Abstract
Nanoscience is a prominent area of modern research due to its potential to revolutionize various fields. Iron-based nanoparticles, with their unique magnetic properties, have found applications in a wide range of scientific and technological disciplines. Iron oxide, a mineral compound, has several polymorphic forms, including hematite (?-Fe2O3), magnetite (Fe3O4), and maghemite (?-Fe2O3). The pharmacokinetic and pharmacodynamic characteristics of many medications have been enhanced through the use of nanoparticle drug delivery systems. This paper investigates recent developments in the synthesis and functionalization of magnetic nanoparticles. There are several ways to create iron oxide nanoparticles, including chemical, biological, and physical approaches. The various techniques for producing iron oxide nanoparticles and managing their size, shape, and magnetic characteristics are outlined in this review. It also covers a variety of characterization methods, including X-ray diffraction (XRD), UV-visible absorption, scanning electron microscopy (SEM), and FTIR analysis. Nanoparticles are increasingly used to target bacteria as an alternative to antibiotics. Nanotechnology offers significant benefits in treating bacterial infections, including the use of nanoparticles in antibacterial coatings for medical materials and implantable devices.
Keywords
Iron oxide nanoparticles, Diamagnetism, ferromagnetism, antibiotics, bacteria, co-precipitation, microemulsion method, Iron deficiency anemia
Introduction
Nanoparticles are submicron particles which diameters ranging from 1 to 100 nm and made up of organic and inorganic material because of distinctive size and physicochemical properties of nanoparticles, using nanoparticles as a material has provide a significant benefit. In many field of research and technology have used iron oxide nanoparticles because of their distinctive magnetic properties.(1) Magnetic Nanoparticles have several application in medical science like targeted drug administration, MRI contrast agent for imaging of magnetic hyperthermia, tissue repair and Hyperthermia.(2) For the MRI application of magnetite nanoparticles, their size should be less than 20nm and show very low toxicity. These specially coated magnetite nanoparticles are approved for use in magnetic Resonance Imaging (MRI). The MRI properties of iron oxide nanoparticles have been extensively studied due to their super paramagnetic character. There are several types of iron oxide nanoparticles are available including haematite (?-Fe2o3), Magnetite (Fe3O4), and maghemite ?-Fe2o3 of these magnetite has been strong biocompatibility.(3) Magnetic nanoparticles are consist of different magnetic elements such as cobalt, Iron, Nickel, and their alloys which shows ferromagnetism and superparamagnetism properties. Due to their safety, biocompatibility and substantial therapeutic value, Iron Oxide (IONPs) have been the subject of extensive research among various MNPs for many years. Data from hospital and community shows that the prevalence of antibiotic resistance are rising. Because of emergency multidrug-resistance (MDR) bacteria traditional antibiotics are losing their bioactivity which makes it essential to find alternate treatment. Several pathogenic microbes have evolved developed resistance to antimicrobial medicines, such as antibiotics, which present a major challenge in treatment of infectious diseases. As a result, bacteria that develop resistance to many drugs possess a serious risk to public health. To overcome medication resistance, it is therefore essential to find a novel and potent agents. In order to address this issue nanotechnology offers new possibilities for the manufacture of antimicrobial nanoparticles.
Because of their established biocompatibility magnetic nanoparticles are the magnetic materials most appropriate for biomedical applications. The development of resistance among pathogenic fungi and bacteria to antimicrobial agents, driven by phenotypic resistance associated with their biofilm state and genetic mutations, has emerged as a significant health concern in recent years. Recent investigations have shown that a variety of metal nanoparticles have antibacterial qualities. These nanoparticles possess the capacity to directly engage with the microorganism cells, resulting in harm to the outer covering of the cell and consequently leaving the bacterial cell vulnerable or, in certain instances, interfering with the biochemical processes and leading to annihilation of the cell.(4)
Nanoparticles are becoming increasingly utilized for the purpose of targeting bacteria, serving as a substitute for antibiotic. The application of nanoparticles in antibacterial coatings for medicinal devices and implantable devices to prevent infection and promote wound healing, in bacterial detection system to prevents microbial diagnostics, and in antibacterial vaccines to manage bacterial infections are some examples of how nanotechnology may helpful in treatment of bacterial infections.(5) Nanoparticles have become a viable approach for effective drug delivery. Current studies in the absorption of nanoparticles in the gastrointestinal tract concentrate on improving absorption of nutrients, medications and vaccinations that are either poorly absorbed or broken down by digestive process. In place of commonly used iron salts, a variety of iron oxide nanoparticles composite were created to treat IDA with excellent effectiveness and little side effects. In contrast to a commercial iron oxide particles, the goal of this study was to create iron oxide nanoparticles and assess their potential as an supplement in the treatment of IDA.(6)
Magnetic nanoparticles can be made in several methods. It is possible to create magnetic nanoparticles by either “top-down” approach or “bottom-up” approach. Using a high energy ball- milling process, a magnetic sample are grounded to required size particles in top-down method. The “top-down” approach has the benefit of producing large number of particles in a single batch, but it also has a drawback of compromising control over the particle size and shape which having a important factor in biological applications. The bottom-up approach required a beginning with a salt of ferrous (Fe2+) or ferrous (Fe3+) ions and going through a distinct chemical process to nucleate and promote seeded development. Various chemical approaches are utilized for synthesis of iron oxide nanoparticles, with co-precipitation being highlighted as a simple and efficient method to produce large quantity of nanoparticles. This method is allowed for scalable production of these nanoparticles for further research and application. With the rise of new bacterial resistance strains, there is growing interest in exploring nanoparticles as a alternative antimicrobial agents. Gram positive and Gram negative pathogenic bacterial strains have been shown to be moderately resistant to iron oxide nanoparticle-mediated antibacterial activity. This suggests that they could be used to treat bacterial infections in the biomedical and pharmaceutical industries.(7) This review examines existing MNPs synthesis techniques and the benefits and applicability they offer for biomedical applications. Associated materials, solvents and functions are discussed because, discussing synthesis necessarily requires a clarification from the biocompatibility outlook. Specific biomedical uses of MNPs from different imaging techniques are also highlighted in dedicated sections, along with potential challenges. This review provide a concise overview of certain synthesis and biomedical applications of iron oxide nanoparticles.(8)
Physical properties of iron oxide nanoparticles:
Physical attributes involve optical characterization such as light transmission, injection and reflection capabilities as well as UV reflection and retention capabilities on the surface of material. The mechanical characteristics of iron oxide nanaoparticles such as their flexibility, elasticity, malleability and plasticity are essential for their use. The properties such as conductivity, semiconductivity and resistivity makes the nanoparticles employed for modern devices efficient for eco-friendly power applications.(9) The physical properties of IONPs such as including their size, shape, surface area, magnetization and susceptibility. The synthesis methods and functionalization techniques plays a very important role in determining these physical properties which in turns influence the magnetic properties of IONPs.(10)
Chemical properties:
The application of nanoparticles is determined by the reactivity of nanoparticles with target, their stability and susceptibility to moisture and other environmental factors such as environmental heat and light. Because of their antimicrobial antifungal sterilizing and toxicological characteristics, they are suitable for used in biomedicine and environmental purpose.
