Abstract
The development and optimization of microemulsions for the intranasal delivery of Amlodipine besylate, an antihypertensive drug, were investigated to address solubility and bioavailability issues commonly associated with oral and solid dosage forms. Given the challenges of first-pass metabolism and the blood-brain barrier (BBB), intranasal delivery offers a promising alternative. This study explored microemulsions as a vehicle for enhancing drug stability and delivery efficiency. Various formulation components were evaluated, including oils, surfactants, and co-surfactants. Oleic acid emerged as the optimal oil due to its high solubilization capacity. Tween 20, with an HLB value of 16.7, and Propylene glycol were selected as the surfactant and co-surfactant, respectively. The pseudo-ternary phase diagrams were used to identify stable microemulsion regions and optimize the surfactant-to-co-surfactant ratio, resulting in a final formulation with a 2:1 ratio. The selected microemulsions were clear, transparent, and stable. Formulation ME-1, characterized by its low viscosity and globule size of 155.4 nm, showed superior performance. In vitro diffusion studies on bovine nasal mucosa indicated that the mucoadhesive properties of chitosan enhanced drug release by increasing the contact time with the mucosa. Stability tests at 40°C and 75% RH confirmed that ME-1 remained stable over one month. This study concludes that microemulsion ME-1, with Oleic acid, Tween 20, and Propylene glycol, represents an effective formulation for intranasal delivery of Amlodipine besylate, offering improved solubility, stability, and drug delivery efficiency.
Keywords
Amlodipine besylate, intranasal delivery, Microemulsion, Oleic acid, Co-surfactant.
Introduction
Nasal drug delivery systems are designed to deliver medication at a controlled rate either locally to the nasal cavity or systemically, offering a non-invasive and convenient alternative to oral or injectable routes. The nasal route is gaining attention due to its ability to bypass first-pass metabolism, resulting in faster absorption and higher bioavailability for certain drugs. This method is particularly useful for drugs that are poorly absorbed orally or for treatments requiring rapid onset, such as in emergency situations (e.g., migraines or seizures). Nasal drug delivery systems are engineered using various formulations and technologies to ensure precise, controlled release or site-specific targeting within the nasal mucosa, enhancing therapeutic efficacy and patient compliance.1-5 In recent years, there has been growing interest in more patient-friendly dosage forms, including nasal sprays and powders. As a result, research and development efforts have shifted toward improving existing drugs' safety, efficacy, and bioavailability while reducing dosing frequency and production costs. Nasal drug delivery systems provide an innovative solution to these challenges by offering a route that allows for direct drug absorption into systemic circulation or localized treatment of nasal disorders, all while minimizing systemic side effects.6-13 A nasal drug delivery system (DDS) refers to a formulation or device that enhances the efficacy and safety of the therapeutic agent by controlling the rate, timing, and site of drug release within the nasal cavity. This ensures that the active ingredient is absorbed effectively, either into the nasal tissue or the bloodstream. The goal of nasal DDS is to deliver an optimal dose of the drug directly to the target area, achieving the desired therapeutic concentration quickly and maintaining it over the required period.14-19 Key aspects of nasal drug delivery include spatial and temporal control. Spatial control involves directing the drug to specific regions of the nasal cavity, such as targeting the olfactory region for brain drug delivery or the respiratory region for systemic absorption. Temporal control refers to modulating the drug release rate, ensuring sustained therapeutic levels in the body. A well-designed nasal DDS maximizes the therapeutic effect by ensuring precise delivery while minimizing side effects.20-23 The effectiveness of nasal drug delivery depends on the ability of the dosage form to deliver the active substance to its target site at a sufficient rate and concentration. For treatments that require rapid relief, such as allergic rhinitis or acute migraine attacks, the rapid absorption offered by the nasal route plays a critical role in determining the drug's success. Compared to other administration routes, nasal delivery offers a unique balance of rapid onset, higher bioavailability, and ease of administration, making it a valuable option in modern drug therapy.24
Microemulsions are thermodynamically stable, isotropic dispersions of two immiscible liquids, such as oil and water, which are stabilized by an interfacial layer of surfactant molecules. These systems consist of an oil phase and a water phase, combined with surfactants, and sometimes cosurfactants, to form very small droplets ranging from 5 nm to 200 nm in size. Due to their droplet size being smaller than 25% of the wavelength of visible light, microemulsions appear transparent. One of the key features of microemulsions is that they can form spontaneously or with minimal energy input, unlike other emulsions that require high energy to be formed.
