangabad. HPMC (E5, 15M and K100) was obtained by Research-lab fine chemicals industries in Mumbai, along with Mannitol and Aspartame. Polyethylene glycol is sourced from Gateefoseeas, a company located in Mumbai. Citric Acid is supplied by Thomas Baker Pvt. Ltd, based in Mumbai. Finally, Vanillin is sourced from Ranbaxy Fine Chemicals Limited, located in New Delhi.
Pre-formulation Study?
Determination of ? max of Montelukast:?
Weigh accurately 10 mg of Montelukast, transfer it into a 100 mL volumetric flask, and make up ? the volume to 100 mL with phosphate buffer (pH 6.8). From this solution, 1 mL was withdrawn ? and added to a 10 mL volumetric flask and diluted up to 10 mL with phosphate buffer (pH 6.8). ? Finally, the sample was scanned in the range of 200-400 nm. The wavelength of the maximum ? absorption was noted, and the UV spectrum was recorded.?
Standard Calibration Curve of Montelukast in Phosphate Buffer (pH 6.8)?:
Accurately weigh 100 mg of Montelukast and add it to a 100 mL volumetric flask. Make up the ? volume to 100 mL with phosphate buffer (pH 6.8) (1000 ?g/mL). From this solution, 1 mL was ? withdrawn and added into a 10 mL volumetric flask, and the volume was made up to 10 mL with ? phosphate buffer (pH 6.8) (100 ?g/mL). This solution was used as the stock solution. From the ? stock solution, 2,4,6,8,10,12 and 14 mL of solution was withdrawn and added into a 10 mL ? volumetric flask and finally diluted up to 10 mL with phosphate buffer (pH 6.8). The absorbance ? was measured for each solution at 345 nm using a UV-Visible spectrophotometer. The graph was ? plotted for absorbance vs. concentration.?
Formulation of Fast Dissolving Films:?
Dose Calculation?
The drug to be loaded in the film was determined by the dose of the drug and the drug loading in ? the glass plate was determined by the area of the glass plate.?
Preparation of films by solvent casting method:?
All the ingredients were weighed accordingly. The polymer was dissolved in ethanol. Kept aside ? for swelling. The drug mannitol, citric acid and vanillin were dissolved separately in ethanol. Then ? polymer solution added to drug solution and plasticizer (PEG-400) added then stirred for 15 ? minutes to produce a clear solution, which kept aside for 15 minutes to get bubble free solution. ? Then solutions were casted slowly with continuous flow on glass plate to prevent formation of ? bubbles then it kept for drying. The dried films were gently separated from glass plate and ? evaluated.
Formulation design:
Fast dissolving oral films were prepared using various grades of HPMC as polymer.

RESULTS AND DISCUSSION:
Standard calibration curve of Montelukast Sodium:
Standard calibration curve of Montelukast Sodium:

Table standard calibration curve of Montelukast Sodium
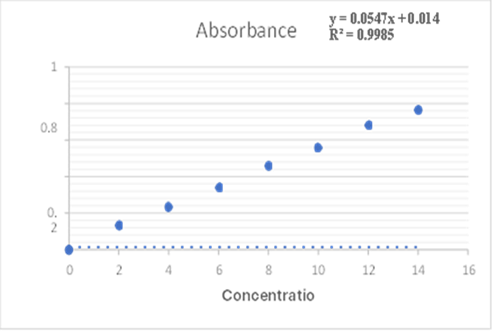
Fig: Calibration Curve of Montelukast Sodium
Evaluation:
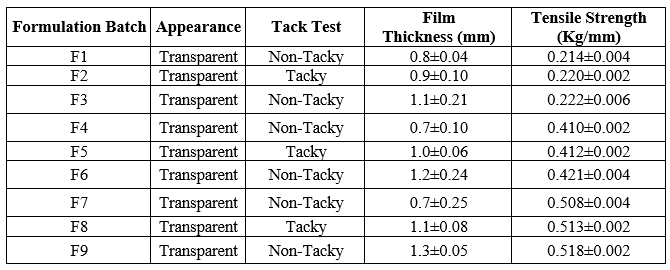
Table: Evaluation of Films for, Appearance Tack test, Thickness and Tensile Strength
Physical Appearance and Surface Texture of Mouth Dissolving films:
These parameters were checked simply with visual inspection of Mouth dissolving film and by feel or touch. The observation suggests that Mouth dissolving films are having smooth surface and they are elegant enough to see.
Tack Test
All Strips were evaluated for tack test out of that only F2 , F5 and F8 batches were found to be tacky and other batches were found to be non-tacky The tack test of all mouth dissolving oral films is given in above table
Tensile Strength:
Tensile strength was found to increase with increase in concentration of the polymers. Tensile strength range of the films varied from to 0.214 ± 0.004 to 0.518±0.002 for HPMC films.
Thickness of Mouth Dissolving films:
The thickness of mouth dissolving Films were measured using screw gauge and the average thickness of Mouth dissolving film given in above Table The thickness of Mouth dissolving film prepared with HPMC E5, 15M K100 respectively.
Thickness of mouth dissolving films were found between 0.8±0.04 to 1.3±0.05.
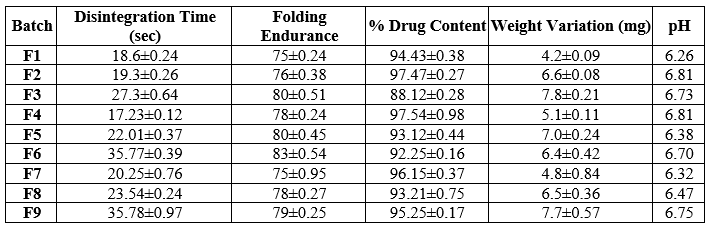
Table: Evaluation parameter of formulation batches
Disintegration Time of Mouth Dissolving film:
Strip of 2 x 2 cm?2; size taken and disintegration time checked visually. In each case three mouth dissolving films were used and the average drug content was calculated, the results were shown in above table.

Fig: Disintegration time prepared films
Disintegration time of mouth dissolving films were found between 17.23±0.12to35.78±0.97
Folding Endurance of Mouth dissolving films:
The folding endurance of Mouth dissolving film was determined by repeatedly folding a small film of Mouth dissolving film at same place till it broke and the average folding endurance of all Films given in Table which ranges between in table sin between range 75±0.24 to 83±0.54.

Fig: Folding Endurance Of Prepared Films
Drug Content Uniformity of Mouth Dissolving Films:
In each case three mouth dissolving films were used and the average drug content was calculated, the results were shown in above table. The drug was dispersed in the range of 88.12±0.28 to 97.54±0.98 Suggesting that drug was uniformly dispersed in mouth dissolving films. The S.D. value calculated for such formulation is very less which suggest that the results are reproducible and accuracy in the method used to prepare mouth dissolving films.
Weight Variation of Mouth Dissolving films:
Weight of Mouth dissolving Films was determined using digital balance and the average weight of Mouth dissolving films were given in above table. The weight variation of formulated films in between 4.2±0.09 to 7.8±0.21
Surface pH of Mouth Dissolving Films:
The surface pH was noted by pH meter near the surface of mouth dissolving film and allowing to equilibrate for 1 min and the surface pH of mouth dissolving films was given in above table the surface pH of mouth dissolving film was found to be in between 6.26 to 6.81 pH (n=3).
In-Vitro Dissolution Studies of Montelukast Sodium
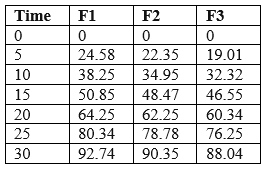
Table: In-Vitro Dissolution studies of Montelukast Sodium
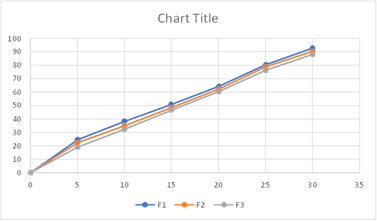
Fig: Cumulative % Drug Release from F1-F3
The better release of drug in batches containing HPMC, E5can be observed and the % drug release of corresponding batches can be ranked in following descending order.
F1>F2>F3

Table: In-Vitro Dissolution studies of Montelukast Sodium
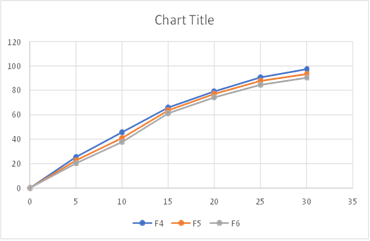
Fig: Cumulative % Drug Release from F4-F6
The better release of drug in batches containing HPMC 15M can be observed and the
% drug release of corresponding batches can be ranked in following descending order.
F4>F5>F6