Rheological properties of iron oxide nanoparticles:
When magnetized particles are added to a non-colloidal suspension, an external magnetic field regulates the rheological properties of the carrier fluid, forming magneto-rheological fluids. Depending on the strength of the applied magnetic field, these fluids can display rheological behaviors that change from Newtonian to plastic or non-Newtonian. The magneto-rheological (MR) effect is defined by changes in rheological properties like yield stress and viscosity. The concentration of suspended particles and the saturation magnetization of the applied magnetic field control the magnitude of this effect. In ferrofluids, the rheological characteristics are influenced by the flow of the fluids; initially, the magnetic moments of suspended particles align with the vortices of the flow, but they reorient when a magnetic field is applied. The viscosity of ferrofluids increases with the applied magnetic field at a fixed shear rate and decreases with increasing shear rate at a constant magnetic field.(11)
Magnetic properties:
The materials are classified based on how their ability to interact to a magnetic field that is applied externally. In nature, there are many different kinds of magnetism. Different types of magnetism found in nature can be identified with the help of description of orientations of magnetic moment in the material. There are five basic forms of magnetism are: diamagnetism, ferromagnetism, paramagnetism, antiferromagnetism and ferrimagnetism. In diamagnetic materials, electrons orbital movement is zero and the magnetic behavior is due to rotating. This kind of magnetism occurs only when the external magnetic field is applied. It occurs as a result of changes of that occur as an orbital motion of electrons in the existence of a magnetic field outside. Diamagnetic materials include Al2O3, Cu, Zn, Si. Paramagnetic materials such as pyrite, have a small positive magnetic susceptibility (C=O) and moment withthey lack long-range order because their atomic magnetic moments are uncoupled. Bulk magnetic materials are ferromagnetic, meaning that their magnetic moments remain aligned even after the external magnetic field is removed. Therefore these “permanent magnets” increase the flux density such as (Fe, Ni and Co) by showing. Hard magnets are characterized by High remnant magnetization (i.e., net magnetization at zero external magnetic field) and coercivity (i.e., requiring a high magnetic field strength in the reverse direction to bring the net magnetization to zero). Even in the absence of an applied magnetic field, these materials maintain their permanent magnetization. Antiferromagnetism are shown by a material with antiparallel atomic magnetic moments of a comparable magnitude (e.g. troilite FeS). Thermal energy is sufficient above the neel temperature to induce random fluctuation in the equal and appositely aligned atomic moment, which causes their long-range order to vanish. The materials behave paramagnetically in this state. (9)-(12)
Factors enhancing the efficiency of iron oxide nanoparticles:
- The precipitation technique is the most efficient, cost-effective and uncomplicated method to acquire magnetic particles, such as magnetic particles.
- Excellent surface-area-to-volume ratios provided by small nanoparticle sizes allow for interaction with a wide variety of gaseous and aqueous chemical species. Materials at the nanoscale are very effective at binding metal ions.
- Properties related to medication administration and magnetofection using magnetically responsive and magnetically guided nanoparticles.
- Proper functionalization of iron oxide nanoparticles post-synthesis is essential to avoid chemical corrosion and instability, resulting in biocompatible and stable nanoparticles with enhanced efficiency.
- The efficiency of iron oxide nanoparticles in a variety of applications is attributed to their higher surface-to-volume ratio, which also enhances their reactivity and potential biochemical activities.
- Surface modification with suitable organic and inorganic molecules is crucial for improving the stability, biocompatibility, and functionality of iron oxide nanoparticles. (13)
- Methods of preparation of iron oxide nanoparticles:
Many studies has been conducted in the past few year to synthesize iron oxide nanoparticles and many reports have provide effective synthesis technique for creating Monodispersed, biocompatible, shape-controlled, and stable iron oxide nanoparticles.(14) There are various methods are available for synthesizing iron oxide nanoparticles about 90% of methods which are used for synthesis of IONPs are chemical method while physical and biological methods accounts for 8% and 2% respectively. Chemical methods encompass a range of techniques, including sol-gel, hydrothermal synthesis, co-precipitation, microemulsion, thermal decomposition, and sonochemical synthesis, microwave assisted synthesis and physical routes include pyrolysis technique and laser ablation in solution method.(15)
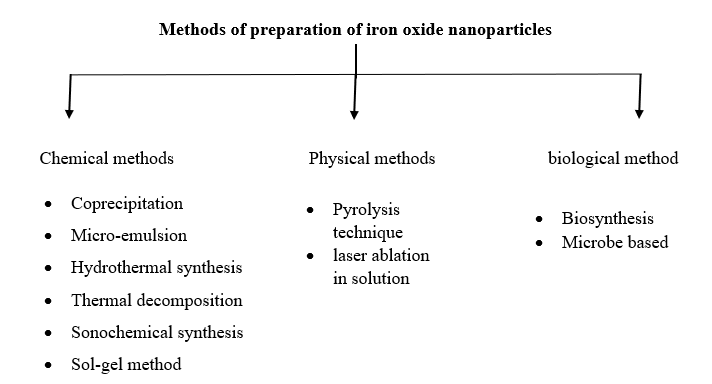
Chemical routes:
Co-Precipitation:
In this experiment, the ?- Fe3O2 nanoparticles was prepared by co-precipitation method. In this method Iron (II) chloride (FeCl2·3H2O) and iron (III) chloride (FeCl3) were dissolved in DI water. Throughout the experiment, the molar ratio of iron (II) chloride to iron (III) chloride in this method was kept constant at 1:2. A hot plate magnetic stirrer was then used to mix the solution together for 30 minutes. Various factors such as processing temperatures, pH level and stirring rate were controlled and regulated within mixing steps. Afterward, the combined solution was centrifuged at 4000 rpm for 15 minutes. After that, the precipitated matter was separated and dried for fifteen minutes at a temperature of 100 in a hot air oven. The dried brown precipitate was obtained using a mortar and pestle, then ground into a powder. Equations 1-3 present the balanced chemical equation for the reaction between FeCl3·4H2O and FeCl3, indicating that ?-Fe2O3 is the final product and water is a byproduct.(16)
Fe2+ +2Fe3+ 8OH- Fe3O4 + 4H2O (1)
Fe3O4 + 0.2502 + 4.5 H2O 3 Fe (OH) (2)
2Fe (OH)3 ?-Fe2O3 + 3H2 (3)
Magnetic nanoparticles are prepared by co-precipitation method. The procedure for synthesis was conducted in following manner FeCl3.6H2O and FeCl2.4H2O, with molar ratio of 2:1, were individually dissolved in distilled water. Then these two solutions were combined into a 500ml glass beaker and subjected to heating until reaching various temperatures for a duration of 10 minute with stirring. A 20mL of 10% Nh4OH were progressively added after the solution had heated and it was vigorously agitated at 300, 400 and 500 rpm with a magnetic stirrer until pH of mixture reached to 10. Then the solution was heated for 30 minutes at temperature of 40°c, 60°c and 80°c. The resultant mixture was subsequently separated into liquid and precipitate using filter paper and rinsed with distilled water. The precipitate was dried at hot air oven at 100°c for the period of 150 minutes.(17)
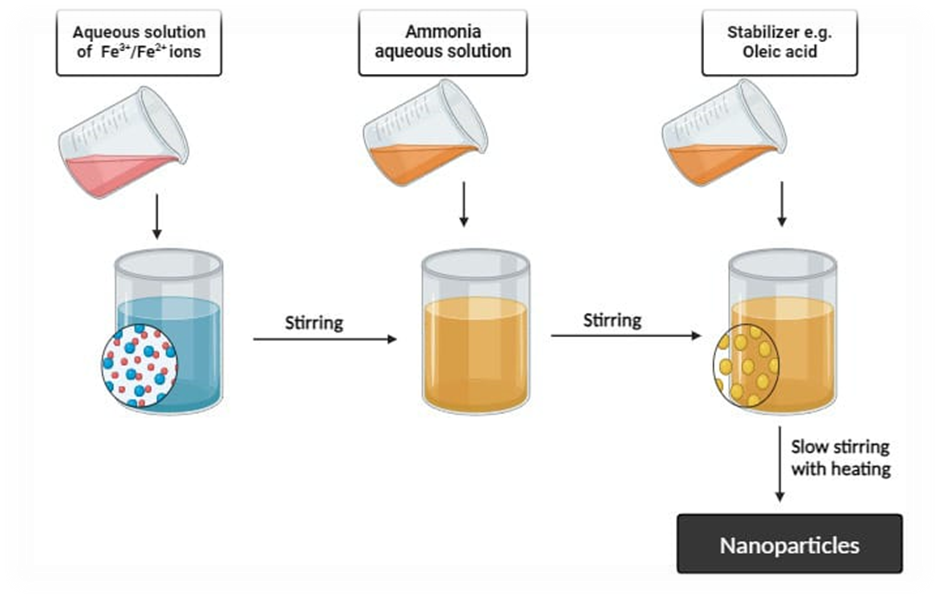
Fig 1: Flow chart for co precipitation method
Micro-emulsion method:
The micro emulsions are transparent system that spontaneously form when a medium chain alcohol (C5-C10) containing ionic surfactant is combined with the oil phase and aqueous phase in comparatively high volume. Catalytic iron oxide is produced using the micro emulsion process, It is preferred because it yields particles with a high surface area and a narrow pore size distribution (between 4 and 15 nm), typically exhibiting cubic or surface morphology.(18) The isotropic thermodynamic dispersal of two distinct phases of oil and water This is referred to as a microemulsion when there is a surfactant present. At the oil-water interface, surfactant molecules can form a monolayer, with the hydrophilic head groups dissolved into the water phase and its hydrophobic tails dissolved in the phase of oil. (1) In this microemulsion system, Tween 80 was used as the surfactant, 1-butanol as the co-surfactant, n-heptane as the continuous oil phase, and an aqueous reactant solution as the dispersion phase. 60 mL of n-heptane and 20 mL of Tween 80/1-butanol were used to maintain a volumetric ratio of 1:1 between the surfactant and co-surfactant.
Fe (NO3)3·9H2O and FeSO4·7H2O are combined in a 2:1 molar ratio in 80 mL of a Tween-80/1-butanol/n-heptane mixture to form the precursor solution (Solution I). This mixing results in the formation of a reverse microemulsion. 50 mL of aqueous NH3 and 80 mL of the Tween-80/1-butanol/n-heptane mixture compose Solution II.
