Abstract
This research focuses on the formulation and evaluation of floating sustained-release tablets of Ranolazine, an anti-anginal agent with poor bioavailability and short half-life. The tablets were prepared using various polymers such as hydroxypropyl methylcellulose (HPMC) and sodium alginate to achieve controlled drug release and prolonged gastric residence time. The formulation was optimized using a 32 full factorial design to study the effect of independent variables like polymer concentration and tablet hardness on drug release kinetics and floating behavior. The prepared tablets exhibited excellent floating properties with an extended-release profile over 12 hours. The optimized formulation showed desirable characteristics, including buoyancy, controlled drug release, and good physicochemical stability. In vitro dissolution studies revealed sustained release of Ranolazine from the tablets, indicating their potential for once-daily dosing regimen, enhancing patient compliance, and improving therapeutic efficacy. The developed floating sustained-release tablets of Ranolazine could be a promising dosage form for the treatment of chronic stable angina pectoris.
Keywords
Floating, Angina, Ranolazine, Extended, Sustained.
Introduction
Drug delivery is the method or process of administering pharmaceutical compound to achieve a therapeutic effect in humans or animals Novel Drug delivery System (NDDS) refers to the approaches, formulations, technologies, and systems for transporting a pharmaceutical compound in the body as needed to safely achieve its desired therapeutic effects.
Gastro retentive Drug Delivery System
The pharmaceutical industry is becoming more interested in oral controlled release drug delivery because to its increased therapeutic benefits, which include patient compliance, convenience of dosage, and formulation flexibility. By releasing the medication gradually into the gastrointestinal tract, this technique seeks to keep an effective concentration in the systemic circulation for an extended period of time, enabling a steady supply to the absorption site. [1] By focusing on site-specific release in the upper gastro intestinal tract, gastro retentive medication administration extends the stomach residence period while producing both local and systemic effects. Numerous strategies, such as systems with a high density, have been created. [2,3,4] The numerous gastro-retentive techniques that have lately emerged as industry leaders in the development of site-specific oral controlled release medication delivery systems are covered in the current study. Because dosage forms stay in the stomach for longer periods of time than conventional dosage forms, the ability to extend and control the emptying time is a major advantage. Gastric emptying of dosage forms is a highly variable process. Creating a regulated delivery method to improve bioavailability and absorption presents a number of challenges. The inability to limit the dose form in the intended gastrointestinal tract region is one of these challenges. The process of absorbing drugs from the gastrointestinal tract is intricate and diverse. It is commonly known that contact time with the small intestine mucosa affects how much a medicine is absorbed via the gastrointestinal system. Therefore, for medications that are not fully absorbed, the small intestine transit time is a crucial component. A summary of basic human physiology is provided, including information on gastric emptying, motility patterns, and physiological and formulation factors that impact stomach emptying. The gastric residence period of medications is further extended by gastro retentive devices, which can stay in the stomach area for several hours. Extended stomach retention increases the solubility of medications that are less soluble in high pH environments, decreases drug waste, and increases bioavailability. Additionally, local medication administration to the stomach and proximal small intestine can be accomplished with its help. [5] Better accessibility to novel products with novel therapeutic potential and significant patient benefits is facilitated by gastro retention. The process of mucoadhesion may be used to accomplish the regulated stomach retention of solid dose forms.[6] Flotation, sedimentation, expansion, altered form systems, and concurrent delivery of pharmaceutical drugs are some of the possible processes involved. [7,8,9]
Basic Physiology of Gastrointestinal Tract:
There are three sections to the stomach: the fundus, the body, and the antrum (pylorus). Undigested matter is stored in the fundus and body, and the stomach is mixed and emptied by the antrum. Both fasting and feeding, the stomach empties. [10] This is known as the interdigestive myoelectric cycle, or migrating myoelectric cycle (MMC), and Wilson and Washington have further split it into the following four phases.
- Phase I or the basal phase, contains contractions and lasts for 40–60 minutes.
- The 40–60-minute Phase II (pre-burst phase) is characterized by sporadic contractions and action potentials. Both the intensity and frequency steadily rise as the phase goes on.
- Phase III (the explosion phase) lasts for four to six minutes. It consists of brief, strong contractions that occur often. All of the undigested material is carried out of the stomach and into the small intestine by means of this valve. Another name for it is the "housekeeper wave."
- Phase IV, which happens in between phases III and I of two consecutive cycles, lasts for 0 to 5 minutes. [11]
FLOATING SYSTEM:
Gastroretentive dosage forms can remain in the gastric region for several hours, significantly prolonging the gastric residence time of drugs. Prolonged gastric retention improves bioavailability, reduces drug waste, and improves solubility of drugs that are less soluble in a high pH environment. Over the past three decades, oral controlled release dosage forms have been developed due to their considerable therapeutic advantages, such as ease of administration, patient compliance, and flexibility in formulation. However, this approach has several physiological difficulties because of variable gastric emptying and motility. [12] Since floating drug delivery systems may solve the disadvantages of conventional drug delivery systems, such as frequent dosing, limited bioavailability, etc., in comparison to quick stomach emptying time, they have attracted a lot of attention in recent decades. One way to characterize an ideal floating drug delivery system is one that stays in the stomach long enough to release the active medication gradually. These continue to float over the stomach contents. Consequently, the pharmacological impact is extended, increasing the drug's bioavailability. This review of the literature on gastroretentive and floating tablets was written with the intention of gathering new information on the topic, including the principles, benefits, and classification of floating tablets, preparation methods, evaluation techniques, list of medications formulated as floating tablets, formulation evaluation, and potential applications of floating tablets in the future. One type of gastroretentive medication administration technique is the floating tablet. Drugs with a limited window of absorption, those less soluble in water in the alkaline pH of the small intestine, and those less stable in the intestinal or colonic environment can all have their bioavailability increased by the use of gastroretentive systems, which lengthen the residence time of dosage forms in the stomach. [13,14]