Table: In-Vitro Dissolution studies of Montelukast Sodium
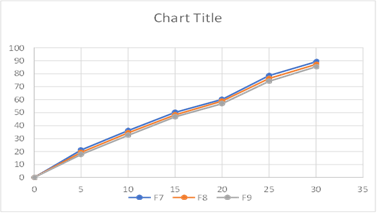
Fig: Cumulative % Drug Release from F7-F9
The better release of drug in batches containing HPMCK100 can be observed and the
% drug release of corresponding batches can be ranked in following descending order.
F7>F8>F9.
In these all aspects the formulation F4 satisfied all the pharmaceutical parameters of mouth dissolving films, and appears to give better therapeutic effects, with disintegration time 17.23±0.12 seconds 97.32 % drug release.
Stability Studies
Stability studies for the optimized formulation (F4) was carried out in order to determine the physical stability of the formulation. The results were shown in there was no significant change in the parameters which are evaluated during the study period in the accelerated conditions.
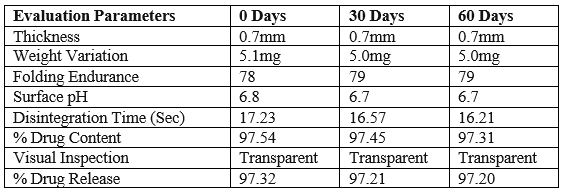
Table: Parameters studies on F4 formulation before and after stability study
There were no considerable changes in physical parameter of film such as Thickness, Weight variation, Folding endurance, Disintegration time, % Drug content of formulation F4 before and after accelerated stability study.
Comparison with Marketed Product:
The drug release profiles of optimized mouth dissolving film formulation and
marketed tablet formulation of Montelukast Sodium10 mg (montair 10 mgTablet) were compared. There was no significant difference between these formulations. The cumulative drug release of Montelukast Sodium for Marketed product was found to be 96.24%and for optimized Batch F4 cumulative drug release was found to be 97.32% at the end of 30 min. So, it was concluded that, drug release from the formulation mouth dissolving Films of Montelukast Sodium was rapid and as good as the marketed conventional tablet and it was a novel approach for delivery of Montelukast Sodium.
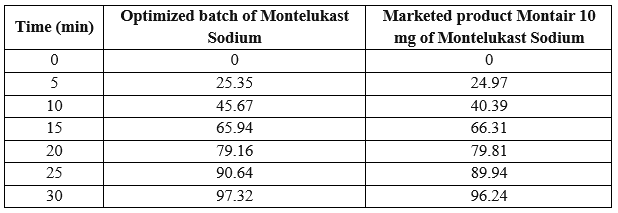
Table: % drug release

