Abstract
Oxiconazole nitrate is used to treat fungal infections. It has a restrictive pharmaceutical role because of its poor aqueous solubility, which reduces the bioavailability of the drug. The present investigation was carried out to formulate and evaluate the Oxiconazole nitrate loaded spanlastic gel. Spanlastic was prepared by Ethanol injection method using Span 60 and Tween 80 as non-ionic surfactant and edge activator. The formulation was evaluated for various parameters like, particle size, transmittance, entrapment efficiency, surface charge and in vitro release studies. The formulation showed the lesser particle size, transmittance of 72.30±0.01%, higher entrapment efficiency of 85.90±0.67% and dissolution of 85.02±0.26%. The vesicles were found to be spherical and tiny in size using optical microscopy. The spanlastic formulation was incorporated into gel prepared using 1% w/w Carbopol 934 and evaluated for various parameters. The prepared gel was homogenous with 90.82±4.14% drug content and % cumulative drug release of 80.10±0.69% at the end of 8hrs. Stability studies indicated that oxiconazole nitrate loaded spanlastic gel was stable. Thus it can be concluded that the developed formulation would be a promising delivery system with improved efficacy, controlled release and patient compliance.
Keywords
Oxiconazole nitrate, Spanlastic, Ethanol injection, Antifungal, Gel.
Introduction
Drug delivery refers to approaches, technologies and systems for transporting a pharmaceutical compound in the body as required to safely achieving its desired therapeutic effect, in the past few decades, considerable attention has been focusing on the development of novel drug delivery system (NDDS). The novel drug delivery system is referred as a rebirth system as it is the most suitable and approachable in developing the delivery system which improves the therapeutic efficacy of new as well as pre-existing drugs thus provides controlled and sustained drug delivery to the specific site and meets the real and appropriate drug demand of the body.1 Pharmaceutical nanotechnology, the most recent addition to the pharmaceutical sciences, offers new capabilities, chances, and instruments that are anticipated to have a big impact on treatment and disease diagnostics.2In the field of nanotechnology, novel vesicular drug delivery systems have advanced significantly. Spanlastics are a novel surfactant based elastic vesicular drug delivery system, which entraps the drug in the core cavity in the form of the bilayer. These are amphiphilic in nature, in which the drug is encapsulated in a vesicle which is made by non-ionic surfactant. They are shown to be chemically more stable.3 These Spanlastics can be incorporated onto topical formulations for prolonged release and enhanced skin retention, thus reducing the variability of drug absorption, improving the patient compliance and have improved several drawbacks of the conventional dosage form.4 The Oxiconazole nitrate is a broad -spectrum imidazole antifungal agent5 belongs to BCS class II having low aqueous solubility and high permeability. Due to its poor solubility in water it leads to low dissolution rate and thus poor therapeutic efficacy if given orally. Low systemic absorption can be overcome by its topical delivery by incorporating drug in spanlastic based gel. Spanlastic will deliver drug by enhancing the solubility of the drug and entrapment increases skin permeability, and incorporation into gel provides prolong retention time due to viscosity of the formulation and better patient compliance.
MATERIALS AND METHODS
Materials:
Oxiconazole nitrate was supplied from Yarrow Chem Products, Mumbai .All other excipients and solvents used were of the analytical pharmaceutical grade.
Methods:
Pre-formulation studies of drug:
Determination of Standard calibration curve of oxiconazole nitrate8:
The solution containing 10 µg/ml concentrationof oxiconazole was prepared and scanned over range of 200-400nm against phosphate buffer of pH 6.8 as blank using double beam U V spectrophotometer and absorbance was measured at screened wavelength by UV spectrophotometer. The calibration curve was plotted against concentration versus absorbance.
Drug- excipient compatibility study9:
FT-IR spectra of pure oxiconazole nitrate, and prepared spanlastic formulation were obtained using FT-IR spectrometer. FT-IR spectra were recorded within the spectral region of 4000 and 400 cm?1 using the instrument Perkin Elmer FTIR.