Disadvantages of floating drug delivery system:
- Drugs with issues with solubility or stability in the gastrointestinal tract cannot be used in a floating system.
- For these systems to function well and allow for the distribution of drugs, there must be a significant volume of fluid in the stomach.
- The only suitable candidates are those medications that have considerable first pass metabolism and are greatly absorbed throughout the gastrointestinal system.
- Some medications that are part of the floating system irritate the stomach mucosa. [15]
Evaluation of extended-release Floating Tablet:
Any USP monograph for a solid oral dosage form that requires drug absorption for the purpose of achieving a therapeutic effect requires in vitro dissolution testing, which is a crucial component of quality assurance. Dissolution testing has drawn attention as a bottom-of-the-predictive tool for in vivo performance following the adoption and implementation of the SUPAC IR/SR initiatives (scale-up and post-approval changes for immediate and modified based systems with a propensity for either sticking to release oral dosage forms). For some post-approval modifications to formulations and manufacturing processes, in vitro dissolution testing may be utilized in lieu of in vivo bioequivalence testing in the approval process, given that a reliable in vivo in vitro correlation has been established. [16] Numerous experimental challenges have come to light as a result of the increasing emphasis being paid to dissolving testing. For example, it has been noted that compendial dissolving medium frequently fail when in vitro–in vivo correlations are tried for Class II medicines, i.e., medications with high permeability but inadequate water solubility to allow the complete dosage to be dissolved under typical GIT circumstances. As opposed to this, more effective medium can reflect the pH and bile salt levels of the small intestine when it is fed and fasted. [17] The suggested implementation of a two-tier disintegration method for goods contained in hard gelatin capsules serves as another illustration. It has been shown that, even if they are bioequivalent, capsules that undergo mild stress and cause gelatin to crosslink frequently fall short of the conditions for compendial disintegration. Pepsin can be added to simulated gastric juice to remedy this problem. [18,19].
MATERIALS AND METHODS
Hydrophilic Polymers:
HPMC K4M, HPMC K15M, HPMC K100M, Carbopol 934P and Chitosan to modify the pattern of Ranolazine release from matrix.
Effervescent agent:
Sodium bicarbonate
Filler:
Micro Crystalline Cellulose
Antiadherant: Talc
Lubricant:
Magnesium Stearate.
Pre-formulation Studies
- Determination of Melting Point:
Melting point of Ranolazine was determined by capillary method. Fine powder of Ranolazine was filled in glass capillary tube (previously sealed on one end). The capillary tube is inserted into the melting point apparatus and observed the temperature at which drug started to melt by using the thermometer which was already immersed into the liquid paraffin in the apparatus.
- Compatibility study:
A successful formulation of a stable and effective solid dosage form depends on careful selection of the excipients that are added to facilitate administration, promote the consistent release and bioavailability of the drug and protect it from degradation. If the excipients are new and have not been used in formulations containing the active substance, the compatibility studies are of paramount importance.
- Angle of Repose:
Angle of response is the angle of inclination, formed to the flat surface by the bulk of granules when it is allowed to flow under gravitational force from a fixed height. It is a characteristic of granule flow properties and is calculated by using the formula.
? = tan -1 (h/r)
Where, ? - Angle of repose, h - Height of granule above flat surface, r - Radius of circle formed by the granule pile
4. Carr’s Index
It is also a characteristic of granule flow properties. The bulk density and tapped density was measured and compressibility index was calculated using the formula,
C.I. = {(Pt – P0) / Pt} × 100
Where, Pt = tapped density
?0 = bulk density
5. Hausner Ratio
Tapped density and bulk density were measured and the Hauser ratio was calculated using the formula,
Hausner ratio = Pt / P0
Where, Pt = tapped density, ?0 = bulk density
6. FT-IR:
Compatibility of the Drug with the excipients was determined by subjecting the physical mixture of the drug and the polymers of the main formulation to infrared absorption spectral analysis. Any changes in chemical composition of the drug after combining it with the polymers were investigated with I.R. spectral analysis.
7. The Standard Calibration Curve of Ranolazine
Ranolazine was quantitatively analysed by various techniques. In the present study, Ranolazine was estimated by UV spectrophotometry method.
Determination of ?max for: Ranolazine
To prepare a ranolazine stock solution, accurately weigh 100 mg of ranolazine using an analytical balance. Dissolve the ranolazine in ethanol, using a sonicator if needed. Fill the flask to volume and dilute with ethanol to create a 1000 µg/mL stock solution. Dilute the stock solution with ethanol to obtain desired concentrations.(10,20,30,40,50,60µg/mL) Calculate the required volumes and use a UV-Vis spectrophotometer to measure the absorbance at the wavelength 272nm to ranolazine. Use ethanol as a blank to zero the spectrophotometer and plot the absorbance values against corresponding concentrations. Calculate the line equation and verify the results. The ?max was found to be 272 nm.
Procedure:
Accurately weighed quantities of polymer and MCC for each batch were taken (in a mortar and mixed geometrically, to this required quantity of Ranolazine was added and mixed slightly with pestle. Accurately weighed quantity of Sodium bicarbonate was taken separately in a mortar and powdered with pestle. The powder is passed through sieve no 40 and mixed with the Ranolazine blend which was also passed through sieve no 40. The whole mixture was mixed for 3 minutes. To this Magnesium stearate was added and mixed for minutes, later Talc was added and mixed for 2 minutes. The mixture equivalent to 850mg was compressed into tablets with 13.5mm capsulate punches (A total weight of 170g powder blend was required in each batch containing 100 tablets). The composition of various formulations was given in Table HPMC- Hydroxy propyl methyl cellulose. All the formulations Contained 1% of Magnesium Stearate and 1% Talc

