Abstract
Attempts to develop a robust RP-HPLC method for estimating Pitolisant in tablet form were made using an Agilent (S.K) Gradient System with a UV Detector. The C18 column employed measured 250 mm x 4.6 mm with a particle size of 5 ?m. The mobile phase consisted of methanol and 0.1% water with OPA in a 45%:55% v/v ratio. Detection was carried out at a wavelength of 266 nm with a flow rate of 0.8 ml/min. The retention times for Pitolisant were found to be 3.934 and 4.627 minutes. The method was validated in accordance with ICH guidelines, demonstrating linearity over the range of 5-25 ?g/ml, and precision with a value of 98.95%. The method's limit of detection (LOD) was determined to be 0.1004 ?g/ml, and the limit of quantitation (LOQ) was 0.304 ?g/ml. Accuracy and recovery percentages were within the 98-102% range. Additionally, the system's robustness met the criteria specified by the ICH guidelines. Therefore, the developed method is simple, accurate, precise, economical, and reproducible, making it suitable for routine quality control analysis of Pitolisant in both bulk drugs and formulations.
Keywords
Pitolisant, Mobile phase, RP-HPLC etc.
Introduction
Pitolisant is an innovative pharmaceutical agent primarily used to manage narcolepsy, a neurological disorder that causes excessive daytime sleepiness and sudden episodes of muscle weakness, known as cataplexy. It operates as a histamine H3 receptor antagonist/inverse agonist, effectively increasing histamine levels in the brain. Histamine is a critical neurotransmitter that promotes wakefulness and regulates the sleep-wake cycle. By modulating histaminergic activity, Pitolisant enhances wakefulness and mitigates the symptoms of cataplexy.1-3 Pitolisant was first approved by the European Medicines Agency (EMA) in 2016 and later by the U.S. Food and Drug Administration (FDA) in 2019. It offers a novel mechanism of action compared to traditional stimulant medications used for narcolepsy, providing an alternative for patients who may not respond adequately to or tolerate conventional treatments. Its approval marked a significant advancement in the therapeutic landscape for narcolepsy, addressing unmet needs in the management of this debilitating condition. The drug is marketed under the trade name Wakix and is known for its favorable safety and efficacy profile, making it a valuable addition to the treatment options available for narcolepsy patients. Its development and approval have provided healthcare professionals with a new tool to improve the quality of life for individuals affected by narcolepsy, highlighting the ongoing advancements in sleep disorder therapeutics.4-5