At room temperature these solutions were mixed for 30 minutes at 300 rpm. After adding Solution II to Solution I, the mixture was stirred continuously at 1000 rpm for a duration of 150 minutes at various temperatures of 30°C, 50°C, and 80°C. The resulting precipitate was repeatedly washed with DI water and acetone to remove any residual surfactant and ammonia. Finally, the precipitate was dried in a vacuum oven to produce the Fe3O4 nanoparticles.(19)
Hydrothermal decomposition method:
The hydrothermal process produces homogeneous iron oxide nanoparticles by subjecting iron precursors to vapor in a sealed container at high pressure and temperature in an aqueous solution.(15) The iron oxide nanoparticles produced in this synthesis process by crystal growth at high temperature and pressure condition (usually below 300°c). Reactors capable of maintaining high temperature and pressure are normally used for this kind of synthesis. High crystallinity is produced at the same time that the reaction rate is increased. Highly crystalline iron oxide nanoparticles, sized between 15 to 25 nm, were synthesizes using an organic solvent and iron precursor solution in pressure resistant reactors at 473 k. However the primary disadvantage of this technique is the need for expensive reactors.(20)
Thermal decomposition method:
Particularly there are two distinct method that can be used to accomplished the thermal decomposition method namely “heating up” and “hot injection”. A pre-mix solution of precursor, compounds, surfactants, and solvent is continuously heated as a part of heating-up process to a temperature where nanoparticles were begins to cluster grow. On the other hand, by injecting chemicals into a hot surfactant solution and then slowing for a controlled growth phase, the hot injection phase approach cause a quick and and uniform nucleation. In any case both procedure based on same principle, which is to heat a nonmagnetic organometallic precursors chemical in the presence of organic solvents and surfactant. Nonmagnetic precursors are often iron carbonyls and acetylacetonates, while fatty acids, rather than oleic acid are commonly utilized as a surfactant. Argon is significant because it helps to keep the atmosphere inert. Temperature ranges between 100°c to 350°c is the optimal temperature required for the production of crystalline MNP’s having size ranged between 4 and 30 nanometert in diameter which exhibits a high degree of uniformity. In this context the control of particle size relies significantly on the temperature and reaction time.(10)
Sono chemical synthesis:
In this method a pure nanosized particles was prepared by utilizing a stoichiometric quantity of sodium hydroxide (NaOH) and ferric chloride (FeCl3·6H2O). After that NaOH was dissolved in a deionized water to produce solution of 3M 50 ml this solution was gradually added drop by drop to an aqueous FeCl3.6H2O (0.1 M, 50 ml) in 30 minutes. Employing a titanium alloy ultrasonic horn submerged in the solution, direct ultrasonic irradiation was applied to the prepared mixture using a Misonix model 3000 sonicator.During the sonication process the colour of the slurry was changed from yellow to reddish-brown. After 30 minute of this process a variety of Fe2O3 samples were produced. To eliminate byproducts the resultant precipitate then obtained, filtered and rinsed with methanol followed by double-distilled water. The obtained sample were dried at RT and after that heated at temperature of 500°c to get crystalline iron oxide nanoparticles.(21)

Fig 2. Flow chart for sonochemical method
Sol-gel method:
For producing the nanoparticles, the sol-gel method employs condensation and hydrolysis process of metal alkoxide or their precursors. The intermediates must get additional treatment in order to obtained nanoparticles with high crystallinity. In this process the sol is prepared by dissolving the precursors in water, stirring, and heating the mixture to generate the sol. The MNPs are then dried to prepare the gel. To obtained the required MNPs, the solvent is eliminated. Although the sol-gel method produces MNPs with great homogeneity and purity, it also creates impurities that are difficult to remove. It offers the high degree of control over the content and the size of particle just like other methods.(22) The solvent used in sol-gel method is commonly consist of water, while the precursors possess the capability to undergo hydrolysis through the application of either an acidic or basic substance. The structures and dimensions of iron oxide are influenced by a variety of factors such as reaction rates, temperatures, the nature of precursor and pH level.(18)
Physical Method:
Pyrolysis Method
Iron oxide nanoparticles IONPs are prepared by pyrolysis process which includes the controlled breakdown of precursor material at high temperature and inert atmosphere. By aerosol pyrolysis of precursor mixtures, this technique can be utilized to generate core-shell nanostructures such as ?-Fe2O3 Nano composites. Additionally, carbon coated IONPs synthesized through pyrolysis exhibit good chemical and thermal stability, high electrical conductivity and improved dispersibility and stability compared to bare IONPs. Laser pyrolysis is another variation of this method, where a gaseous mixture of iron precursors is heated by a laser to produce small, narrow sized and non-aggregated IONPs. Overall, the pyrolysis method provides the versatile approach for the synthesis of magnetic IONPs with various structures and properties, making it valuable technique for application in fields of biomedicines and nanotechnology.(23)
Laser ablation in solution method:
In this method by using a solution of sodium dodecyl sulphate (SDS CH3(CH2)11OSO3Na) soluble in DI water at room temperature, iron oxide (?-Fe2O3) nanoparticles were prepared with 99.99% purity by laser ablation of iron pressed pallets. Six milliliters of the solution with the iron target at the bottom was put into a quartz container. The laser beam was focused on the surface of the target by positive lense of 120 mm focal length.(24) The colloidal solutions were obtained through irradiation of an iron pellet using a pulsed Neodymium-doped Yttrium aluminium garnet (ND:YAG) laser operating at a wavelength of 1064 nm. The laser was operated at RT with a pulse width of 9 ns and a repetition rate of 1 Hz. Four different laser frequencies such as 20 mj, 40mj, 60mj and 80mj were used to create iron oxide nanoparticles. In this experiment the ablation was performed for 30 minute.(25)
Biological method:
In biological synthesis methods various resources are used such as plant, plant extract, fungi, bacteria and viruses. Various methods are used by biological resources for producing nanoparticles. The stabilizing and reducing agents are chemicals made by plant and algae, fungus and bacteria which are used in the biological synthesis of nanoparticles. Toxic and organic materials are avoided in the biological synthesis of nanoparticles.(26)
Biogenic synthesis
To produce iron oxide nanoparticles (Fe3O4) using biogenic synthesis 0.1 m solution of ferric chloride hexahydrate (FeCl3.6H2O) was mixed with 0.1 g/10ml of prepared plant extract in 1:1 v/v ratio. Fe3O4 nanoparticles were obtained by reduction process. After 60 minutes of stirring the mixture was allowed to equilibrate at RT for an additional time of 30 minutes.(27)
The colloidal suspension was obtained under centrifugation at the speed of 10,000 rpm for a duration of 20 minutes and washed several time with ethanol then the obtained colloidal suspension was vacuum-dried at 40°C to obtain Fe3O4 nanoparticles that had been washed.(28)
Microbe based
Because of its adaptability, capability to withstand harsh environments and ecofriendly nature bacterial assisted synthesis has emerged as a viable method for green synthesis of an extensive range of nanoparticles. Nanoparticles can be synthesized intracellularly and extracellularly depending on the bacterial strain used.(29)
Biosynthesis of iron oxide nanoparticles
Fresh B. Subtilis were cultured on nutrient broth medium and after incubating for 1 day at 37°C, the culture was centrifuged at 6000 rpm for 12 minutes to harvest the freshly developed cells. Subsequent processing was done on the supernatant. collected and used for the preparation of nanoparticles.