Evaluation of Tablets
- Hardness:
Tablet hardness has been defined as the force required for breaking a tablet in a diametric compression test. A tablet was placed between two anvils of the hardness tester (Monsanto type), force was applied to the anvils, and the crushing strength that caused the tablet to break was recorded.
- Thickness:
The tablet thickness is essential for consumer acceptance and to maintain tablet to tablet uniformity. The thickness of the tablets was measured using vernier calliper. It is expressed in mm. 5 tablets of each batch were picked randomly and its thickness were measured individually. The thickness of the tablet is mostly related to the tablet hardness3. Friability: Tablets require a certain amount of strength, or hardness and resistance to friability, to withstand mechanical shocks of handling in manufacture, packaging and shipping. Pre-weighed tablet samples (20 tablets) were placed in the friabilator, which was then operated for 100 revolutions, dropping the tablets a distance of 6 inches with each revolution. The percentage friability was calculated using the formula.
% Friability = [(W1 – W2) / W1] × 100
Where, W1 = Initial weight of 20 tablets and W2 = Weight of the 20 tablets after testing
- Weight variation test
20 tablets were selected at random and weighted individually. The average weight of each batch of tablet was calculated. Individual weights of the tablets were compared with the average weight. Since the tablets weighed over 100 mg, I.P. specifies that the tablets pass the test if not more than two of the individual weights deviate from the average weight by more than 7.5%.
?viation = (Individual weight – Average weight / Average weight) × 100
- Drug content:
To evaluate tablets potential for efficacy, the amount of drug per tablet needs to be monitored from tablet to tablet, and batch to batch. To perform the test, ten tablets from each batch were weighed and powdered. Powder equivalent to the average weigh of the tablet was accurately weighed and transferred into a 100 ml volumetric flask and dissolved in a suitable quantity of distilled water. The solution was made up to the mark and mixed well. A portion of the sample was filtered and analysed by a UV spectrophotometer at 272 nm.
- In vitro Buoyancy Study:
The time taken for dosage form to emerge on surface of medium called floating lag time (FLT) and duration of time by which the dosage form constantly emerges on surface of medium called total floating time. (TFT).
Procedure:
One tablet from each formulation batch was placed in USP type II dissolution apparatus containing 900 ml of 0.1N HCl using paddle at a rotational speed of 50 rpm. The temperature of medium was maintained at 37±2 oC. the time taken for tablet to emerge on surface of medium and the duration of time by which the
RESULT AND DISCUSSION
FTIR Spectral Analysis
The different peaks of drug, polymer and their physical mixture indicate all groups and characteristics of the drug were not altered. There is no significant interaction in drug and polymer.