There are three main types of microemulsions based on the composition: (1) oil-in-water microemulsions, where oil droplets are dispersed in a continuous aqueous phase; (2) water-in-oil microemulsions, where water droplets are dispersed in a continuous oil phase; and (3) bicontinuous microemulsions, where microdomains of oil and water are interdispersed within the system.25 The formation and stability of microemulsions can be understood through a simplified thermodynamic perspective. The free energy of microemulsion formation depends on the reduction in surface tension at the oil-water interface, achieved by surfactants, and the increase in entropy resulting from the formation of numerous small droplets. The favorable entropic contributions, primarily from the mixing of small droplets and dynamic processes like surfactant diffusion, offset the small positive surface tension, resulting in a negative free energy of formation. This makes the formation of microemulsions spontaneous and ensures their thermodynamic stability. Various surfactants are used to stabilize microemulsions, including non-ionic, zwitterionic, cationic, and anionic surfactants, each playing a role in reducing surface tension and stabilizing the dispersed droplets within the system. Amlodipine besylate, a calcium channel blocker, is widely used in the treatment of hypertension and chronic stable angina. Despite its effectiveness, amlodipine besylate faces limitations such as poor aqueous solubility and low bioavailability, which reduce its therapeutic potential. To overcome these challenges, innovative drug delivery systems, such as microemulsions, have been explored as promising alternatives. Microemulsions are isotropic, thermodynamically stable systems composed of oil, water, surfactants, and co-surfactants. These systems offer several advantages, including enhanced solubilization of poorly water-soluble drugs, improved drug absorption, and increased bioavailability. Due to their small droplet size and high surface area, microemulsions facilitate rapid drug diffusion across biological membranes, making them ideal for delivering drugs like amlodipine besylate.26
The formulation of microemulsions involves selecting appropriate components and optimizing their ratios to achieve stability, desired drug release profiles, and efficient drug loading. This process requires a systematic approach, often utilizing techniques such as phase diagrams and response surface methodology to identify the optimal formulation conditions.
MATERIALS AND METHODS:
Materials:
The formulation and optimization of microemulsions for improved amlodipine besylate delivery required a range of high-quality materials sourced from reputable suppliers. Each material played a critical role in the formulation, contributing to the stability, effectiveness, and performance of the microemulsion system.
Amlodipine besylate, the active pharmaceutical ingredient, was obtained from Yarrow Chem Pharmaceutical Pvt. Ltd., Mumbai, ensuring the purity and efficacy needed for therapeutic delivery. Various oils, such as isopropyl myristate, oleic acid, triacetin, and castor oil, sourced from Research Lab and Thomas Baker in Mumbai, were essential in forming the oil phase of the microemulsion, influencing droplet size and drug solubility. Polyethylene glycol 400 (PEG-400) and propylene glycol, supplied by Venus Chemicals and Thomas Baker respectively, served as cosurfactants to enhance the solubilization and stability of the formulation.27-38
Surfactants such as Tween 20 and Tween 80, obtained from Thomas Baker and Research Lab, played a pivotal role in reducing surface tension between the oil and water phases, facilitating the formation of stable microemulsions. Methanol, chitosan, and other excipients, including potassium dihydrogen orthophosphate and sodium hydroxide, were utilized to adjust the pH, improve viscosity, and further stabilize the formulation.
These carefully selected materials contributed to the creation of a stable, isotropic system capable of enhancing the bioavailability of amlodipine besylate, ensuring consistent and controlled drug release.
Methods:
Preformulation studies
Preformulation studies are a crucial part of the drug development process, where the physical, chemical, and mechanical properties of the drug substance are analyzed to ensure the creation of an effective, stable, and safe dosage form. These studies help in understanding the behavior of the drug under various conditions and are essential for proper drug delivery system design. In this project, key preformulation studies included the description of the drug’s physical characteristics, determination of its melting point to assess thermal stability, solubility analysis in different solvents, identification of the drug sample to confirm its authenticity, and drug-excipient compatibility studies to prevent any adverse interactions that could affect the stability and efficacy of the formulation.39-48
Identification of Drug Sample
To identify the drug sample, the absorption maxima (? max) of the drug was determined using ultraviolet-visible (UV-Vis) spectrophotometry. This technique provides specific information about the chromophoric part of the molecule by measuring the absorption of light at particular wavelengths. For Amlodipine besylate, the UV spectrum of a 100 µg/ml solution in methanol was scanned between 200-400 nm, revealing a peak at 360 nm, consistent with previous literature. To study Beer’s Lambert’s Law, drug solutions of various concentrations were prepared by diluting a stock solution (10 mg of the drug diluted to 100 ml with phosphate buffer at pH 6.4) to concentrations of 2, 4, 6, 8, 10, and 12 µg/ml, with absorbance measured at 360 nm. Additionally, Fourier Transform Infrared (FTIR) spectral analysis was conducted to identify functional groups in the drug. The drug, mixed with a KBr disk, was scanned at a resolution of 2 cm??1; over a wavenumber range of 400 to 4000 cm??1; to record its characteristic peaks.49-53
Drug-Excipient Compatibility Studies by FT-IR Analysis
The infrared (IR) spectrum of a compound provides valuable information about the functional groups present within it. In this study, the IR absorption spectra of the pure drug, Amlodipine besylate, as well as physical admixtures of the drug with various excipients, were recorded in the range of 4000-400 cm??1; using the KBr disc method. This analysis was conducted to identify characteristic peaks and assess drug-excipient compatibility. Initially, IR spectra of the pure drug and individual excipients-Oleic acid, Tween-20, and propylene glycol were obtained. Subsequently, admixtures of the drug with these excipients were prepared, and their IR spectra were analyzed. The spectral data indicated no significant interaction between Amlodipine besylate and the excipients, confirming their compatibility for formulation purposes.54-56
Selection of components57-62
The selection of components for the microemulsion (ME) system was guided by the drug's miscibility with the chosen oils and the Hydrophilic-Lipophilic Balance (HLB) values of the emulsifiers.