Preparation of oxiconazole nitrate spanlastic by ethanol injection method10:
Spanlastics was prepared by ethanol injection method. Tween 80 was accurately weighed and dissolved in 40mL of distilled water heated to the temperature of 70?C. Span 60 were accurately weighed and dissolved in 10 mL of ethanol and Oxiconazole nitrate was weighed and added to the span solution. Span solution was then injected using a 30- gauze syringe at a fixed rate of 1 mL/min to the pre-heated tween solution which was continuously being stirrer on a magnetic stirrer at 500 rpm, Stirring was continued for 30 minutes at 70?C. The solvent will be evaporated by heating to obtain drug-loaded spanlastic vesicles. The resulting vesicles at room temperature produced a homogenous milky white suspension of Spanlastics free of visible particles. It will be subjected to probe sonication by transferring the colloidal suspension onto a beaker. Finally, the spanlastic suspension will be stored at 4°C

Table 1: Formula for Oxiconazole nitrate spanlastic
Preparation of Oxiconazole nitrate loaded spanlastic gel11:
The Carbopol 934 (1%w/w) was dissolved in appropriate amount of deionized water. The resultant mixture was stirred for 2 hrs to ensure complete dissolution and homogeneity of gel. Appropriate amount of spanlastic was added to the mixture and the mixture was stirred for 30 minutes to achieve uniform dispersion. Two drops of triethanolamine was added while stirring to neutralize Carbopol. The propylene glycol was added as penetration enhancer, methyl paraben was added as preservative.
Characterization of oxiconazole nitrate loaded spanlastic gel: 12-20
Physical appearance:
The prepared gel was examined for clarity, color, homogeneity, and the presence of foreign particles.
pH determination:
1g of gel was accurately weighed and dispersed in 100 ml of distilled water. The pH of dispersion was measured by using digital pH meter (Systronics).
Determination of drug content:
One gram of gel was dissolved in a 100 ml of methanol. From this 1 ml of solution is taken and made up to 25 ml with methanol. The resultant solution was analysed by U.V spectrophotometer at ?max.
Determination of viscosity:
The viscosity of formulated gel was determined using Brookfield viscometer using Spindle no.64 at 50 rpm. Measurements were done in triplicate and the average of the readings was taken.
Spreadability test:
Spreadability of the gel formulation was determined by taking two glass slides(14*5cm)of equal length.On one glass slide,1gm gel was applied.To the other slide,weights (1000g) are added and the time taken for the second glass slide to slip off from the first glass slide was determined.A shorter interval indicates better spreadability.Spreability was calculated by using the formula,
S= ML/T
Where, S=spreadability
M = weight kept on upper slide
L = length of the glass slide
T = time taken toslip off the slides completelyfrom each other
In-vitro diffusion study:
The in-vitro diffusion of drug from gel formulation was studied across cellophane membrane using a Franz-type diffusion cell. The cellophane membrane was mounted between the donor and receptor compartments of the diffusion cell. The receiver compartment was filled with phosphate buffer pH 6.8 to ensure sink condition. The donor compartment of the cell was filled with 1g of the gel. The medium was agitated using a magnetic stirrer (200 rpm) at a temperature of 37 ± 0.5°C. 5ml sample was withdrawn at an interval of 1 hour for a period of 8 hours, and each time the equal volume was replaced with drug-free receptor fluid. The aliquots were suitably diluted with receptor medium and analyzed by UV-visible spectrophotometer at ?max.
Stability study:
The optimized oxiconazole nitrate loaded spanlastic gel formulation was subjected to stability testing for a period of 3 months as per ICH norms at a temperature 25°C ± 2°C and relative humidity 60% ± 5% RH. Samples were taken at regular time intervals of 1month for over a period of 3 months and analyzed for the change in pH, viscosity, drug content, and in-vitro drug release by the procedure stated earlier. Any changes in evaluation parameters, if observed was noted.