Graph No.1: FTIR Spectral Analysis of Ranolazine

DISCUSSION:
Physical mixture of drug and polymer was characterized by FTIR spectral analysis (Graph 1) for any physical as well as chemical alteration of drug characteristics. From results, it was concluded that there was no interference in the functional group as the principal peaks of Ranolazine were found to be unaltered in the drug polymer physical mixture.
Standard Calibration Curve of Ranolazine
Graph No.2: Calibration curve of Ranolazine in 0.1 N Ethanol

From the scanning of drug in ethanol it was concluded that the drug had ? max of 272.0 nm and which was exactly similar as reported. From the standard curve of ethanol, it was observed that the drug obeys Beer – Lambert’s law in concentration range of 0 – 60µg / ml in the medium.
Differential Scanning Colorimetry And Thermogravimetric Analysis
Graph No. 3: DSC and TGA of Ranolazine

By understanding the thermal behaviour of ranolazine, This discussion outlines the general interpretation of DSC results for ranolazine. DSC curves and data would provide a more detailed analysis tailored to the observed thermal behaviour. Understanding the thermal properties ensures that Ranolazine maintains its efficacy and safety throughout its shelf life and under various storage conditions.
Pre-compression Parameters: Charecterstic of Final Blend

DISCUSSION:
Table indicates the powder characteristics of various batches of floating tablets. Various formulations show good flow properties. Results of Angle of repose (210.45? – 280.69?), Carr’s index (13.25 – 16.48), Hausner’s ratio (1.12-1.167) shows satisfactory results, which is required for better bioavailability
Evaluation of Formulated Tablet
Post Compression Parameter:

DISCUSSION:
From above table physical parameters of each batch, it was concluded that the tablets of all batches had desirable physical characteristics. Results of Hardness of various batches of prepared formulations (7.24 –7.85 kg / sq. cm.) and Friability (0.53 – 0.76 %) indicates that the tablets having sufficient strength to withstand physical abrasion. Tablets of all batches pass the weight variation test as per the limits prescribed in IP. (5?viation is allowed for average weight of tablet X ? 250 mg).
In-vitro Buoyancy study of Ranolazine Floating Tablets

DISCUSSION:
Results of floating properties study reveal that all batches had good floating capability. This might be due to the presence of gas generating agent, i.e., sodium bicarbonate. Formulations from B1 to B2 decreased total floating time which may be due to high affinity of Carbopol towards water, which promotes water penetration into tablet matrices, leading to increased density. Formulations B3 to B5 showed satisfactory total floating time. The formulation B4 showed maximum floating time.

