Abstract
Prolonged usage of proton pump inhibitors (PPIs) has been linked to a higher likelihood of specific health problems, such as bone fractures and deficits in essential nutrients. The attention on floating systems led to a decrease in synthetic polymers by effectively using natural polymers, which possess desired characteristics such as biocompatibility and biodegradation features derived from HPMC. It is even feasible to alter its properties by adjusting the level of deacetylation in order to get reduced density, swellability, and enhance its suitability for drug delivery by floating methods. The present work successfully achieved its desired goal by developing a floating drug delivery system for Omeprazole. The experimental section yielded satisfactory results, which are summarised below. The floating tablets were synthesised using the wet granulation method. The characteristics assessed for granules included bulk density, tap density, compressibility index, Hausner's ratio, and angle of repose. The evaluated flow parameters and derived attributes for all 9 formulations were shown to be within acceptable ranges, indicating favorable flow characteristics. The physicochemical properties of floating tablets of all formulations were investigated by measuring parameters including thickness, diameter, hardness, friability, weight change, and drug content. Observed average values and variances for the above parameters were within the specified limits. The floating characteristics, including floating lag time and total floating time, were measured for all available tablets, revealing that all the tablets produced exhibited favorable floating features. The presence of the gas producing agents NaHCO3 and citric acid may be the cause of this observed phenomenon. The buoyant properties of the floating tablets were determined by the swelling of hydrocolloid particles on the surface upon contact with the stomach fluid, as well as the existence of empty space or porosity in the dry centre of the tablet. An In vitro drug release laboratory investigation showed that the manufactured tablet F1 exhibited the highest percentage of cumulative drug release after 12 minutes. This might be attributed to the presence of HPMC K100. The stability tests were carried out following the ICH recommended protocols for duration of 6 months. These findings indicate that HPMC with Omeprazole as floating tablets exhibit similar floating and drug release properties, suggesting that it may have moderate clinical effectiveness.
Keywords
Proton pump inhibitors (PPIs), HPMC, Omeprazole.
Introduction
Omeprazole is a proton pump inhibitor (PPI) medication that works by inhibiting the proton pump in gastric parietal cells, reducing the secretion of gastric acid. This makes it effective in treating conditions like gastroesophageal reflux disease (GERD), peptic ulcers, and Zollinger-Ellison syndrome (1). Omeprazole alleviates symptoms like heartburn and promotes healing of esophageal and gastric mucosal damage. Studies have shown that patients with GERD experience significant improvement in symptom control and quality of life when treated with omeprazole compared to placebo treatments. However, healthcare professionals should exercise caution when prescribing this medication due to potential side effects and long-term risks associated with prolonged use (2). Recent studies have raised concerns about the long-term use of PPIs like omeprazole, including an increased risk of kidney disease, bone fractures, and gastrointestinal infections like Clostridium difficile (3). Therefore, clinicians must carefully evaluate the risk-benefit profile when considering long-term therapy with omeprazole. The main goal of creating a floating tablet formulation is to prolong gastric retention time, improve drug absorption and therapeutic efficacy, and optimize omeprazole's pharmacokinetics. This is achieved by using specific excipients that facilitate buoyancy and controlled release. Key parameters during the formulation process include selecting appropriate polymers, such as hydrophilic polymers like HPMC or sodium alginate, and gas-generating agents like sodium bicarbonate. In vitro studies are crucial to assess the tablets' floating behavior and dissolution rates under simulated gastrointestinal conditions. The success of the formulation and evaluation depends on balancing sustained release kinetics and patient compliance. The development process includes rigorous testing for quality attributes like hardness, friability, uniformity of content, and stability. By addressing these objectives through systematic research, pharmaceutical scientists can significantly improve therapeutic outcomes for patients managing acid-related disorders.
MATERIALS AND METHODS
Drugs and Chemicals
Omeprazole from Microlabs, HPMC KM 100 from SD fine chemicals, Talc from Central Drug House, Magnesium Stearate from Central Drug House, and Microcrystalline Cellulose from Central Drug House.
Preformulation Studies
Characterization and Identification of the Drug
Organoleptic Properties
The drug samples were studied for appearance, color, and odor (4).
Determination of Melting Point
Melting point of Omeprazole was determined by capillary method. Fine powder of individual drug was filled in glass capillary tube (previously sealed on one end). The capillary tube was dipped in liquid paraffin inside the melting point apparatus the powder at what temperature it will melt, was noticed. Average of triplicate readings was taken (5).
Solubility
Solubility of drug was determined in various buffers of various pH values 1.2, 5.4 and 6.8. Solubility studies were performed by taking excess amount of these drugs in different beakers containing solvents. The mixtures were shaken for 24 hrs at regular intervals. The solutions were filtered by using Whattmann’s filter paper grade no. 41. The filtered solutions are analysed spectrophotometerically. Average of triplicate readings was taken (6).