RESULTS AND DISCUSSION
Determination of Standard calibration curve of Oxiconazole nitrate:
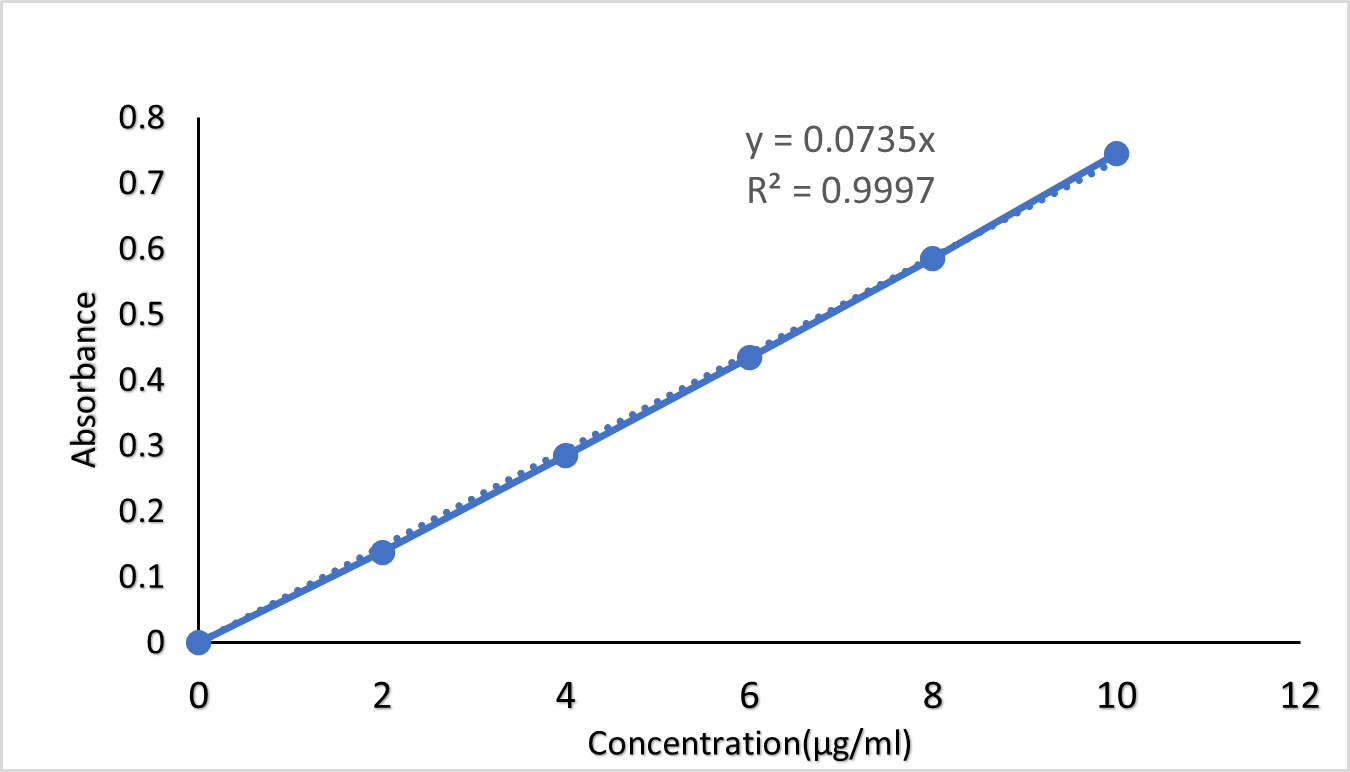
Fig 1:Standard calibration curve of oxiconazole nitrate in phosphate buffer pH6.8
The calibration curve of Oxiconazole nitrate with slope, intercept and regression co-efficient was determined the absorbance value remained linear and obeyed Beer’s Lamberts Law in the range of 0-10?g/ml with the R2 value of 0.9991.
Drug – excipient compatibility studies:
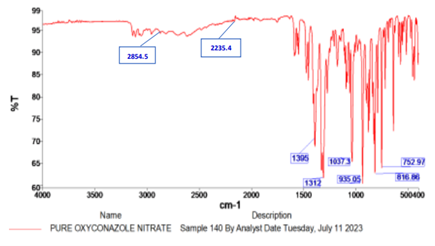
Fig 2:FTIR spectra of pure oxiconazole nitrate
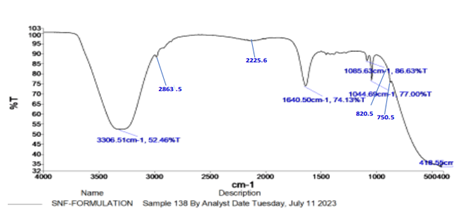
Fig 3: FTIR spectra of spanlastic formulation
All the characteristics of IR peaks related to pure drug Oxiconazole, have also appeared in the FT-IR spectrum of optimized spanlastic formulation. This result could infer that there was no chemical incompatibility between the drug and excipients.