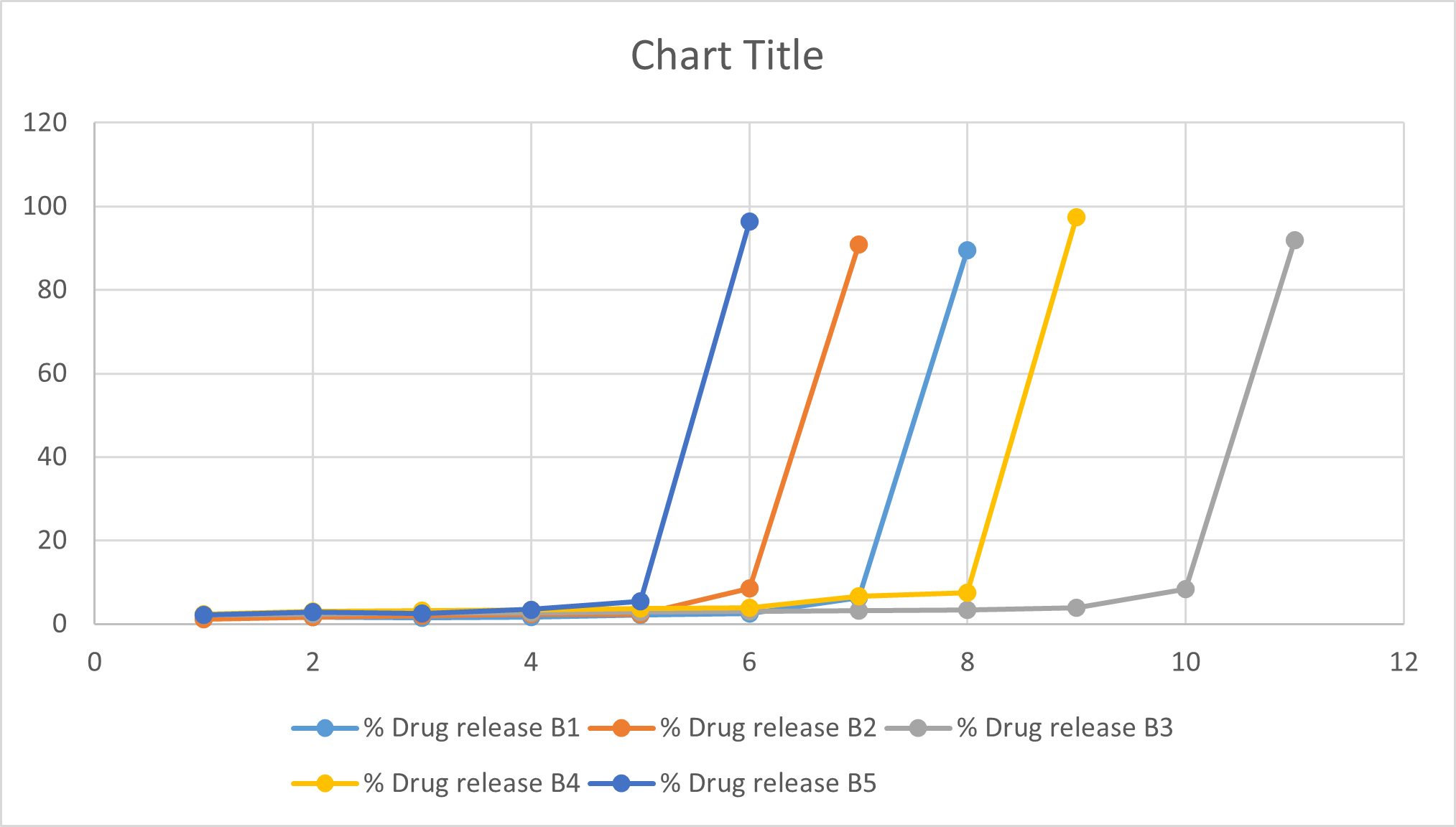
DISCUSSION:
In the dissolution study of Floating sustained release tablet, the percent drug release from batch B1 to B5 was found to be 89.52, 90.8, 91.98, 97.45, 96.32 respectively. In that batch no. B4 show more drug release compared to other batches. For the treatment of angina, it is necessary to release of tablet required release of drug after lag time that is 8 hrs. Graph of drug release show that in the batch no B4 release of drug after 8 hrs. Because of more drug release of batch, no B4 that is 97.45 and release of drug after 8 hrs. Hence batch no B4 is optimized batch.
CONCLUTION
The study presents a promising approach for enhancing the therapeutic efficacy and patient compliance in managing chronic angina. A floating sustained release tablet of Ranolazine was developed using a combination of HPMC K15M and Chitosan, ensuring a steady release of the drug over an extended period. The tablet's release rate was significantly influenced by the proportion and viscosity of the polymer used. The formulation achieved a controlled release profile, maintaining therapeutic drug levels for extended periods, which is critical for chronic conditions requiring steady plasma concentrations. The study identified optimal conditions for achieving the desired floating and release characteristics, enhancing patient adherence to the medication regimen. The formulation was well-tolerated within the gastric environment, and stability studies indicated that the tablet maintained its integrity and drug release profile over its shelf life. Overall, the floating sustained release tablet of ranolazine offers a significant advancement in angina pharmacotherapy, aligning with the goal of improving clinical outcomes through innovative drug delivery systems. Future studies could focus on in vivo evaluation and long-term clinical benefits to fully establish the efficacy and safety profile of this formulation in the target patient population.
REFERENCES
- Nayak AK, Maji R, Das B. Gastroprotective drug delivery system: A review ISSN 0974- 2441.
- Streubel A, Siepmann J. Bodmeier R. Multiple unit Gastroretentive drug delivery: A new preparation method for low density microparticles. J Microcapsule 2003;20:329- 47.
- Goole J, Vanderbist F, Aruighi K. Development and Evaluation of new multipleunit Levodopa sustainedrelease floating dosage forms. Int J Pharm 2007:334:35-41.
- Sharma S, Pawar A. Low density multiparticulate system for pulasatile release of Meloxicam. Int J.Pharm 2006:313:150-58.
- Santus G, Lazzarini G, Bottoni G, Sandefer EF, Doll WJ, Ryo UY, Digenis GA. An in vitro / in vivo investigation of oral bioadhesive controlled release furosemide formulations. Eur J Pharm Biopharm1997:44:39-52