Selection of Oils
To identify the most suitable oil for the microemulsion that would enhance the nasal permeation of Amlodipine besylate, the solubility of the drug in various oils, including castor oil, isopropyl myristate, triacetin, and oleic acid, was evaluated at 25°C. The procedure involved weighing 10 grams of each oil into a 25 ml glass beaker, adding 100 mg of Amlodipine besylate, and stirring with a magnetic stirrer until the drug was fully dissolved. Additional quantities of the drug were added until saturation was achieved, and the total amount of drug dissolved was quantified using UV spectrophotometry at 360 nm. Among the oils tested, oleic acid demonstrated the highest solubility for Amlodipine besylate and was therefore selected as the oil phase for the microemulsion.
Selection of Surfactants and Co-Surfactants
Non-ionic surfactants, which are less likely to ionize and are compatible with both anionic and cationic substances, were considered for the formulation. Surfactants such as Tween-20 and Tween-80, along with co-surfactants like PEG-400 and propylene glycol, were evaluated through titration. Based on their performance, Tween-20 and propylene glycol were chosen as the optimal surfactant and co-surfactant for the microemulsion system due to their effective stabilization properties.
Microemulsion formulation and phase diagram study
A hypothetical pseudo-ternary phase diagram for an oil/water/surfactant/co-surfactant (SCoS) system is used to illustrate the relationship between these components. In this diagram, two of the corners represent pure components oil and water, while the third corner represents a binary mixture of surfactant and co-surfactant. The diagram includes dilution lines to assess: (a) the efficiency of the surfactant/co-surfactant mixture in solubilizing equal amounts of oil and water (SCoSmin, indicated by the dashed line) and (b) the water solubilization capacity of the surfactant/co-surfactant/oil mixture at a constant SCoS/oil weight ratio of 1:1 (Wmax, shown by the solid line). When SCoS is insufficient or when water concentrations exceed Wmax, a two-phase system (2?) is observed. Conversely, when SCoS concentrations are above SCoSmin and water concentrations are below Wmax, a single-phase region (1?) is present. At very high concentrations of SCoS, liquid crystals (LC) are formed.63-66
Water titration method and phase diagram construction
To construct the pseudo-ternary phase diagrams, the water titration method was employed to determine the optimal component concentrations for forming a stable microemulsion. Oleic acid, Tween 20 (non-ionic surfactant), and Propylene glycol (co-surfactant) were used. Transparent oil-Smix (Tween 20/Propylene glycol) mixtures at varying weight ratios (1:9 to 9:1) were prepared and titrated with water to observe phase clarity and flow. Three phase diagrams were created for S/CoS ratios of 1:1, 1:2, and 2:1. The diagrams were plotted with oil, Smix, and water at the triangle’s apex, with their respective concentrations as 100% and 0% at the opposite ends. The microemulsion region was identified by the area under the curve. Selected microemulsion compositions were prepared by mixing the weighed components and stirring to achieve a clear microemulsion.67-68
Evaluation of microemulsions
The prepared microemulsions were thoroughly evaluated based on several critical parameters to assess their quality and effectiveness. These evaluations included testing for optical transparency to ensure clarity, measuring pH to confirm that the microemulsions are within a suitable range for stability and compatibility, and assessing conductivity to gauge the ionic content of the formulations. Viscosity was evaluated to understand the flow properties of the microemulsions, while globule size was measured to ensure uniformity and stability. The refractive index was determined to provide insights into the optical properties of the microemulsions. Additionally, in-vitro drug diffusion studies were conducted to assess the release profile and efficacy of the drug delivery, and stability studies were performed to evaluate how the microemulsions maintain their properties over time under various conditions. These evaluations collectively ensure that the microemulsions are well-formulated, effective, and stable for their intended use.69-71
In-vitro drug diffusion study
The nasal cavity's highly vascularized mucosa allows drugs to enter systemic circulation directly, bypassing first-pass metabolism. It comprises a thin layer of about 100µm, containing ciliated and non-ciliated columnar cells, goblet cells, and basal cells. The mucus layer, produced by goblet cells and moved by ciliated epithelium, is typically washed away during equilibration. However, challenges in nasal drug delivery include low residence time and variable absorption due to factors like mucus, epithelial barriers, mucociliary clearance, and enzymatic activity. To address these issues, formulations with high mucoadhesive properties can enhance drug retention and absorption. Specifically, Amlodipine besylate, when delivered nasally, suffers from low retention and bioavailability due to rapid mucociliary clearance. Thus, a formulation that increases nasal residence time and enhances absorption is highly beneficial. The use of bioadhesive polymers can significantly improve both residence time and bioavailability. Using Franz diffusion cells, the diffusion kinetics of Amlodipine besylate microemulsion through bovine nasal mucosa was studied in phosphate buffer (pH 6.4) over 3 hours. Tissue samples were placed in diffusion cells, and the diffusion process was monitored by sampling the acceptor chamber at intervals. The amount of drug diffused was analyzed using UV-visible spectrophotometry at 360 nm, with a linearity range of 2µg/ml to 12µg/ml and an r?2; value of 0.989.72
Stability study
In the design and evaluation of dosage forms, the stability of the active ingredient is crucial for determining the product's viability. Stability studies typically involve exposing the product to standard temperature and humidity conditions, but these can be time-consuming. To expedite the process, accelerated stability studies are conducted by storing the product under extreme temperature conditions. In this study, the microemulsion formulation was stored at 40±2°C and 75±5% relative humidity for one month. After this period, the formulation was assessed for viscosity, pH, conductivity, and refractive index.73-74
RESULTS AND DISCUSSION:
Preformulation Studies
The physicochemical characteristics of Amlodipine besylate indicate that it is a white crystalline powder with a faint odor and a bitter taste. Its melting point ranges from 178ºC to 179ºC, which aligns with the standards set by the Indian Pharmacopoeia (I.P.), confirming the drug’s purity. Solubility studies show that Amlodipine besylate is readily soluble in methanol and ethanol, only sparingly soluble in water, and fully soluble in a phosphate buffer at pH 6.4. This solubility profile is crucial for understanding the drug's behavior in different solvents and its suitability for various pharmaceutical formulations.