Characterization of Oxiconazole nitrate spanlastic:

Table 2: Determination of particle size, transmittance and entrapment efficiency
The results of study indicated that the particle size was 452.2 nm lesser particle size. The % transmittance of was 72.3±0.01% and entrapment efficiency was 85.90±0.67%.
Zeta potential
The zeta potential of optimized spanlastic formulation was determined by malvern zeta sizer instrument. The Zeta potential of oxiconazole nitrate loaded spanlastic formulation was found to be -5.74 mV (Fig 10) which indicates good stability of spanlastic formulation.
In-vitro release studies:
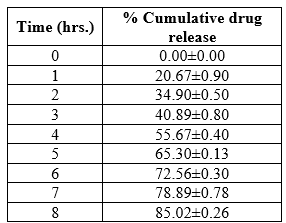
Table 3: In -vitro release studies of spanlastic
The Drug and excipients composition influences in-vitro release rate of spanlastic.The formulation showed a biphasic release profile, an initial faster release phase up to 3 hrs followed by a controlled release over period of 8hours. This biphasic release pattern seemed to be a characteristic of bilayered vesicles. Rapid drug leakage was observed during the initial phase due to the presence of drug adsorbed on the surface of the vesicles. After that the tentrapped drug would show a controlled release profile. Differences in the in- vitro release profiles might be due to vesicle size, lamellarity and membrane fluidity as a function of chain length of surfactant.
Characterization of Oxiconazole nitrate loaded spanlastic gel:

Table 4: Physicochemical evaluation data of spanlastic gel
Physical examination:
The prepared gel was off-white in color.The clarity of the gel was determined by visual inspection under black and white background and it was graded as Clear.The prepared gel was evaluated for physical appearance. The formulation was homogenous without any gritty particles or aggregates.
Determination of pH:
The pH of the gel containing oxiconazole nitrate loaded spanlastic was found to be 5.61±0.52. which is related to the pH of the skin; hence it causes no irritation. Thus, the gel was found to be compatible with the skin pH.
Determination of drug content:
The drug content of oxiconazole nitrate loaded spanlastic gel was found to be 90.82 ± 4.14%, The results of the drug content illustrated the suitability of the prepared oxiconazole nitrate loaded spanlastic gel for pharmacological action.
Determination of viscosity:
The viscosity was measured at 50 rpm using a spindle number 64. The viscosity of the prepared gel was found to be 2320.67 ± 9.07 cps.