B. Subtilis supernatant had been used to produce a 2 mm aqueous solution. FeCl3 was added by a ratio of 1:1 to a subtilis supernatant solution in 250 ml conical flask and the PH was adjusted to 9. The change in colour was indicated that the production of Fe3O4 nanoparticles. The resultant solution was incubated at 35°C for 48 hours and 2000 rpm at dark condition. By using a biotech spectrophotometer, UV Visible spectroscopy was carried out in the range of 200-100 nm is used to evaluate the production of Fe3O4 nanoparticles.(30)
Characterization technique:
UV-Visible spectra analysis:
The The reaction medium's UV-visible spectrum carried out to determine the reduction of pure Fe3+ ions after subsequent dilution of just a little of sample aliquots in distilled water at specific wavelength range of 330-450 nm. The analysis of UV-Visible spectrum was conducted employing a UV-Spectrophotometer.
Particle size determination:
In order to ascertain the mean distribution of particle size the pulverized iron oxide nanoparticles were analyzed using ZETA Sizer Nanoseries (Malvern instrument Nano ZS). The sample holder initially held a liquid dispersant that was made up of 25 milliliters of sodium hexametaphosphate and 500 milliliters of deionized water. Iron oxide nanoparticles were then dissolved in deionized water and ultrasonically treated.
This technique based on measurement of time dependant fluctuation in the intensity of scattered light, which occurs because particles are under Brownian motion.(7)
Fourier Transformed Infra-Red Spectroscopy:
IR spectroscopy is a useful method for determining and validating functional groups. molecular interactions within compounds. Molecular groups responsible for capping and functionalization of iron oxide nanoparticles were determined by using Fourier transformed infrared spectroscopic analysis. The FTIR spectra were recorded with a vertex broker transformed infrared spectrophotometer in the range between 4000-400 cm-1(31)
Scanning electron microscopy:
SEM is employed to observe the surface morphology of nanoparticles and provide information about particle size and shape. The morphology of prepared IONPs were identified by using SEM analysis. The shape of iron oxide nanoparticles were determined by result of SEM analysis. The SEM result indicates there are agglomeration of magnetite material and the uneven amount of particles.(32)
X-Ray Diffraction:
X-Ray diffraction is a technique which is utilized for determining the crystallinity level of this magnetite manufacturing product. The objective of XRD study was to characterize the crystal and examined the structure of the sample. In XRD study if the diffraction peak is larger then it indicates the greater number of crystals compounds in the sample. A panalytical system diffractometer operating at an accelerating voltage of 40 kV was utilized to perform crystallographic analysis on samples within the range of 10° to 70°.(17)
Screening of antimicrobial activity:
For screening of antimicrobial activity different pathogenic bacteria like Bacillus licheniformis (MTCC 7425) and Staphylococcus aureus (MTCC 1144), Vibreo cholera (MTCC 3904), Psuedomonas aeuroginosa (MTCC1034), Streptococcus aureus, and Ecoli. were used. These organisms were subcultured before use and kept at 4°C on nutrient agar slopes.(7) Magnetic iron oxide nanoparticles were designed to enhance the sensitivity and detection limit and to increase the performance of microbial application.(33)
Agar well diffusion method:
The iron oxide nanoparticles have ability to prevent growth of bacteria when applied to pathogenic bacteria. Using a sterile gel puncher (cork borer), a wells of 6mm diameter were punched over the agar plates the wells will be filled with 100 µL of nanoparticles (50 mg/mL) in sterile distilled water. The plates were incubated at 37°C for 24 hours. The antibacterial effect of the iron oxide nanoparticle extract was confirmed by the presence of a zone of inhibition around each well after the incubation period.(34) -(35)
Minimal Inhibitory Concentration (MIC):
Minimal inhibitory concentration test will be performed in 96 multi-well plates using liquid medium two-fold microdilutions as their basis. For this purpose 200 µl volumes of nutritional broth for (bacteria) or YGP ((for fungus) will be used to produce Serial binary dilutions of the tested chemical compound, ranging from 5 to 0.01 µg/mL, were prepared.Each well was inoculated with 20 µL of a 0.5 McFarland density inoculum. For twenty-four hours, the plates were incubated at 37°C. The resultant culture's absorbance at 620 nm was used to measure the antimicrobial effect; lower absorbance values indicated a stronger antibacterial effect.
Biomedical Applications of iron oxide nanoparticle:
MNPs for cancer therapy and diagnosis:
After considerable preclinical research and development, cancer nanomedicine has produced a number of products that have enhanced cancer care. However, others believes that cancer medicines because of small number of approved nanomedicine and their poor clinical outcomes. Even though anticancer medication, radiation and surgery can save lives when treating solid tumors, they still have a great deal of negative side effects. Magnetic nanoparticles are also used as a anticancer medicines from past 20 years. They emerged as a novel therapeutic approach to cancer therapy and magnetic resonance imaging. Because of their small size and they may easily interact with biomolecules with inside as well as outside of cell. This avoiding a magnetic hyperthermia, tissue tagging, targeted drug administration, and magneto-mechanical actuation of cell surface receptors. Only iron oxide nanoparticles can be used to treat humans with magnetic fluid hyperthermia, a cancer treatment that uses magnetic hysteresis heating. Early research revealed that nanoparticles have strong interaction with human immune system. Since then, efforts have been made to reduce the interaction in the development of cancer nanomedicines, with the goal of improving medication delivery to solid tumor.(36)
Biomedical applications in CNS:
Since iron oxide nanoparticles have the ability to cross the blood brain barrier (BBB) and deliver medications or therapeutics substance to brain they are particularly appropriate for applications involving the central nervous system. In order to increase the effectiveness of medication delivery, methods intended to treat CNS diseases, researchers are working to built tailored IONPs with improved physicochemical features. This ongoing refinement of IONPs underscores their versatility and adaptability for CNS applications.(10)
Functionalization for biomedical use:
Enhancing the biocompatibility and efficiency of INOPs for biomedical applications in the central nervous system requires their functionalization with different polymers or chemical compounds. Though surface property modification IONPs can be more optimized for targeted drug administration and CNS imaging applications by tailoring their interactions with biological system.(10)
MRI as a biomedical imaging technique:
A prominent biomedical imaging method used in diagnosing medicine is magnetic resonance imaging (MRI). Its main application is to produce a 2D and 3D images of human tissues. MRI operates on a principle of nuclear magnetic resonance imaging and requires a contrast agent (CAs) to enhance sensitivity for detecting various pathological processes. In MRI applications especially iron oxide nanoparticles, in particular magnetic nanoparticles are discussed about a contrast agent. When in a superparamagnetic, domain these nanoparticles are exhibit good performance as a contrast agent due to their T1 and T2 relaxation time and high proton magnetic alignment time, resulting in improved magnetic resonance imaging. When an external magnetic field is present, the gradients created by the magnetic nanoparticles enhance the quality of the images. Because of their exceptional colloidal stability and low toxicity in biological environments, Iron oxide nanoparticles are an excellent choice for contrast agents in MRI. Their performance in biological imaging applications can be enhanced by further modifying their surface to improve their qualities as a contrast agents.(37)
Hyperthermia:
Using heat to target kill and the tumor is known as a hyperthermia in cancer therapy, and magnetite nanoparticles are essential for this process. By heating tumor cells with a high frequency alternating magnetic field (AFM), these nanoparticles serve as a thermal seed. The target temperature range is 42- 45°c which precisely causes the malignant tissue to undergoes cell apoptosis while leaving healthy cells unharmed. Thermo-ablation happened at temperature higher than 48°c, which cause tissue necrosis. Anisotropy, saturation magnetization values, magnetic field parameters and particle size are some of the variables that show how effectively magnetite nanoparticles dissipate during hyperthermia treatment. Important factors that influence how magnetite nanoparticles disperse heat are specific absorption rate (SAP) and specific loss power. In order to treat hyperthermia, Brownian and Neel relaxation processes are crucial since they enhance local heating. The most common method of treating magnetically generated hyperthermia currently is local hyperthermia therapy, which focuses on particular body parts. This approach is frequently used in cancer treatment utilizing hyperthermia because it enables precise targeting of tumor sites. The specific absorption rate (SAR) for individual Fe3O4 nanoparticles and their clusters is determined using hyperthermia tests, this is because of collective magnetic behavior that arises from several Fe3O4 nanoparticles resulting from each cluster. (37)-(10)
Biosensors:
The integration of iron oxide nanoparticles into biosensors plays a crucial role in enhancing the detection of biomolecules and pathogens with high sensitivity and specificity. Biosensors are analytical devices that integrate a biological sensing element with a physicochemical detector to convert a biological response into a measurable signal. By incorporating iron oxide nanoparticles into biosensors, several advantages are realized:
- High sensitivity: iron oxide nanoparticles possess unique properties that can be exploited to enhanced the sensitivity of biosensors. Their interaction with target biomolecules or pathogens can lead to measurable changes in magnetic signals, allowing for the detection of even low concentration of the analyte.
- Specificity: To selectively bind to particular biomolecules or pathogens of interest, iron oxide nanoparticles can be functionalized or modified. By ensuring that the biosensor react only to the target analyte, this specificity lowers the possibility of false positive result and improves detection accuracy.
- Rapid detection: iron oxide nanoparticles are used in biosensors to quickly detect biological material. Fast and effective transduction is made possible by the magnetic characteristics of the nanoparticles, which enables prompt identification of the target analyte in the sample.
The integration IONPs into biosensors enhances the performances of these analytical devices by improving sensitivity and specificity.(36)
Tissue Engineering and regenerative medicine
Tissue engineering and regenerative medicine are interdisciplinary fields that aims to restores, maintain or improve the function of damaged or diseased tissue or organs through the use of biological and engineering principle. Iron oxide nanoparticles play a significant role in these areas, offering unique advantages for tissue engineering and regenerative medicine application.
Cell labelling and tracking: Researchers can observe and follow the activities of cells in real time by using iron oxide nanoparticles to label cells. Researchers are able to monitor important processes like mitigation, proliferation, and differentiation within tissues or organs by attaching these nanoparticles to cells. This labelling method contributes to our understanding of mechanism behind tissue regeneration by offering insightful information about cellular responses and dynamic.
Drug delivery: Iron oxide nanoparticles act as a effective contrast agent in imaging techniques such as MRI. Iron oxide nanoparticles labeling allows researchers to track the position and migration of transplanted cells throughout the body in non-invasive manner. This tracking capacity is essential for determining the effectiveness of cell-based treatments, and analyzing the integration of transplanted cells into host tissue and gaining insights into the progress of tissue regeneration proceess.
Tissue regeneration: There are several benefits from incorporating iron oxide nanoparticles to the matrices of scaffolds used in tissue engineering. These nanoparticles can enhance the mechanical properties of scaffolds, providing structural support, and promote cell adhesion and growth. Furthermore, adding bioactive compounds to iron oxide nanoparticles allows for the stimulation of particular cellular responses that support tissue regeneration. Through the utilization of iron oxide nanoparticles, distinctive characteristics, can develop advanced biomaterials that promote tissue restoration and renewal providing promising prospects for application in regenerative medicine.(38)
Barriers and consideration in iron oxide-based drug delivery:
Several physiological barriers such as vascular epithelium prevents the SPIONs from reaching their targeting cells. In cancer treatment drug delivery system can help the medication to accumulate in the targeted area but it should be recognized that a very tiny portion of intravenously administered dose is actually deposited the tumor. Unlike other drug delivery systems, Superparamagnetic Iron Oxide Nanoparticles have the advantage of magnetic properties that can be used to apply a localized external magnetic field to increase targeting efficiency. The blood brain barrier is permeable only to particles with the appropriate physical and chemical properties and sufficiently small particle size.(39) Iron oxide nanoparticles are administered intravenously, where they are distributed throughout the body’s peripheral tissues and organs. Serum proteins have the ability to absorb or opsonize the nanoparticles. Changes in nanoparticles size and surface chemistry have significant effect on their in vivo kinetic characteristics. One of the most common method for enhancing the pharmacokinetic behavior of nanoparticles is molecular attachment o polyethylene glycol to the particle surface group.(40) The first step in magnetically-guided drug targeting (MGDT) is to make drug immobile within the magnetic nanoparticles. The medication or carrier mixture is then administered intravenously or intra-arterially into the patient. In order to guide and concentrate the complex to specified areas, a rare earth permagnet produces a high gradient external magnetic field. After the complex has been accumulated in vivo at the target, the therapeutic drug is released from the magnetic nanoparticles either by changes in physiological factors such as temperature, osmolarity, and pH, or by enzyme activity. As a result the medication is more frequently absorbed by tumor cells at the designated sites.(41)
