With the potential to treat a variety of genetic illnesses, malignancies, and chronic aliments, cell and gene therapy (CGT) is a revolutionary method in contemporary medicine. However, because biological materials are complicated, precision is requiring, and many therapies are highly customized, the production of CGT products is fraught with difficulties. This procedure entails the controlled, scalable, and repeatable isolation, alteration, growth, and delivery of cells or gene. Maintaining product quality, making sure regulations are followed, controlling supply chains, and creating economical manufacturing technique are some of the primary obstacles. Although CGT manufacturing has greatly benefited from advancements in bioprocessing automation, quality control, and cryopreservation, the sector still faces challenged in reaching the scalability, consistency, and cost required for broad clinical use. The present state of CGT production, the technologies influencing its future, and the tactics required to get over current obstacles to the effective commercialization of cell and gene therapies.
Cell therapy, Gene therapy, Viral vector, good manufacturing practices (GMP), Scalability
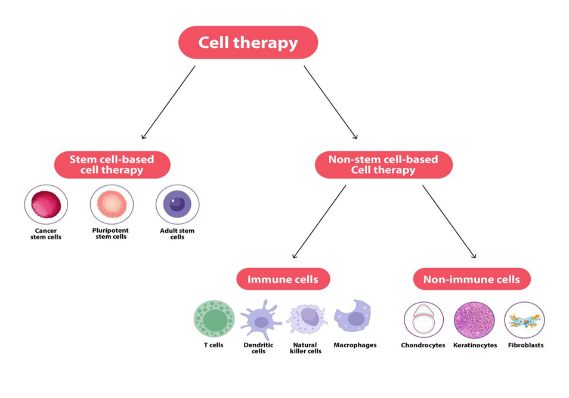
An organism embryos and adult cells contain stem cells, which are a sort of unspecialized, self-renewing cell that can differentiate into any type of cell or a variety of cell types. The developmental potency of stem cells determines the number of cell types they can differentiate into human stem cells are used in stem cell-based therapies to cure illnesses. Although all three of primary types of stem cells are able to differentiate and self-renew, their capacities for development vary.
Three types of stem cells are utilized or targeted in cell therapy: Cancer stem cells (CSCs), Pluripotent stem cells (PSCs), and adult stem cells (ASCs) [5].
- Cancer stem cell (CSCs) is one of the biggest threats to global health is still cancer. Treatment resistance, metastasis, and cancer recurrence are thought to be caused by CSCs. Certain markers, including CD133, CD44, CD90, CD34, ALDH1 and EpiCAM, are used to identify CSCs. By separating CSCs from malignancies, these indicators make targated treatments possible. Because of their special abilities to self-renew, differentiate, and evade the immune system, CSCs play a key role in the development of cancer and treatments resistance [6].
- Pluripotent stem cell with exception of those needed for the placenta, pluripotent stem cells (PSCs) can differentiate into almost any type of cell in the body. PSCs come in three primary varieties [7].
a) Embryonic stem cell inner mass cell of blastocytes give rise to ESCs, which have enormous promise for medicinal treatments like heart disease or diabetes cell regeneration. However, because extracting then damages the embryo, their usage raises ethical questions.
b) Epiblast stem cell these cells are pluripotent like ESCs but have different characteristics. They are found in the epiblast layer of a blastocyst following implantation. Their pluripotent differs from that of ESCs, yet they can develop into a variety of cell types [8].
c) Induce pluripotent stem cell are essential for drug development, disease modeling, and possible treatments. Although their application is still mostly in the experimental stage, it has potential for the treatment of disease like heart failure and macular degeneration [9].
- Adult stem cell
The bone marrow, fetal liver, spleen, umbilical cord, and placenta all contain hematopoietic stem cells (HSCs), which are special adult stem cells. They have the ability to move through blood and have the capacity to grow into tissues other than the blood system, such liver cells. Interesting questions concerning the functions of HSCs in reacting to illness and tissue damage are brought up by this adaptability. HSCs may even develop into neurons, suggesting that they have the capacity to differentiate into variety of cell types in different stages of the embryo [10].
2.2 Non-stem-based cell therapy
Somatic cells from the human body are used in non-stem-based cell therapies These cells are obtained, grow, and gene for medical, prophylactic or diagnostic reasons [11].
The following are two categories of non-stem-based cell therapy:
- Immune cells
To induce an immunological response against a tumor, immune cell ACT entails injecting patients with altered peripheral or tumor-resident immune cells [12].