Determination Of ?max
Amlodipine besylate exhibited a maximum absorbance wavelength at 360 nm, which corresponds with the standard value. Therefore, the drug used in the formulation meets the purity criteria outlined in the I.P. specifications.
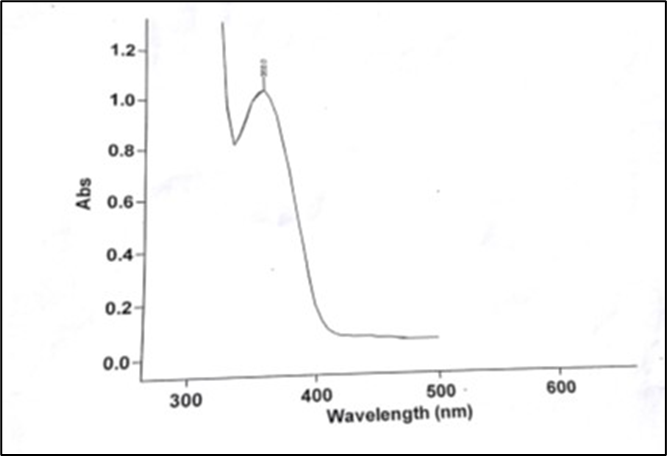
Figure 1: Spectra of Amlodipine Besylate

Figure 2: Calibration curve of Amlodipine besylate
FTIR study
Interaction studies were conducted to determine any potential interactions between the drug and excipients used in the preparation of intranasal drug delivery systems. The IR spectra revealed no significant changes in the main peaks of the pure drug compared to those of the drug with the polymer, indicating the absence of physical interactions or new band formations between the drug, solvent, and polymer. The key peaks for Amlodipine besylate were observed at 3439, 1616, 1676, and 1036 cm??1;.

Figure 3: FTIR spectra of Amlodipine besylate
FTIR study of Compatability of Amlodipine besylate with Excipients (Oleic acid + Tween20 + Propylene glycol)

Figure 4: Compatability of Amlodipine besylate with Excipients
(Amlodipine besylate + Oleic acid + Tween-20 + Propylene glycol)
Formulation Development
Pharmaceutical development studies aim to select the appropriate dosage form and ensure formulation stability. They detail each step in the development process, identifying and controlling critical parameters to produce a reliable and reproducible quality product.
Selection of components
The selection of components for the microemulsion (ME) system was based on the miscibility of the drug and the hydrophilic-lipophilic balance (HLB) values of the emulsifiers. Oleic acid was chosen as the oil due to its suitable drug solubility, with a reported solubility of 40 ± 1.16 mg/ml. For the surfactant, Polyoxyethylene sorbitan mono-laurate (Tween 20) was selected because of its high HLB value of 16.7, which indicates its strong emulsifying properties. Propylene glycol was chosen as the co-surfactant based on its solubility with the drug and its effectiveness in the titration method. Together, Tween 20 and propylene glycol were determined to be ideal for forming a stable microemulsion system.
pseudo- ternary phase diagrams
The pseudo-ternary phase diagrams were used to identify the stable microemulsion regions, which were characterized by transparency and low viscosity, with no noticeable transition between oil-in-water and water-in-oil microemulsions. Outside these regions, the microemulsions either appeared turbid or formed gels. The effect of varying the surfactant (Tween 20) to co-surfactant (PEG-400, Propylene glycol) ratio (1:1, 1:2, 2:1) using Oleic acid as the oil phase was studied. The phase diagrams revealed that increasing the surfactant concentration expanded the microemulsion region and improved water solubilization. The 1:1 surfactant to co-surfactant ratio had the smallest microemulsion region, while the 2:1 ratio showed the best water solubilization and stability. This suggests that the optimal ratio for achieving a suitable HLB value and stable microemulsion is 2:1 for surfactant to co-surfactant.

Table 1: Formulation containing Smix composition (Tween 20: PG) (2:1)
Oil content of Microemulsion
The oil content in the microemulsion formulation is critical for achieving the therapeutic dose of Amlodipine besylate. To ensure that the nasal dose of 5-10 mg is administered effectively, each 500 µl dose of the microemulsion must contain 10 mg of the drug. To achieve this, the formulation must include up to 12% oil content, which is sufficient to solubilize the 10 mg dose in 500 µl of the microemulsion for effective delivery per nostril.
Formulation batches
The formulation batches were prepared to optimize the microemulsion system for Amlodipine besylate. The compositions of the different formulations are detailed in Table 2. These formulations varied in the percentages of oleic acid, Tween 20, propylene glycol (PG), water, and the amount of Amlodipine besylate. The selection criteria focused on stability and clarity, with Tween 20 and PG used at varying ratios. The objective was to achieve a formulation with low viscosity and minimal surfactant use. Ultimately, the formulation with the lowest viscosity and optimal clarity was chosen as the final optimized batch.