Future prospects of iron oxide nanoparticles:
Because of their magnetic properties iron oxide nanoparticles have been successfully applied in different areas of biomedicine including MRI scanning for detection of brain disorder, cardiovascular disorder, liver disease. The production of iron oxide nanoparticles under different conditions still requires a investigation and complete control over the shape and size distribution of IONPs remains a difficulty(23)
Although magnetic hyperthermia and medication drug delivery through MNPs have been widely studied in animal models it remains extremely difficult to translate this research into effective treatment for human patients. The effect of MNPs size shape and replacement and dosage needs to be studied before they are used extensively. Toxicity issues of MNPs needs to be addressed and resolved. To design and fabricate MNPs for different application in diverse fields creative and constructive research is necessary to overcome obstacles.(42) Multifunctional IONPs were being developed for phototherapy, chemotherapy, targeting diagnosis and nanocarrier therapies in order to further address the challenges associated with cancer and multidrug resistance diseases. Specially in the fields of disease diagnosis, early detection and cellular and deep tissue imaging, the future of iron oxide nanoparticles, the future of IONPs in biomedical application is quite promising.(36) Because they are easily implanted into the body and easily removed after their purpose is achieved, IONP based MOFs (metal-organic frameworks) may be interesting candidates in biomedical applications. Their interconnected porosity from the detection perspective enables them to target (pathogens/ viruses/ biomolecules) in an optimal and extensive manner. Apart from these previously mentioned innovative uses, it is essential to create marketable solutions based in IONPs for diverse domains including healthcare, electronics and catalysis among others.(43)
CONCLUSION:
The conclusion of this article emphasizes the importance of achieving controlled and reproducible synthesis of IONPs to unable successful application in various field. Controlling the size, shape, characteristics and phase purity of IONPs requires an understanding of their formation mechanism. In this review article a wide range of IONPs applications are covered such as medication administration, tissue repair MRI, biosensing, hyperthermia, and environmental remediation. The review also discusses the different ways that the inorganic nanoparticles can be synthesized, including microwave synthesis, pyrolysis, coprecipitation, microemulsion, sosnochemical synthesis techniques. It also mentioned the significance of using multiple characterization technique to confirm the oxide phase of iron in nanoparticle synthesis. In conclusion the article underscores the ongoing research efforts aimed at advancing the understanding of IONPs, optimizing synthesis techniques and exploring their potential in various applications. Iron oxide nanoparticles are employed as MR contrast agent for gastrointestinal and vascular imaging as well as imaging of liver tumors, metastases, and lymph node metastasis.
The demand for high quality nanoparticles had led to a wide range of production methods, particularly in medical and industrial field. The article highlights the impact of different preparation methods on the chemical effectiveness of Fe3O4 nanoparticles and emphasizes the continuous advancements in nanoscience to improve quality of life. The study showed that in magnetic hyperthermia, larger ferromagnetic particles perform better at heating than superparamagnetic particles at particular magnetic field intensities additionally nanoparticles with a broader size distribution showed higher heating power in the transition range between superparamagnetism and ferrimagnetism. When these iron oxide nanoparticles are customized to particular magnetic field values, this result indicates potential beneficial feature for their use in magnetic hyperthermia.
REFERENCE
- Majidi S, Sehrig FZ, Farkhani SM, Goloujeh MS, Akbarzadeh A. Current methods for synthesis of magnetic nanoparticles. Artif Cells, Nanomedicine Biotechnol. 2016;44(2):722–34.
- Bolden NW, Rangari VK, Jeelani S, Boyoglu S, Singh SR. Synthesis and Evaluation of Magnetic Nanoparticles for Biomedical Applications. J Nanoparticles [Internet]. 2013 Jan 15;2013:1–9. Available from: https://www.hindawi.com/journals/jnp/2013/370812/
- Lodhia J, Mandarano G, Ferris NJ, Eu P, Cowell SF. Development and use of iron oxide nanoparticles (Part 1): Synthesis of iron oxide nanoparticles for MRI. Biomed Imaging Interv J. 2010;6(2).
- Prodan AM, Iconaru SL, Chifiriuc CM, Bleotu C, Ciobanu CS, Motelica-Heino M, et al. Magnetic properties and biological activity evaluation of iron oxide nanoparticles. J Nanomater. 2013;2013.
- Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int J Nanomedicine. 2017;12:1227–49.
- Hashem F, Nasr M, Ahmed Y. Preparation and Evaluation of Iron Oxide Nanoparticles for Treatment of Iron Deficiency Anemia. Int J Pharm Pharm Sci. 2018;10(1):142.
- Behera SS, Patra JK, Pramanik K, Panda N, Thatoi H. Characterization and Evaluation of Antibacterial Activities of Chemically Synthesized Iron Oxide Nanoparticles. World J Nano Sci Eng. 2012;02(04):196–200.
- Anik MI, Hossain MK, Hossain I, Mahfuz AMUB, Rahman MT, Ahmed I. Recent progress of magnetic nanoparticles in biomedical applications: A review. Nano Sel. 2021;2(6):1146–86.
- Hemeg HA. An Overview of Iron Oxide ( Fe 3 O 4 ) Nanoparticles?: and Anticancer Applications. 2022;
- Ansari SAMK, Ficiarà E, Ruffinatti FA, Stura I, Argenziano M, Abollino O, et al. Magnetic iron oxide nanoparticles: Synthesis, characterization and functionalization for biomedical applications in the Central Nervous System. Materials (Basel). 2019;12(3).
- Baabu PRS, Kumar HK, Gumpu MB, Babu K J, Kulandaisamy AJ, Rayappan JBB. Iron Oxide Nanoparticles: A Review on the Province of Its Compounds, Properties and Biological Applications. Materials (Basel). 2023;16(1).
- Dubuisson J, Fehlmann A, Petignat P. Management of presumed benign giant ovarian cysts: A minimally invasive technique using the alexis laparoscopic system. J Minim Invasive Gynecol. 2015;22(4):540.
- Ali A, Zafar H, Zia M, ul Haq I, Phull AR, Ali JS, et al. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol Sci Appl. 2016;9:49–67.
- Wu W, He Q, Jiang C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res Lett. 2008;3(11):397–415.
- Arias LS, Pessan JP, Vieira APM, De Lima TMT, Delbem ACB, Monteiro DR. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics. 2018;7(2).
- Hui BH, Salimi MN. Production of Iron Oxide Nanoparticles by Co-Precipitation method with Optimization Studies of Processing Temperature, pH and Stirring Rate. IOP Conf Ser Mater Sci Eng. 2020;743(1).