Determination of spreadability:
The spreadability of the prepared oxiconazole nitrate loaded spanlastic gel was determined and its spreadability factor was found to be 11.83 ± 0.35gcm/sec. This showed that gel has a good spreadability which ensures the ease of application.
In-vitro diffusion study:
The in-vitro drug diffusion study of optimized Oxiconazole nitrate loaded spanlastic gel the was carried out using Franz diffusion cell which showed a cumulative % drug release of 80.10% at the end of 8 hours.

Table 5: In-vitro drug diffusion study of Oxiconazole nitrate loaded spanlastic gel
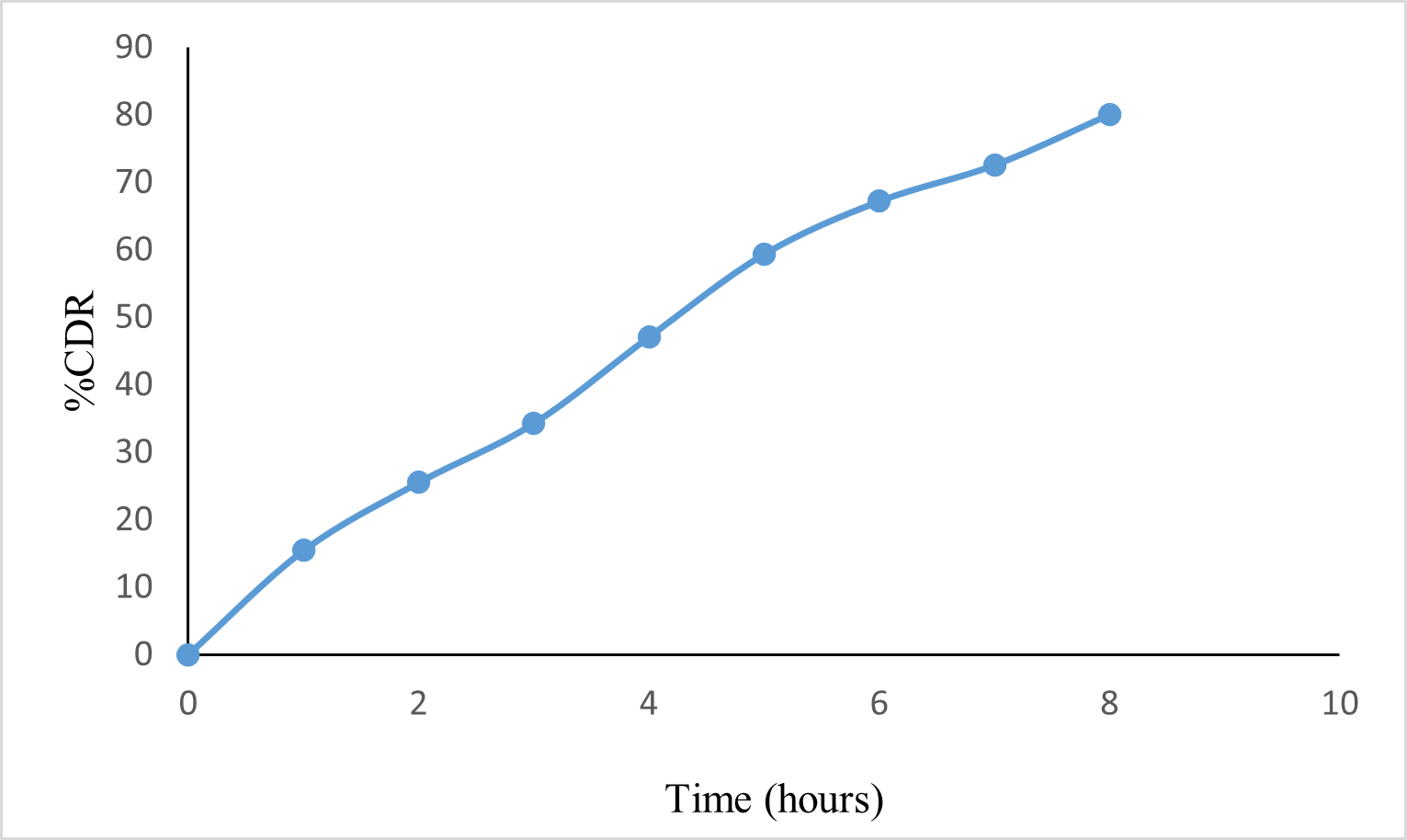
Fig 4: In -vitro release profile of Oxiconazole nitrate from spanlastic gel
The spanlastic gel formulation showed drug release of 80.10% at the end of 8 hours. The release pattern of spanlastic gel showed controlled release as that of spanlastic.

Table 6: Stability studies
From the stability study, it was found that the evaluated formulation showed, there was no influence of variety of environment factors such as temperature, humidity, and light, and during storage conditions or shelf life of drug in Table no 35. The data showed that there were no significant changes in pH, viscosity, % drug content and in-vitro drug release after 90 days
CONCLUSION
The present study has been satisfactory attempt to formulate spanlastic gel for the controlled topical delivery of oxiconazole nitrate using span 60 as a non-ionic surfactant tween 80 as edge activator and ethanol as a solvent. From the reproducible results of the executed experiments, it can be concluded that prepared Oxiconazole nitrate loaded spanlastic gel has a great potential to improve the topical delivery of oxiconazole nitrate and has a potential to treat fungal infection with patient compliance as compared with conventional formulations.
ACKNOWLEDGMENT
I would like to express my sincere gratitude to the management of Srinivas college of Pharmacy for providing necessary facilities to carry out my research work.
REFERENCES
- Verma A, Chauhan M. Spanlastics-future of drug delivery and targeting. World J Pharma Res., 2017; 6(12): 429-46.
- Imam SS. The future of non-invasive ways to treat cancer. Int J Pharma Sci. Res. 2021; 12(9): 4684-96.
- Almuqbil RM, Sreeharsha N, Nair AB. Formulation by Design of Efinaconazole Spanlastic Nanovesicles for Transungual Delivery Using Statistical Risk Management and Multivariate Analytical Techniques. Pharmaceutics, 2022; 3(14):1-19 .