- Klausner EA, Lavy E, Friedman M, Hoffman A. Expandable gastroretentive dosage forms. J control Release 2003:90:143-62
- Deshpande AA, Shah N, Rhodes CT, Malik W. Development of novel controlled release system for gastro retention. Pharm res 1997:14:815-19
- Park K. Enzyme digestible swelling as platforms for long term oral drug delivery: synthesis and characterization. Biomaterials 1988:9:435
- Radi Hejazi, Mansoor Amiji. Chitosan based gastrointestinal delivery systems. Journal of Controlled Release 203:89:151-165.
- [11] Benchgaard H, Ladefoged K. Distribution of pellets in gastrointestinal tract: The influence on transit time exerted by the density or diameter of pellets. J Pharm Pharmacol 1978:30:690Y692
- [12] Vantrappen GR, Petters TL, Janssens J. The secretory component of inter digestive migratory motor complex in man. Scand. J Gastroenterol. 1979:14663:Y667.
- Garg R, Gupta GD. Progress in controlled gastrointestinal delivery systems. Tropic J Pharm Res 2008;7:1055-66.
- Fukuda M, Peppas NA, McGinity JW. Floating hot-melt extruded tablets for gastroretentive controlled drug release system. J Control Release, 2006; 115(2): 121–129. [PubMed].
- Hancock BC, Christensen K, Clas SD; Microscale Measurement of the Mechanical properties of compressed Pharmaceutical powders, part 1: The Elasticity and Fracture Behavior of Microcrystalline Cellulose. Int. j. Pharm., 2000; 209: 27-35.
- Manish Rane, JayeshParmar, & Ali Rajabi-Siahboomi. Hydrophilic Matrices for Oral Extended Release: Influence of Fillers on Drug Release from HPMC Matrices. Pharma Times 2010; 42 (4): 41-5.
- Anon, Guidance for industry. Modified release solid oral dosage forms: scale up and post approval changes: chemis-try, manufacturing and controls, in vitro dissolution testing and in vivo bioequivalence documentation. US Department of Health, Food and Drug Administration, Center for Drug Evaluation and Research, September 1997.
- E. Galia, E. Nicolaides, D. Horter, R. Lobenberg, C. Reppas, J.B. Dressman, Evaluation of various media for predicting in vivo performance of class I and II drugs, Pharm. Res. 15 (1998) 698–705.
- J. Brown, N. Madit, E.T. Cole, I.R. Wilding, D. Cade, The ´ effect of cross-linking on the in vivo disintegration of hard gelatin capsules, Pharm. Res. 15 (1998) 1026–1030.
- Gelatin Capsule Working Group, Collaborative development of two-tier dissolution for gelatin capsules and gelatin coated tablets using enzyme containing media, Pharm. Forum 24 (1998) 7045–7050.


 Samrudh kiran Shinde*
Samrudh kiran Shinde*
 Shivaji Patil
Shivaji Patil









 10.5281/zenodo.12788828
10.5281/zenodo.12788828