Immune cells that have been altered:
- T Cells: Immunological memory, homeostasis, and the establishment and maintenance of immune response all depend on T cells. They have receptors that provide self-tolerance while simultaneously recognizing a broad variety of antigens from cancers and infections. The bone marrow produces the progenitors of T selection before being released into the body. T cells are found in almost every organ and tissue in the body, including mucosal locations, main and secondary lymphoid tissues, and depending on the tissue and life stages, their functions can differ greatly. T cells are linked to a number of autoimmune and inflammatory conditions, underscoring their vital role in both health and illness There are numerous important T cell substrates.
1.Native T cells are prepared to react to novel antigens.
2.Memory T cells provide long-term protection and are produced from previous antigens exposures.
3.Regulatory T (Treg) cells assist in controlling and reducing immunological responses to avoid hyperactivity
b) Dendritic cells: An essential component of antigen-presenting cells (APCs) that connect innate and adaptive immunity as dendric cells (DCs). They are essential for triggering T lymphocytes, such as helper and cytotoxic T cells, to fight cancer and infections. In addition to simulating immunological responses, DCs help the body develop immune tolerance by assisting in the
differentiation of self from non-self, thereby averting autoimmune reactions. DC therapy has demonstrated promise in treating a number of illnesses, include autoimmune disease (like melanoma, prostate cancer, endometrial cancer, non-small cell lung cancer, hepatocellular carcinoma, colorectal cancer, and mesothelioma) transplantation (particularly kidney and diseases (such as leishmaniasis, COVID-19, hepatitis B, and HIV). DCs have the ability to modify immune responses for improved tolerance and protection again a variety of illnesses in addition to improve anti-tumor responses [14].
c) Natural killer (NK) cells: A unique subset of large granular lymphocytes known for their capacity to generate cytokines and their inherent cytotoxicity against tumor cells are known as natural killer (NK) cells. It has been discovered over time that NK cells have complex systems in place to regulate cytotoxic reactions and reduce tissue damage. NK cells use their connections with neighboring cells to maintain a balance between activation and inhibition. In order to prevent possible autoimmunity and maintain efficient immune surveillance, this complex identification mechanism is necessary. [15].
d) Macrophages: Macrophages (MCs) are found in all tissues and are essential for organogenesis, post-injury regeneration, disease processes, and homeostasis, their origin, tissue type, and polarization in response to different environmental stimuli all contribute to their diversity as a cell population. MCs can take on a variety of phenotype, including anti-inflammatory M2 macrophages that promote wound healing and repair by interacting with stem cells in their niches and fibrosis. Developing novel therapeutic approaches of diseases requires an understanding of their phenotypic diversity of macrophages in both health and disease, emphasizing their systemic contributions to tissue homeostasis and repair mechanisms [16].
- Non-immune cells
It is anticipated that non-immune cells, such as neurons, mesenchymal cells, stromal cells, epithelial cells, epidermal keratinocytes, and synoviocytes, will contribute to the host’s defense system not only as structural components but also as regulators and effectors of its defense immune response. Numerous immune response types can be impact by nonimmune cells [17].
- Chondrocytes: The main cartilage cells, chondrocytes, are embedded in a rich extracellular matrix (ECM), Pericellular matrix (PCM), which has a particular structure, molecular makeup, and physical characteristic that are crucial for manufacturing chondrocyte’s function, envelops a chondrocyte in each chondron. [18].
- Keratinocytes: In addition to being utilized in cell therapy for burns and genetic skin problems, keratinocytes cultures including stem cells have been essential in expanding our knowledge of stem cell and keratinocytes biology, human keratinocytes stem cells were first recognized as holoclones by clonal analysis. This allowed for image - based identification and a non-invasive evaluation of their proliferation potential applications of keratinocytes. [19].
Fibroblast: Fibroblast cell are non-immune cells found in connective tissue that are used in fibroblast therapy to encourage tissue regeneration and healing. These cells produce collagen and extracellular matrix components, which are essential for wound healing. Fibroblast therapy can aid in tissue repair, scar reduction, and skin renewal in both medical and cosmetic settings. The therapy, which is a promising treatment for a number of aliments, involves isolating and injecting fibroblasts into damaged areas in an effort to promote repair processes independently of immune reactions [20].
- Gene Therapy of Manufacturing
In order to repair to reconstruct the disease-causing mutation, a healthy gene is inserted into the patient’s DNA using a procedure called gene therapy. A normal gene integrates into a different chromosomal position than the defective allele when it is introduced into q nucleus of a mutant cell. A new mutation arises as a result of integrating a normal gene into another functional gene, however the mutation may also be restored. Gene therapy’s cere tenet is to address genetic problems at their root [21].