Fig: Comparative Drug Release Study of Montelukast Sodium in Montair 10 mg vs F4 Batch
SUMMARY & CONCLUSION:
Montelukast Sodium is the drug used in Asthma which is very common. The absolute bioavailability of Montelukast Sodium it is 64%. To overcome the above-mentioned problems an attempt was made to develop and to improve the solubility of drug and reduce side effects, it was attempted to develop mouth dissolving films using different film forming polymers. FTIR spectroscopic studies were carried out in order to establish compatibility between drug and excipients. The results were concluded that there were no chemical interactions between drug and the excipients used, so they could be used for the formulation of mouth dissolving films. Total 9 formulations of mouth dissolving films were developed using various excipients which were found to be compatible using FTIR of films. Formulations were prepared using three different polymers such as HPMC E5, HPMC 15Mand HPMC K100. Films were evaluated for quality control tests such as Appearance, Tack test, Thickness, Tensile strength, disintegration time, folding endurance, % Drug content, Weight variation, in-vitro dissolution, Comparison with marketed product and stability study.
The appearance of the formulations was found to be transparent. Results of tack test shown F2, F5 and F8 were tacky and all the other formulations were non tacky. The thickness of formulations was between 0.7±0.10 to 1.3±0.05. Tensile Strength of all 9 formulations was found to be in between 0.214±0.004 to 0.518±0.002. The Disintegration time of the formulations was found to be between17.23± 0.12 to 35.78± 0.97. The surface pH of all formulations was found to be in between 6.26 to 6.81. The Folding endurance of the formulations was found to be in between 75± 0.24 to 83± 0.54 The % drug content of all the formulations was found be in between 88.12±0.28 to 97.54±0.98. The Weight variation of the formulations was found to be in between 4.2± 0.09 to 7.8± 0.21. batch F1, F2 and F3 the % release of Montelukast Sodium was found to be 92.74 %,90.35 % and 88.04 % respectively. In this study we observed that as we increase the concentration of Polymer then percentage of drug release decreases. Montelukast Sodium in batches F4, F5, and F6, the drug release percentages were found to be 97.32%, 93.35%, and 90.35% respectively. It was observed in this study that an increase in the concentration of the polymer resulted in a decrease in the percentage of drug release. Montelukast Sodium in batches F7, F8, and F9 the drug release percentages were found to be 89.25 %, 87.21 %, and 85.38% respectively. It was observed in this study that an increase in the concentration of the polymer resulted in a decrease in the percentage of drug release. In these all aspects the formulation Batch F4 satisfied all the pharmaceutical parameters of mouth dissolving films. So, the F4 batch was selected as optimized batch. The optimized batch which is F4 was then compared with the marketed product (Montair 10 mg). the Montelukast Sodium the % drug release in F4 was 97.32 where for Montair 10 mg% drug release was found to be 96.24.
CONCLUSION
Overall, this study successfully developed mouth dissolving films for Montelukast Sodium which showed desirable physical characteristics and drug content. The findings suggest that the formulation approach using these film-forming polymers can be a promising strategy to enhance the solubility and minimize side effects of the drugs in the treatment of Asthma, allergic rhinitis. Further investigations, including in vivo studies and clinical trials, may be warranted to assess the efficacy and safety of these formulations for potential therapeutic applications.
REFERENCE
- T. UshaKiran Reddy, et al, “A Detailed Review On Mouth Dissolving Oral Films”Indo American Journal of Pharmaceutical Research 2018:8(06). Vol 8 Issue 06,2018.Page No. 1315-1326.
- Mukem Bhattarai, et al, “Mouth Dissolving Oral Films: A Novel Trend To Oral Drug Delivery System” Sunsari Technical College Journal 2015; 2(1):58-68
- Muhammad Bilal Hassan Mahboob, et al, “Oral Films: A Comprehensive Review” International Current Pharmaceutical Journal, November 2016, S(12):111-117.
- M. Sri rekha, et al, “Novel Oral Drug Delivery System: Mouth Dissolving Buccal Films” American. J. Pharmacy and Health Research 2014;2(12) Page No.18-41.
- Loveleen Arora, et al, “A Review On New Generation Oro-dispersible Films And Its Novel Approches Indo American Journal of Pharmaceutical Research,2017,Page No.7451-7470.
- Devender Sharma, et al. “Update Review On Oral Disintegrating Film” International Journal of Creative Research Thoughts, Volume 6, Issue 1 January 2018,Page No.92- 102
- Reza KH, Chakraborthy P. Recent industrial development in oral thin film technology: an overview. Pharma Tutor, 2016; 4: 17-22.
- Mary Elizabeth RN, Martelli BS. Sublingual and buccal medication administration. Encyclopedia of Nursing and Allied Health, 20050229.
- Galey, W.R., H.K. Lonsdale and S. Nacht, The in vitro permeability of skin and buccal mucosa to selected drugs and tritiated water. J. Investigative Dermatol., 1976; 67(6): 713-717.
- Malke, M., S. Shidhaye and V.J. Kadam, Formulation and evaluation of Oxacarbazine fast dissolve tablets. Indian J. Pharmaceutical Sci., 2007; 69(2): 211- 214.
- Frey P. Films strips and pharmaceuticals, pharm mf. & package. Sourcer, winter; 2006; 92-93.
- Siegel, I.A. and H.P. Gordon, Surfactantinduced increase of permeability of rat oral mucosa to non-electolytes in vivo. Archives of Oral Biol., 1985; 30:


 Angali Swati*
Angali Swati*
 Sontake Abhay
Sontake Abhay



















 10.5281/zenodo.12680731
10.5281/zenodo.12680731