Table 2: Composition of the selected Microemulsion ratio
Evaluation Of Microemulsions:
The ME formulation were evaluated for the various parameters.
Optical transparency
All formulations, ME-1 through ME-6, were clear and transparent. The optical transparency results indicate that all formulations are stable and visually clear, with no observable haze or cloudiness.
pH determination
The pH values of the microemulsions, ranged from 6.23 to 6.80 across different formulations. ME-1 had a pH of 6.23, while ME-6 had a pH of 6.80. These values are consistent with those reported in previous literature. All formulations fall within the pH range compatible with nasal secretions, which typically have a pH of 6.4. Additionally, the pH of the formulations remained stable during storage at room temperature.
Conductivity measurements
Conductivity measurements for the microemulsions are summarized in Table 3. The conductivity values for the formulations were found to be negative, with ME-1, ME-2, ME-4, and ME-5 showing -0.3, and ME-3 and ME-6 showing -0.2. This negative conductivity is attributed to the high solubilization of the water phase by the emulsifiers and co-emulsifiers. Consequently, the oil globules, which carry minimal charge, result in overall negative conductivity readings.
Viscosity measurements
The viscosity values increased from ME-1 to ME-6, primarily due to the higher concentrations of oil and surfactant mixture in the formulations. The surfactant mixture, being the most viscous component of the microemulsion, contributes significantly to the overall viscosity. As the concentration of the surfactant mixture rises, the viscosity of the formulation increases accordingly. For example, ME-1, which contains 30% surfactant mixture, has a viscosity of 205 cps. This trend highlights the direct relationship between surfactant concentration and the formulation's viscosity.
Globule size determination
Globule size was measured using a Malvern Zetasizer with disposable cuvettes. The microemulsion particles had sizes ranging from 10 to 100 nm. The size distribution analysis revealed a particle diameter of 155.4 nm and a width of 35.65 nm at 100% intensity, confirming the isotropic nature of the O/W microemulsion.
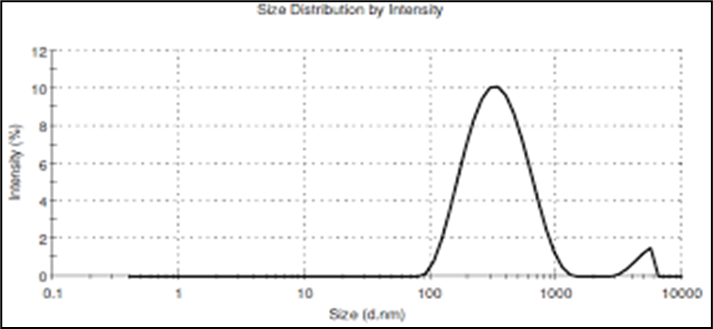
Figure 5: Size distribution by intensity graph of final selected formulation ME-1
Refractive Index determination:
The refractive index (R.I.), an important stability parameter for microemulsions, was measured using an Abbey’s Refractometer, calibrated with cyclohexane. The refractive indices of various formulations are detailed in Table 3. Formulations ME-1 through ME-6 exhibited refractive indices ranging from 1.432 to 1.557. According to the literature, an increase in R.I. typically corresponds to a decrease in formulation transparency. The higher R.I. values observed in these formulations can be attributed to the high concentration of oleic acid used in the formulations.

Table 3: Evaluation Parameters
In - vitro drug diffusion study:
In the drug diffusion study through bovine nasal mucosa, the release pattern of the drug exhibited an initial lag time of approximately 30 minutes, after which the release rate increased linearly. The results are detailed in figure 6, which includes absorbance, drug concentration, and percentage of drug released from the formulation. Formulation ME-1 was selected for the study due to its low viscosity. According to the data, drug release started after 30 minutes and continued to increase, with only 23.24% of the drug released by 90 minutes. This limited release could be attributed to the keratinization of the bovine nasal mucosa.
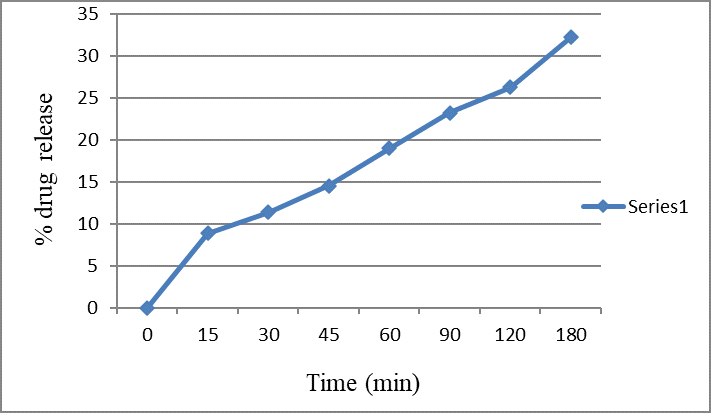
Figure 6: % Drug diffused with time (min) from Amlodipine besylate
Stability Study
Stability studies were conducted on the formulation containing the drug over a period of one month. After this duration, the formulation was re-evaluated for several parameters, including viscosity, conductivity, pH, and clarity. The results of these evaluations are summarized in Table 4, which compares the parameters before and after the stability study. The pH of the formulation slightly decreased from 6.23 to 6.20. Conductivity showed a minimal change, decreasing from -0.3 to -0.2. The viscosity decreased marginally from 205 cps to 201 cps. However, the refractive index increased from 1.432 to 1.453. These results provide insights into the formulation's stability and its performance over the study period.