3.1. Gene therapy using a somatic cell: This kind targets somatic cells with genetic alterations. It is safer since these cells are non-reproductive, meaning that the effects are not passed on the subsequent generations. Since most tissues will be replaced by new ones, the short-term consequences of somatic cell treatment are a drawback.
3.2. Gene treatment using a germ cells: This kind of gene therapy involves introducing germ cells, such as sperm or ova. Gene therapy using cells. This kind of gene therapy involves introducing germ cells – either sperm or ova – to transport vectors to the target cells. Gene therapy is further divided into ex-vivo and in-vivo therapy.
a) Gene therapy Ex-vivo: Ex-vivo gene therapy involves removing the faulty cells from the body and introducing a therapeutic gene to them. After being effectively altered, they are cultivated outside of the body and then returned to the host where are altered gene now reproduces.
b) Gene therapy In-vivo: This technique involves injecting a normal gene into host cells using a vector that can contain the therapeutic gene. Gene therapy can be classified as either gene addition or gene replacement depending on the sort of alteration made to the defective gene. Replacement of genes replacing a damaged gene with a repaired one is known as gene replacement.
3.3. Gene therapy based on gene addiction: Gene addition is the process of introducing a functional or normal copy of aa gene into the genome to restore the cell’s normal function. This idea is mostly applied in different cancer research projects that involved gene therapy. Because both laypeople and biologist frequently use terms like genetic engineering, which are categorized into gene therapy of somatic cells and gene therapy of germ cells based on the modification of cells by therapeutic genes, it is crucial to comprehend some of the terms that are frequently used in relation to gene therapy [22].
3.4. Somatic cell gene therapy: This type targets genetic alterations in some cells. Since somatic cells are not reproductive, the effect won’t be passed on to the progeny, making it relatively suffer.
3.5. Germ cell gene therapy: This type targets genetic alterations in germ cells. Because the germ cells (either sperm or egg) are inserted into the therapeutic gene, genetic alterations in this kind of gene therapy are transmissible. Future generations may be impacted by this kind. It can be categorized as either in vivo or ex vivo according on how the vectors are delivered into the target cells.
a) Ex-vivo type gene therapy: This technique involves removing damaged cells from the body and targeting them with a therapeutic gene. The cells are cultured in an ex-vivo environment before being returned to the host, where the changed gene is replicated.
b) In-vivo type gene therapy: In this kind of gene therapy, the healing gene is transferred to the host cells by means of an injection of a vector. Based on the kind of modifications made to the faulty gene during gene replacement and gene addition treatment, it can be categorized.
3.6. Replacement of genes: This approach substitutes a modified gene for the defective one.
3.7. Treatment for gene addiction: By introducing a functional or standard copy of the gene into the genome, this techniques restores the cell’s normal functions [23].
3.8. Requirements of gene therapy: The foundations of gene therapy include determining which delivery which delivery system is best for the gene (often a virus, also known as viral vector) confirming that the gene being transported can be expressed in the host cells, and establishing that the technology being utilized is safe. Clinical studies fir human gene therapy seldom pass all these tests, usually because the cell does not express the gene or the delivery device does not reach the cell. Improved gene therapy method is being developed through the use of nanotechnology. One promising use of this study is to specifically eliminate cancer-causing cells while shielding healthy cells from harm by encapsulating genes into nanoparticles that target cancer cells [24].
3.9. Gene treatment vector: There are several ways to transfer cells to DNA using a material called a vector. There are two primary types of vectors; vital and non-viral vector.
a) Vector of viral: A virus uses its genetic material to create viral proteins by injecting it into a host cell during replication. By incorporating their genetic material into the host’s DNA, retroviruses go one step further. By substituting therapeutic DNA for the virus’s genetic material, scientists take advantage of this. Gebe therapy makes use of variety of viruses, such as retroviruses and adenoviruses. Theoretically, this therapeutic DNA can either permanently integrate into the host’s DNA or sever as a temporary blueprint [25].
b) Vector that is not viral: Large-scale production and less host immunogenicity are features of non-viral vectors; yet, their gene expression and transfection may be lower. Magneto transfection, DNA injection, and naked gene guns are examples of non-viral techniques. Startups tacking non-viral delivery issues include gene Edit, Spotlight Therapeutics, and Sixfold bio. In the future, non-viral delivery techniques might take the place of virus-based ones due to their versatility and recurrent administration. Some businesses concentrate on non-viral delivery systems for siRNA and non-viral transport of MRNA payloads, while others concentrate on non-viral gene editing technologies [26].