Figure 1:Structure of Pitolisant (1-[3-[3-(4-chlorophenyl) Propoxy] Propyl] Piperidine.)
The development and validation of an analytical method for Pitolisant using Reverse Phase High-Performance Liquid Chromatography (RP-HPLC) is essential for ensuring the quality and efficacy of this drug in tablet form. The RP-HPLC method, utilizing an Agilent Gradient System with a UV detector, employs a C18 column (250 mm x 4.6 mm, 5 ?m particle size) and a mobile phase comprising methanol and 0.1% water with ortho-phosphoric acid (45:55 v/v). Detection is performed at a wavelength of 266 nm with a flow rate of 0.8 ml/min.6-7 The RP-HPLC method's simplicity, accuracy, precision, and cost-effectiveness make it highly suitable for routine quality control of Pitolisant in both bulk drug and formulated tablet forms, ensuring consistent therapeutic performance and safety.
Experimental:
Experimental Work for Analytical Method Development and Validation of Pitolisant Drug Using RP-HPLC Method.8-11
Materials and Reagents:
- Pitolisant standard: Obtained from a certified supplier.
- Methanol: HPLC grade.
- Ortho-Phosphoric Acid (OPA): Analytical grade.
- Water: HPLC grade, filtered through a 0.45 ?m membrane filter.
- Tablet formulation: Containing Pitolisant.
- Instrumentation:
HPLC System:
Agilent (S.K) Gradient System,Detector: UV detector, Column: C18 column (250 mm x 4.6 mm, 5 ?m particle size), Data Analysis Software: For chromatogram analysis.
Chromatographic Conditions:
Mobile Phase:
Methanol and 0.1% water with OPA (45:55 v/v), Flow Rate: 0.8 ml/min, Detection Wavelength: 266 nm, Injection Volume: 20 ?l, Column Temperature:
Ambient.
Preparation of Standard and Sample Solutions:
- Standard Solution:Accurately weigh and dissolve an appropriate amount of Pitolisant standard in methanol to prepare a stock solution.Further dilute the stock solution with the mobile phase to obtain working standard solutions in the range of 5-25 ?g/ml.
- Sample Solution:Crush tablets to a fine powder.Weigh an equivalent amount of Pitolisant from the tablet powder and dissolve in methanol.Sonicate for complete dissolution and filter through a 0.45 ?m membrane filter.Dilute appropriately with the mobile phase to match the concentration range of the standard solutions.
Method Development:12-15
Optimization of Chromatographic Conditions:Various mobile phase compositions, flow rates, and detection wavelengths were tested.The final optimized conditions were chosen based on peak resolution, retention time, and overall chromatographic performance.
Method Validation:
The method was validated according to ICH guidelines for the following parameters:
- Linearity:
Prepare standard solutions at concentrations of 5, 10, 15, 20, and 25 ?g/ml.Plot the peak area versus concentration and calculate the correlation coefficient.
- Precision:
Evaluate intra-day and inter-day precision by analyzing standard solutions atdifferent concentrations multiple times within the same day and on different. Calculate the % RSD (Relative Standard Deviation).
- Accuracy:
Perform recovery studies by spiking known amounts of Pitolisant into the sample matrix.Calculate the percentage recovery.
- LOD and LOQ:
Determine the LOD and LOQ based on the standard deviation of the response and the slope of the calibration curve.
- Robustness:
Assess the effect of small deliberate changes in chromatographic conditions (e.g., flow rate, mobile phase composition) on method performance.
- System Suitability:
Evaluate system suitability parameters such as theoretical plates, tailing factor, and retention time by analyzing standard solutions.15-20
RESULTS AND DISCUSSION:
Preliminary studies on Pitolisant:
Melting point: The procured reference standard of Pitolisant was found to reported melt in the range of 190-1930C respectively.Solubility:The drug was found to be freely soluble in DMSO, Acetone, ethanol, Methanol, DMF, water and insoluble in Cyclohexane.
UV Spectroscopy:
UV absorption of 20 µg/mL solution of Pitolisant in methanol was generated and absorbance was taken in the range of 200-400 nm. 266 nm ?max.

Figure 2: UV spectrum of Pitolisant in methanol.
Analytical of Method Validation:
- Linearity:
From Pitolisant standard stock solution, different working standard solution (5- 25?g/ml) were prepared in mobile phase 20 ?L of sample solution was injected into the chromatographic system using mixed volume loop injector. Chromatograms were recorded. The area for each concentration was recorded (Table No. 1). The Calibration curves are shown in [Fig. No.3 and 4].

Figure 3: Thus, from the above, it has been observed that, using mobile phase of Methanol+0.1% (OPA) water,(50:50 % v/v),PH2.7.,266 nm, Flow rate 0.8 ml gave adequate retention at 4.627 min with good peak shape (Theoretical plates Pitolisant 8617).

Figure 4: Chromatogram of overlay linearity.
Table 1: Result of Linearity range:


Figure 5: Calibration curve of Pitolisant.
- Accuracy:
Recovery studies were performed to validate the accuracy of developed method. To preanalyzed Tablet solution, a definite concentration of standard drug (80%, 100%, and 120%) was added and then its recovery was analyzed (Table No.39). Statistical validation of recovery studies shown in (Table No. 40).Accuracy 80%

Figure 6: Chromatogram of overlay accuracy 80%, 100% and 120%.
Table 2: Result of recovery data form Pitolisant:

Table 3: Statistical validation of recovery studies of Pitolisant:

- System suitability parameters :( Repeatability):
To ascertain the resolution and reproducibility of the proposed chromatographic system for estimation of Pitolisant system suitability parameters were studied. The result shown in below (Fig. No. Table No.33)
Figure 7: System Suitability test after 1 hr and 2hrs.