Table 4: parameters studied on ME-1 optimized formulation before and after stability studies results
CONCLUSION:
The study focused on developing and optimizing microemulsions for the intranasal delivery of Amlodipine besylate, an antihypertensive drug. The findings indicate that Oleic acid, when used as the oil component, provided the best water solubilization with minimal Tween 20, the chosen surfactant. Propylene glycol proved to be an effective co-surfactant among those tested. The selected microemulsions, composed of Oleic acid, Tween 20, and Propylene glycol, were clear, transparent, and stable. Among the formulations, ME-1, ME-2, ME-3, ME-4, ME-5, and ME-6 were evaluated for clarity, transparency, and stability, with ME-1 being the optimal formulation due to its low viscosity. ME-1 demonstrated a globule size of 155.4 nm, confirming its isotropic nature. The microemulsion was capable of solubilizing 12% oil at a 30% surfactant mixture concentration. In vitro diffusion studies on bovine nasal mucosa showed that the formulation had an increased drug release due to the mucoadhesive properties of chitosan, enhancing contact time with the mucosa. Stability studies at 40°C and 75% RH over one month confirmed that the formulation remained stable throughout the study period. Thus, ME-1, with its optimal formulation and stability, is recommended for further development in intranasal drug delivery systems.
FUNDING:
None.
AUTHORS CONTRIBUTIONS:
All authors have contributed equally.
CONFLICTS OF INTERESTS:
All authors have declared no conflict of interest.
REFERENCE
- Remington: The Science and Practice of Pharmacy. 20th ed. Mack Publication Company; Eastern Pennsylvania: 2002. Vol. 1, p. 903.
- Brahmankar DM, Jaiswal SB. Biopharmaceutics and Pharmacokinetics: A Treatise. Delhi: Vallabh Prakashan; 1995. p. 282.
- Henary RC. Intranasal delivery: Physicochemical and therapeutic aspects. Int J Pharm. 2007; 337:1-24.
- Jain KK. Drug Delivery Systems. Edited by Jain Pharma Biotech, Switzerland. Humana Press; p. 8.
- Chawala V. Nasal drug delivery: A review. Indian Drugs. 2001;38(6):283.
- Satyanarayana S. Intranasal drug delivery systems: An overview. Indian J Pharm Sci. 1996; 58(1):1.
- Talegaonkar S, Mishra PR. Intranasal delivery: An approach to bypass the blood-brain barrier. Indian J Pharmacol. 2004; 36:140-7.
- Ugwoke MI, Agu RU, Verbeke N, Kinget R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv Drug Deliv Rev. 2005; 57:1640-65.
- Mygind N, Dahl R. Anatomy, physiology and function of the nasal cavities in health and disease. Adv Drug Deliv Rev. 1998; 29:3-12.
- Khanvilkar K, Donovan MD, Flanagan DR. Drug transfer through mucus. Adv Drug Deliv Rev. 2001; 48:173-93.
- Alpara HO, Somavarapu S, Atuahb KN, Bramwell VW. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv Drug Deliv Rev. 2005; 57:411-30.
- Martin L, Wilson CG, Koosha F, Tetley L, Gray AI, Senel S, Uchegbu UF. The release of model macromolecules may be controlled by the hydrophobicity of palmitoyl glycol chitosan hydrogels. J Control Release. 2002; 80:87-100.
- Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: A review. J Pharma Pharmaceut Sci. 2003; 6(2):252-73.
- Begley DJ. The blood-brain barrier: Principles for targeting peptides and drugs to the central nervous system. J Pharm Pharmacol. 1996; 48:136-46.
- Pardridge WM. Recent advances in blood-brain barrier transport. Annu Rev Pharmacol Toxicol. 1988; 28:25-39.
- Siegal T, Zylber-Katz E. Strategies for increasing drug delivery to the brain: Focus on brain lymphoma. Clin Pharmacokinet. 2002; 41:171-86.
- Lin JH, Lu AY. Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol Rev. 1997; 49:403-49.
- Dwibhashyam VSNM, Nagappa AN. Strategies for enhanced drug delivery to the central nervous system: Review article. Indian J Pharm Sci. 2008; 145-54.
- Madrid Y, Langer LF, Bram H, Langer R. New directions in the delivery of drugs and other substances to the central nervous system. Adv Pharmacol. 1991; 22:299-324.
- Pardridge WM. Recent advances in blood-brain barrier transport. Annu Rev Pharmacol Toxicol. 1988; 28:25-39.
- Kumar S, Bapu S. Novel strategies in drug delivery to brain. Pharma Buzz. 2008;3(5):14-21.
- Patil SB, Sawant KK. Nasal delivery of anti-hypertensive drugs. J Microencapsulation: Micro and Nano Carriers. 2009;26(5):432-43.
- Fisher N, Brown K, Davis SS, Parr GD, Smith DA. The effect of molecular size on the nasal absorption of water soluble compounds in the albino rat. J Pharm Pharmacol. 1985;37:38-41.
- Tengamnuay P, Mitra AK. Transport of tyrosine and phenyl alanine across the rat nasal mucosa. Life Sci. 1988; 43:585.