4. Manufacturing Process of Cell and Gene Therapy
The equipment required and its possible effects on the cleanroom environment must be taken into consideration when developing a cleanroom for cell and gene therapy. Among the main dander of cleanroom equipment are:
- Heat Emission: take into account proper ventilation or cooling systems as they may impact temperature regulation.
- Contaminant release: Use HEPA filters and make sure that the right cleaning procedures are followed because equipment may introduce particles.
Chemical exposure: Use caution when handling Potentially dangerous products.
4.1. Equipment
This section outlines the essential equipment needed for culturing and characteristic Cell and Gene therapy.
- Tissue culture laboratory: Class-ll biosafety cabinet, CO2 incubator, pipettors, low speed centrifuged, water bath (370C).
Microscopy: Phase-contract microscope, dissecting microscope.
- Storage: Cabinets/Shelves, Refrigerator (40C), Freezer (-200C), Low Temperature Freezer (-70 to 850C), Cryogenic Freezer (Below-1400C).
- Molecular: RT-PCR, Flow Cytometer, Fluorescence Microscope, Confocal Microscope.
- Quarantine Laboratory: Same as Tissue Culture Laboratory (with additional sink).
- Access to core facility: Microscopy, Flow Cytometer, Microarray, Genomics, Proteomics, Virus Production, Vivarium [27].
4.2. Manufacturing strategy and concept of design
One batch at a time, small-scale production is necessary for customized cellular products, such as TILs for cancer treatment. The production process has been enhanced by closed-system technology and single use disposable consumables. Hospital departments have been able to create effective plans for producing customized goods in a small GMP facility by combining closed- system technologies with isolator systems [28].
1) MSC Based cell therapies: - MSC based cell cancer these treatments typically involve T cells, dendritic cells, and natural killer (NK) cells, while stem cell-based treatments concentrate on hematopoietic stem cells (HSCs) or MSCs themselves. MSCs can be obtained from bone marrow, adipose tissue, or Umbilical cord tissue, and they primarily exert their therapeutic effects through paracrine signaling instead of direct cell replacement. The manufacturing process involves tissue procurement, MSC isolation, and expansion in culture media, with quality control ensuring the cell identify, potency, and safety. However, issues such as donor variability and culture conditions and effect MSC characteristic, making effective large-scale expansion crucial for clinical applications while controlling costs and safety concerns.
2) Gene therapies based on AAV and LV: -Two important vectors for gene therapy are lentivirus (LV) and adenoassociated virus (AV). Approved for diseases such as retinal degeneration, AAVs are low immunogenic and carry only one DNA strand. LVs integrates RNA genomes into the host for long-lasting expression, targeting conditions including beta thalassemia and B-cell lymphoma. Whereas LVs are created by co-transfecting several plasmids, AAV are created utilizing certain plasmids. To guarantee safety, both need intricate purification that takes into account problems like contamination. Although stable producer cell lines can lower expenses and increase scalability, production optimization is still difficult.
3) Treatments for CAR-T Cell Manufacturing: -Cell and gene production are combined in CAR-T cell manufacturing which is mainly used for cancer immunotherapy against leukemia and B-cell lymphoma. To produce CARs that recognize certain cancer antigens, the procedure starts with leukapheresis to gather T cells, which are subsequently genetically altered using viral vectors, usually lentiviruses (LVs). In order to maximize their activity. T cells are best grown and activated in serum-free medium. Because impurities might reduce effectiveness, quality control focuses on guaranteeing the potency and purity of T cells as well as CAR expression. The goal of automated system innovations is to cut the vein-to-vein time of CAR-T infusion to two weeks, which would improve patient results and manufacturing consistency [29].
4.5. Quality control testing [30]
For clinical application, the intricate CGT manufacturing process necessitates extensive quality control and validation. Important characteristic is evaluated, including identity potency purity, and safety. It is essential to perform quick quality control assay such as, mycoplasma nucleic acid amplification. To ensure the safety viral vectors must not contain replication-competent vectors.
Table no1: Measurement of quality control and specification


 Radha Virulkar*
Radha Virulkar*
 Sayali Ganjiwale
Sayali Ganjiwale
 Sachin Dighade
Sachin Dighade
 10.5281/zenodo.14584479
10.5281/zenodo.14584479