- Fadli A, Komalasari, Adnan A, Iwantono, Rahimah, Addabsi AS. Synthesis of Magnetite Nanoparticles via Co-precipitation Method. IOP Conf Ser Mater Sci Eng. 2019;622(1).
- Campos EA, Pinto DVBS, de Oliveira JIS, Mattos E da C, Dutra R de CL. Synthesis, characterization and applications of iron oxide nanoparticles - A short review. J Aerosp Technol Manag. 2015;7(3):267–76.
- Asab G, Zereffa EA, Abdo Seghne T. Synthesis of Silica-Coated Fe3O4 Nanoparticles by Microemulsion Method: Characterization and Evaluation of Antimicrobial Activity. Int J Biomater. 2020;2020.
- Samrot A V., Sahithya CS, Selvarani A J, Purayil SK, Ponnaiah P. A review on synthesis, characterization and potential biological applications of superparamagnetic iron oxide nanoparticles. Curr Res Green Sustain Chem [Internet]. 2021;4:100042. Available from: https://doi.org/10.1016/j.crgsc.2020.100042
- Hassanjani-Roshan A, Vaezi MR, Shokuhfar A, Rajabali Z. Synthesis of iron oxide nanoparticles via sonochemical method and their characterization. Particuology [Internet]. 2011;9(1):95–9. Available from: http://dx.doi.org/10.1016/j.partic.2010.05.013
- Kritika N, Roy I. Therapeutic applications of magnetic nanoparticles: recent advances. Mater Adv. 2022;3(20):7425–44.
- Wu W, Wu Z, Yu T, Jiang C, Kim WS. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater. 2015;16(2).
- Khashan KS, Sulaiman GM, Mahdi R. Preparation of iron oxide nanoparticles-decorated carbon nanotube using laser ablation in liquid and their antimicrobial activity. Artif Cells, Nanomedicine Biotechnol [Internet]. 2017;45(8):1699–709. Available from: http://dx.doi.org/10.1080/21691401.2017.1282498
- Ismail RA, Sulaiman GM, Abdulrahman SA, Marzoog TR. Antibacterial activity of magnetic iron oxide nanoparticles synthesized by laser ablation in liquid. Mater Sci Eng C [Internet]. 2015;53:286–97. Available from: http://dx.doi.org/10.1016/j.msec.2015.04.047
- Chokriwal A, Sharma M, Singh A. Green Nanoparticle Synthesis and Their Applications. Int J Pharmacogn [Internet]. 2015;2(3):110–5. Available from: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.2
- Mahdavi M, Namvar F, Ahmad M Bin, Mohamad R. Green biosynthesis and characterization of magnetic iron oxide (Fe 3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules. 2013;18(5):5954–64.
- Alam T, Khan RAA, Ali A, Sher H, Ullah Z, Ali M. Biogenic synthesis of iron oxide nanoparticles via Skimmia laureola and their antibacterial efficacy against bacterial wilt pathogen Ralstonia solanacearum. Mater Sci Eng C [Internet]. 2019;98(April 2018):101–8. Available from: https://doi.org/10.1016/j.msec.2018.12.117
- Nadeem M, Khan R, Shah N, Bangash IR, Abbasi BH, Hano C, et al. A review of microbial mediated iron nanoparticles (IONPs) and its biomedical applications. Nanomaterials. 2022;12(1).
- Jubran AS, Al-Zamely OM, Al-Ammar MH. A study of iron oxide nanoparticles synthesis by using bacteria. Int J Pharm Qual Assur. 2020;11(1):88–92.
- Eid MM. Spectroscopic Characterization of Iron Oxide Nanoparticles Functionalized with Chitosan Biosynthesis by a Clean one Pot Method. 2015;18–22.
- Prodan AM, Iconaru SL, Ciobanu CS, Chifiriuc MC, Stoicea M, Predoi D. Iron oxide magnetic nanoparticles: Characterization and toxicity evaluation by in vitro and in vivo assays. J Nanomater. 2013;2013.
- Abu-huwaij R, Al-assaf SF, Mousli F, Kutkut MS, Al-bashtawi A. Perceptive Review on Properties of Iron Oxide Nanoparticles and Their Antimicrobial and Anticancer Activity. 2020;11(8):418–31.
- Thukkaram M, Sitaram S, Kannaiyan SK, Subbiahdoss G. Antibacterial efficacy of iron-oxide nanoparticles against biofilms on different biomaterial surfaces. Int J Biomater. 2014;2014.
- Sangwan P, Kumar H. SYNTHESIS CHARACTERIZATION AND ANTIBACTERIAL ACTIVITY OF IRON OXIDE NANOPARTICLES AGAINST STAPHYLOCOCCUS EPIDERMIDIS. 2020;13(9):13–6.
- Schneider MGM, Martín MJ, Otarola J, Vakarelska E, Simeonov V, Lassalle V, et al. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics. 2022;14(1).
- Ganapathe LS, Mohamed MA, Yunus RM, Berhanuddin DD. Magnetite (Fe3O4) nanoparticles in biomedical application: From synthesis to surface functionalisation. Magnetochemistry. 2020;6(4):1–35.
- Elahi N, Rizwan M. Progress and prospects of magnetic iron oxide nanoparticles in biomedical applications: A review. Artif Organs. 2021;45(11):1272–99.
- Laurent S, Saei AA, Behzadi S, Panahifar A, Mahmoudi M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: Opportunities and challenges. Expert Opin Drug Deliv. 2014;11(9):1449–70.
- Longmire M, Choyke PL. C835B99424409B80Acbbd3C6D1C678D2.Pdf. 2008;3:703–17.
- Estelrich J, Escribano E, Queralt J, Busquets MA. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int J Mol Sci. 2015;16(4):8070–101.
- Vallabani NVS, Singh S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech [Internet]. 2018;8(6):1–23. Available from: http://dx.doi.org/10.1007/s13205-018-1286-z
- Ajinkya N, Yu X, Kaithal P, Luo H, Somani P, Ramakrishna S. Magnetic iron oxide nanoparticle (Ionp) synthesis to applications: Present and future. Materials (Basel). 2020;13(20):1–35.


 Gajanan Sormare
Gajanan Sormare


 10.5281/zenodo.13192998
10.5281/zenodo.13192998