- Wheatley MA, Dent J, Wheeldon EB, Smith PL. Nasal drug delivery: An in vitro characterization of transepithelial electrical properties and fluxes in the presence or absence of enhancers. J Control Release. 1988; 8:176.
- Banker GS, Anderson NR. In: Lachman L, Liberman HA, Kanig JL, editors. The Theory and Practice of Industrial Pharmacy. 3rd ed. Bombay: Vaghesse Publishing House; 1990. p. 296-302.
- Aulton ME. The Science of Dosage Form Design. 2nd ed. Churchill Livingstone; 2002. p. 414-8.
- Patil LD, Gudi SV, Jadav DD, Kadam YA, Dalvi SD, Ingale PL. Development and validation of UV-spectrophotometric methods for simultaneous estimation of amlodipine besylate. Der Pharma Chemica. 2013; 5(4):282-7.
- Haubing C, Xueling C, Weng T, Xiaozhi Z, Zhonghong G, Yajing Y, Huibi X. A study of microemulsion systems for transdermal delivery of triptolide. J Control Release. 2004; 98:427-36.
- Constantinides PP, Scalart JP. Formulation and physical characterization of water-in-oil microemulsions containing long- versus medium-chain glycerides. Int J Pharm. 1997; 158:57-68.
- Li L, Nandi I, Kim KH. Development of an ethyl laurate-based microemulsion for rapid-onset intranasal delivery of diazepam. Int J Pharm. 2002; 237:77-85.
- Charlton ST, Davis SS, Illum L. Nasal administration of an angiotensin antagonist in the rat model: Effect of bioadhesive formulations on the distribution of drugs to the systemic and central nervous systems. Int J Pharm. 2007; 338:94-103.
- Dhamankar AK, Manwar JV, Kumbhar DD. The novel formulation design of O/W microemulsion of ketoprofen for improving transdermal absorption. Int J Pharmtech Res. 2009;1(4):1449-57.
- Djordjevic Lj, Primorac M, Stupar M, Krajisnik D. Characterization of caprylocaproyl macrogoglycerides-based microemulsion drug delivery vehicles for an amphiphilic drug. Int J Pharm. 2004; 271:11-9.
- El-Laithy HM. Preparation and physicochemical characterization of dioctyl sodium sulfosuccinate (Aerosol-T) microemulsion for oral drug delivery. AAPS PharmSciTech. 2003; 4(1):11.
- Clausse M, Morgantini LN, Zradba A, Touraud D. Water/ionic surfactant/alkanol/hydrocarbon system. In: Rosano HL, Clausse M, editors. Microemulsion Systems. New York, NY: Marcel Dekker; 1987. p. 15-63.
- Silvander M, Hellstom A, Warnheim T, Claesson PM. Rheological properties of phospholipid-stabilized parenteral oil-in-water emulsions – Effects of electrolyte concentration and presence of heparin. Int J Pharm. 2003; 252:123-32.
- Acharya SK, Sanyal SP, Moulik SP. Physicochemical investigations on microemulsification of eucalyptol and water in presence of polyoxyethylene (4) lauryl ether (Brij-30) and ethanol. Int J Pharm. 2001; 229:213-26.
- Martin A. A Text Book of Physical Pharmacy. 4th Ed. New Delhi: B. I. Waverly Pvt. Ltd.; p. 95-96.
- Constantinides PP, Yiv SH. Particle size determination of phase-inverted water-in-oil microemulsions under different dilution and storage conditions. Int J Pharm. 1995; 115:225-34.
- Richter T, Keipert S. In vitro permeation studies comparing bovine nasal mucosa, porcine cornea, and artificial membrane: And rostenedione in microemulsions and their components. Eur J Pharm Sci. 2004; 58:137-43.
- Pisal SB, Shelke V, Mahadik SK, Kadam SS. Effect of organogel components on in vitro nasal delivery of propranolol hydrochloride. AAPS PharmSciTech. 2004; 5(4):63.
- Datta R, Bandyopadhyay AK. A new nasal drug delivery system for diazepam using natural mucoadhesive polysaccharide obtained from tamarind seeds. Saudi Pharm J. 2006; 14(2):112-118.
- Shinde JV, Mali KK, Dias RJ, Havaldar VD, Mahajan NS. Insitu mucoadhesive nasal gels of metoclopramide hydrochloride: Preformulation and formulation studies. J Pharm Res. 2008; 1:88-95.
- Majithiya RJ, Ghosh PK, Umrethia ML, Murthy SRR. Thermoreversible-mucoadhesive gel for nasal delivery of sumatriptan. AAPS PharmSciTech. 2006;7(3):67-74.
- Bhanushali RS, Bajaj AN. Design and development of thermoreversible mucoadhesive microemulsion for intranasal delivery of sumatriptan succinate. Indian J Pharm Sci. 2007; 69(6):709-715.
- Yadav AV, Mote HH. Development of biodegradable starch microspheres for intranasal delivery. Indian J Pharm Sci. 2008; 70(2):170-174.
- Dandagi PM, Mastiholimath VS, Gadad AP, Iliger SR. Mucoadhesive microspheres of propranolol hydrochloride for nasal delivery. Indian J Pharm Sci. 2007; 69(3):402-409.