- Witika BA, Mweetwa LL, Tshiamo KO, Edler K, Matafwali SK, Ntemi PV. Vesicular Drug Delivery for the Treatment of Topical Disorders: Current and Future Perspectives. J Pharm Pharmacology, 2021; 73(11): 1427-41.
- Dombe S, Disale A, Pandhare R, Sutar S. Development and evaluation of antifungal gel by using natural polymer. World J Pharm Res., 2018; 7(2):960-77.
- Rathi J, Bhowmick M, Sharma R, Panse S, Pandit R, Gupta S. Development and In-vitro characterization of floating drug delivery system of ketoconazole. J Drug Deliv Ther., 2019;9(1):22-29.
- Shailaja D,Chen D,Jiang L,Pan Y,Monsanto DF et al., Characterization and pharmacokinetics of cyadox nanosuspensions. Scientific reports, 2017;7(2289):1010-38.
- Kamble AV, Awandekar NB, Trivedi RV, Umekar MJ. Development and Evaluation of Microemulsion Based Topical Gel Containing Oxiconazole Nitrate.Int J Pharm Pharm Res. 2017;11(1):241-61.
- Ansari MD, Khan I, Solanki P, J Pandit J, Jahan RS, Mohd Aqil, Yasmin S. Fabrication and optimization of Raloxifene loaded spanlastics vesicle for transdermal delivery. J Drug Deliv Scie Tech., 2022; 68: 1-12.
- Dubey V, Paswan SK, Soni PK. Formulation Designing and optimization of Loteprednol-loaded Spanlastic Nanocarriers For Treatment of Ocular Inflammation. Asian J Pharm., 2023;17(2):233-44.
- Kakkar S, Kaur IP. Spanlastics—A novel nanovesicular carrier system for ocular delivery. Inter J Pharma., 2017;10: 413:202–10.
- Fardin H, Akram P, Hamed H.Effect of surfactant and oil type on size droplets of betacarotene-bearing nanoemulsions. Int J Curr Microbiol App Sci.2015; 20 4:146-55.
- Iriventi P, Gupta NV, Osmani RA, Balamuralidhara V. Design & development of nanosponge loaded topical gel of curcumin and caffeine mixture for augmented treatment of psoriasis. J Pharm Sci., 2020; 29:112-18.
- Badria F,Mazyed E. Formulation of Nanospanlastics as a Promising Approach for Improving the Topical Delivery of a Natural Leukotriene Inhibitor (3-Acetyl-11-Keto -?-Boswellic Acid): Statistical Optimization, in vitro Characterization, and ex vivo Permeation Study. Drug Design, Develop Therapy. 2020;14: 3697–721.
- Alnusaire T S, Sayed AM, Elmaidomy A H,Al-Sanea M M,Albogami S, Albqmi M,et al. An In Vitro and In Silico Study of the Enhanced Antiproliferative and Pro-Oxidant Potential of Olea europaea L. cv. Arbosana Leaf Extract via Elastic Nanovesicles (Spanlastics). Antioxidants. 2021;10,1860:1-18.
- Kumar JR, Muralidharan S, Parasuraman S. In vitro and in vivo evaluation of microspheres loaded topical gel delivery system of ketoconazole in male rats against candida glabrata. J Pharma Scie Res. 2014; 6(11):376-83.
- Yu Y, Tian Y, Zhang H, Jia Q, Chen X, Kang D, et al. The Evaluation of Meloxicam Nanocrystals by Oral Administration with Different Particle Sizes. Molecules., 2022;10;27(2):1-17.
- Varghese J, Sufairath ,Mahendran S. Formulation and evaluation of cubosomes loaded emulgel of oxiconazole nitrate.European J Biomed Pharma Sci.2022;9(5): 188-96.
- Khan WA, Kannojia P, Sen R, Bijauliya RK , Yadav V. Pre-formulation Studies For Formulation And Development Of Ethosomal Gel Of Oxiconazole Nitrate. Indo American J Pharma Res., 2020;(05):705-13.
- Pranali S, Charushila S, Sayali C, Namrata M. Design and Characterisation of Emulgel of an Antifungal drug. J Pharm Sci Res., 2019;11(6):2357-61.


 Vindhya V S*
Vindhya V S*
 Krishnananda Kamath K
Krishnananda Kamath K
 Shripathy D.
Shripathy D.










 10.5281/zenodo.10967697
10.5281/zenodo.10967697