Chromatogram System Suitability Results was found to be mean of five determination were also satisfactory ,hence the analytical method would be concluded that result shown in (Table No :4) Repeatability studies Pitolisant was found to be ,The %RSD was less than 2, which shows high percentage amount found in between 98% to 102% indicates the analytical method that concluded .
Table 4: Repeatability studies of Pitolisant:

- Precision:
The method was established by analyzing various replicates standards of Pitolisant. All the solution was analyzed thrice in order to record any intra-day & inter-day variation in the result that concluded. The result obtained for intraday is shown in (Table No. 5) respectively.
Table 5: Result of Intraday and Inter day Precision for Pitolisant:

- Robustness:
The Robustness of a method is its ability to remain unaffected by small deliberate changes in parameters. To evaluate the robustness of the proposed method, small but deliberate variations in the optimized method parameters were done. The effect of changes in mobile phase composition and flow rate, wavelength on retention time and tailing factor of drug peak was studied. The mobile phase composition was changed in (±1 ml/min-1) proportion and the flow rate was varied by of optimized chromatographic condition. The results of robustness studies are shown in (Table No. 6).Robustness parameters were also found satisfactory; hence the analytical method would be concluded.
Table 6: Result of Robustness study of Pitolisant:
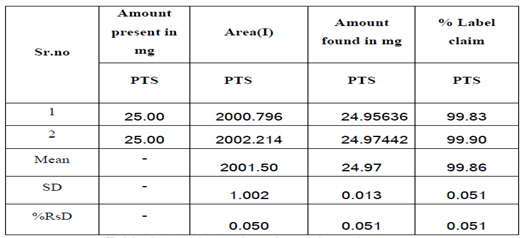
- Limit Detection:
The LOD is the lowest limit that can be detected. Based on the S.D. deviation of the response and the slope the limit of detection (LOD) may be expressed as,
LOD = 3.3 (SD)/S
= 3.3 X 2.39/ 78.45
= 0.1004
Where, SD = Standard deviation of Y intercept, S = Slope
The LOD of Pitolisant was found to be 0.1004 (?g/mL) analytical methods that concluded.
- Limit Quantification
The LOQ is the lowest concentration that can be quantitatively measured. Based on the S.D. deviation of the response and the slope, the quantitation limit (LOQ) may be expressed as:
LOQ = 10 (SD)/ S
=10 X 2.39/ 78.4
= 0.3043
Where, SD = Standard deviation Y intercept, S = Slope
The LOQ of Pitolisant was found to be 0.3043(?g/mL) analytical methods that concluded.
Analysis of Tablet formulation
Weigh 20 Pitolisant Tablets and calculated the average weigh, accurately weigh and transfer the sample equivalent to 18.15 mg Pitolisant into 10 ml volumetric flask. Add about 10 ml of diluents and sonicated to dissolve it completely and make volume up to the mark with diluents. Mix well and filter through 0.45 ?m filter. Further pipette 0.5 ml of the above stock solution into a 10 ml volumetric flask and dilute up to the mark with diluents. (25 ?g/ml). The simple chromatogram of test Pitolisant Shown in the amounts of Pitolisant per Tablet was calculated by extrapolating the value of area from the calibration curve. Analysis procedure was repeated five times with Tablet formulation. Tablet Assay for % Label claim for %RSD Calculated, Result was shown in (Table No. 7).
Brand Name : Wakix 4.45 mg (Cipla)
Total weight of 20 Tab Powder wt. = 319.6 gms
Avg Powder Weight = 15.98 gms./Tab
Eq.Wt for 5 mg = 5 x 15.98 / 4.4= 18.15 mg
Take 18.15 mgs in l0 water i.e = 500 ?g/ml Pitolisant
Analysis of marketed formulation were also % Label Claim was found to be 98- 102% Satisfactory are concluded.
Table 7: Analysis of Marketed formulation:

Tablet Assay for % Label Claim: Tablet Assay for % Label claim for was also was found to be 100.99% and %RSD are less than 2 satisfactory result that concluded.
CONCLUSION:
The developed RP-HPLC method is simple, accurate, precise, economical, and robust, making it highly suitable for routine quality control analysis of Pitolisant in both bulk drug and tablet formulations.
CONFLICT OF INTEREST:
Writers don’t have any conflict of interest
ACKNOWLEDGEMENT:
I would like to express my heartfelt gratitude to ARACOP, including all the teaching and non-teaching staff, and to Principal Dr. R.D. Wagh for their unwavering support throughout this research. A special thanks to my research guide, Dr. V. L. Badgujar, for his invaluable guidance and encouragement. Without their collective assistance, this work would not have been possible.
REFERENCES:
- Anjeneyulu, Y.; Chandasekhar, K.; Manikam, V.A. Textbook of Analytical Chemistry, Pharm Book Syndicate,2005, pp3.
- Christan, D.G. Analytical Chemistry, 5th ed.; John Welly and sons INC; pp.1-3
- Skoog, D.A, Leqary, J.J Principal Instrument Analysis ,54th ed, Thomson Asia Pvt Ltd.; Singapore ,2004, pp3-8.
- Settal, F.A. Handbook of Instrumental Techniques of Analytical Chemistry, 1st ed;.2004, pp,19-21.
- Swartz,M.E.; Krill, I.S. Analytical Method Development and validation India ed.: MarcelDekker; New York,2009, pp-27-54.
- World Health Organization Stability Testing of Active Substances and Pharmaceutical Product,2006, pp.1-33
- Willard, H.H, Merritt, L.L, Dean, J.A,Settle.F.A. AInstrumental Method of Analysis,7th ed;CBS Publishers and Distributors: New Delhi,1986, pp.1-3
- Brawn R.D, Introduction to Instrumental Analysis,1st ed.: Pharma Book Syndicate; Hyderabad,2006, pp.1-7
- Sharma, Y.R. Elementary Organic Spectroscopy: Principle and Chemical Applications, 4th ed.; S. Chand and Company Limited: New Delhi, 2007. Pp.11-12.
- Sharma, B.K. Instrumental Method of Chemical Analysis, 21h ed., Goel Publishing Housing: 2002, pp. 3.
- Conner’s, A.K. Textbook of Pharmaceutical Analysis, 3rd ed.; A Wiley- Intersciences Publication: 1999, pp. 616.
- Beckett, A.H.; Stenlake, J.B.: Practical Pharmaceutical Chemistry, 4h ed., Part II, CBS Publications and Distributors: New Delhi, 1997, pp. 275-277.
- Willard, H.H;Metritt, L.L:Dean, J.A.; Settal, F.A. Instrumental methods of Analysis, 7h ed.: CBS Publishers and Distributers: 1986, pp. I18.
- Katz, E. Quantitative Analysis Using Chromatographic Techniques, Wiley India Pvt. Ltd.: 2009, pp. 193 -211.
- Cazes, J.; Scott, R.P.W. Chromatography Theory, Marcel Dekker INC: New York, 2002, pp. 3-17.
- Wall, P.E. Thin Layer Chromatography (A Modern Practical Approach), VWR International ltd.: Poole, Dorset, 2005, pp.1-3.
- Katz, E. Quantitative Analysis Using Chromatographic Techniques, Wiley India Pvt. Ltd.: 2009, pp. 193 -211.
- Heftman, E.Chromatography- Fundamentals & Applications of Chromatography and Related Differential Migration Methods, 6th ed.; Elsevier:Amstered, 2004, pp.253-291.
- Conners, K.A. A Textbook of Pharmaceutical Analysis, 3rd ed.; A Wiley-Intersciences Publication:1999, pp.373-374.
- Stahl, E. Thin Layer Chromatography: A laboratory Handbook, 2nd ed.;Springer International: pp. 1


 Dipak R. Borase *
Dipak R. Borase *
 Vilas. L. Badgujar
Vilas. L. Badgujar











 10.5281/zenodo.13273666
10.5281/zenodo.13273666