- Shinde JV, Mali KK, Dias RJ, Havaldar VD, Mahajan NS. Insitu mucoadhesive nasal gels of metoclopramide hydrochloride: Preformulation and formulation studies. J Pharm Res. 2008; 1(1):1-8.
- Kantarci G, Ozguney I, Karasulu HY, Arzik S, Guneri T. Comparison of different water/oil microemulsions containing diclofenac sodium: Preparation, characterization, release rate, and skin irritation studies. AAPS PharmSciTech. 2007;8(4):91-98.
- Vyas TK, Babbar AK, Sharma RK, Singh S, Misra A. Preliminary brain-targeting studies on intranasal mucoadhesive microemulsions of sumatriptan. AAPS PharmSciTech. 2006; 7(1):8-15.
- Jansson B. Models for the transfer of drugs from nasal cavity to the central nervous system. Acta Universitatis Uppsaliensis. Uppsala: Uppsala University; 2004.
- Kumar M, Misra A, Babbar AK, Mishra AK, Mishra P, Pathak K. Intranasal nanoemulsion-based brain targeting drug delivery system of risperidone. Int J Pharm. 2008; 27(4):345-351.
- Rao M, et al. Stability studies of rifampicin mucoadhesive nasal drops. Indian J Pharm Sci. 1999; 61(6):366-370.
- Patel DM, Patel MR. Development and evaluation of nasal drug delivery system for anti-migraine drugs. Drug Dev Ind Pharm. 2008; 34(4):380-388.
- Singh S, Saini S, Sharma V, Sharma R, Rajput K. Optimization of intranasal delivery system for targeted drug delivery. J Drug Del Sci Technol. 2008; 18(3):189-195.
- Banerjee S, Kar D, Gupta U, Khare P, Singh S. Formulation and evaluation of mucoadhesive nasal gels containing anti-allergic drugs. J Pharm Sci. 2008; 12(2):55-60.
- Ghosh D, Vora S, Dey S. Formulation and in vivo evaluation of mucoadhesive nasal tablets for enhanced drug delivery. Int J Pharm. 2008; 12(5):172-180.
- Desai N, Gupta V, Khatri P, Yadav S. Development of nasal microemulsion for enhanced bioavailability of antihypertensive drugs. AAPS PharmSciTech. 2008; 9(2):123-130.
- Kumar A, Dey R, Bandyopadhyay A, Jain S. Nasal drug delivery systems: A review on recent advances and future prospects. Drug Dev Ind Pharm. 2008; 34(2):115-123.
- Sharma S, Kumar V, Ghosh D, Agarwal A, Gupta P. Development and characterization of nasal mucoadhesive gels for the delivery of anti-inflammatory drugs. J Pharm Pharmacol. 2008; 60(8):1045-1052.
- Chaudhary D, Mehta M, Kumar S. Preparation and evaluation of nasal microemulsions for improved systemic absorption of anti-diabetic agents. J Control Release. 2008; 131(2):187-193.
- Agarwal M, Verma S, Madaan R. Design and development of nasal delivery systems for local and systemic effects. Int J Pharm Sci. 2008;6(3):232-240.
- Patel S, Singh P, Yadav N, Mehta S. Evaluation of nasal mucoadhesive microspheres for sustained drug delivery. J Pharm Res. 2008;7(1):67-73.
- Kumar P, Jain A, Ghosh P, Kaur M. Development and evaluation of mucoadhesive nasal systems for improved drug delivery. Eur J Pharm Biopharm. 2008; 68(1):28-34.
- Bhatia N, Mody N, Hegde S, Arora S. Nasal drug delivery systems: Current status and future prospects. J Drug Deliv Sci Technol. 2008; 18(4):265-274.
- Sharma S, Kumar M, Verma D, Gupta R. Formulation and characterization of mucoadhesive nasal gels for the delivery of anti-nausea drugs. J Pharm Sci. 2008; 12(6):87-94.
- Patel J, Shah S, Bhatt P, Doshi N. Comparative evaluation of different mucoadhesive polymers in nasal gel formulations. AAPS PharmSciTech. 2008; 9(4):123-130.
- Patel S, Yadav N, Kumar P. Development of nasal microemulsions for effective delivery of anti-hypertensive drugs. Drug Dev Ind Pharm. 2008; 34(5):543-550.
- Singh R, Sharma R, Saini S, Dey S. Nasal delivery systems for rapid onset of action: A review. J Control Release. 2008; 131(3):221-229.
- Jain N, Patel S, Kumar V. Preparation and evaluation of mucoadhesive nasal formulations for controlled drug delivery. Int J Pharm. 2008; 357(1-2):221-229.
- Ghosh P, Singh R, Kumar D, Agarwal M. Development of mucoadhesive nasal formulations for sustained drug delivery. Eur J Pharm Sci. 2008; 35(4):305-311.
- Desai S, Mistry R, Singh S. Comparative studies of nasal drug delivery systems: Microemulsions versus gels. J Pharm Pharmacol. 2008; 60(10):1307-1314.
- Yadav K, Agarwal P, Kumar S, Sharma D. Formulation and characterization of nasal formulations for improved therapeutic efficacy. J Pharm Res. 2008; 7(5):78-85.


 Mahadeo khose*
Mahadeo khose*
 Thakursing Dinesh Pawar
Thakursing Dinesh Pawar










 10.5281/zenodo.13754880
10.5281/zenodo.13754880