Characterization of Omeprazole
Identification of Omeprazole by UV spectroscopy
Preparation of calibration curve of Omeprazole
Dissolve 28.20 g of anhydrous disodium hydrogen phosphate and 11.45 g of potassium dihydrogen phosphate monohydrate in 1000 ml of water. 3 mg of drug was accurately weighed and transferred into 100 ml of volumetric flask and solved in 15 ml of methanol. The volume was made up to 100 ml by using phosphate buffer 6.8 get concentration of 200 µg/ml. From the prepared stock solution 10 ml solution was withdrawn and transferred to another 100 ml volumetric flask and volume was made up to 100 ml to get 20 µg/ml. From above solution 1,2,3,4,5 ml of solution were separately transferred to 10 ml volumetric flask and volume made up to 10 ml to get concentration of 2,4,6,8,10 µg/ml (7).
Identification of Omeprazole by Fourier Transform Infrared (FTIR) Spectroscopy
The infrared spectra of the pure drug were recorded by Perkin Elmer FT-IR spectrometer. Samples were prepared by KBr disc method (2 mg sample in 100 mg KBr) and examined in the transmission mode. Each spectrum was measured over a frequency range of 4000-400 cm?1 (8).
Determination of Drug-Excipients Compatibility by FT-IR analysis
Omeprazole, a widely used proton pump inhibitor, exhibits significant therapeutic benefits in the treatment of gastric acid-related disorders. The compatibility of omeprazole with hydroxypropyl methylcellulose (HPMC) K100 is essential to ascertain its stability and efficacy in pharmaceutical formulations. Fourier-transform infrared spectroscopy (FTIR) serves as a pivotal analytical technique for evaluating the interaction between these compounds. This study aims to elucidate the results of the compatibility assessment of omeprazole with HPMC K100 via FTIR analysis (9).
Pre-Compression Evaluation of power blend for preparing the Floating Tablets containing Omeprazole
Floating tablets are designed to improve the bioavailability of drugs with solubility issues in the gastrointestinal tract. Key parameters evaluated during pre-compression include bulk density, tapped density, Hausner's ratio, and Carr's index, which provide insights into the flow properties and compressibility of the powder blend. Moisture content and particle size distribution also play a significant role in determining the stability and release profile of omeprazole within floating matrices (10).
Preparation of Floating Tablets containing Omeprazole by Direct compression
Floating Tablets containing Omeprazole were prepared by direct compression method according to the formula (Table 1). A total number of four formulations (F1 to F9) of Floating Tablets (250 mg) containing Omeprazole were prepared using only 1 disintegrants namely HPMC KM 100 with different concentrations. All the ingredients were passed through mesh no 60 separately and collected. The drug, Antiadherant (Talc), Effervescent agent (Sodium bicarbonate), Filler (Micro Crystalline Cellulose) and Hydrophilic Polymers (HPMC KM 100) and Lubricant (Magnesium Stearate) were mixed uniformly with gentle triturating using mortar and pestle to get a uniform mixture. Required quantity of superdisintegrants and aspartame were taken for each specified formulation and mixed with the above mixture (11).
Postcompression Evaluation of Powder Blended Characteristics of Floating Tablets containing Omeprazole
This study examines the postcompression evaluation of powder blended characteristics in floating tablets containing Omeprazole, a proton pump inhibitor. The study focuses on factors such as hardness, friability, disintegration time, and drug release kinetics. Understanding these characteristics is crucial for optimizing tablet performance and ensuring effective therapeutic outcomes. Factors like particle size distribution and moisture content also impact these evaluations. Comprehensive postcompression evaluations are essential for developing effective floating tablets that meet pharmacological standards (12).
In vitro dissolution studies
In- vitro drug release studies were carried out by using USP (TDT067) Type II (paddle type) dissolution test apparatus at 50 rpm using pH 6.8 phosphate buffer as dissolution media maintained at the temperature at 37±0.5°C. Samples were withdrawn at specific time intervals and replaced with fresh media and filtered. The amount of drug dissolved was determined by spectrophotometer at 250 nm. The experiments were conducted in triplicate (13).
Stability Studies
In the present study the stability study was conducted in accordance with ICH guidelines by keeping the sample at 40±2 0C and 75±5 % RH for six months in a stability chamber. The high density Polyethylene bottle is the container closure system used in this study. The selected study intervals were the 1st and 3rd months from the initial time (14).
RESULTS AND DISCUSSION
Preformulation Studies
Organoleptic characterization
Organoleptic characterization, melting point determination, and solubility check are essential techniques in the field of pharmaceutical sciences. These methods provide valuable information about the physical and chemical properties of drugs, which are crucial for their formulation and efficacy. Organoleptic characterization involves the sensory evaluation of a drug using our five senses – sight, smell, taste, touch, and hearing. This method helps in identifying any physical changes such as discoloration, odor, or taste alterations that may occur during storage or handling (Table 2).
Melting point determination
Melting point determination is another important technique used to assess the purity and identity of a drug. By measuring the temperature at which a solid substance melts, we can determine its melting point range and compare it with known values to confirm its identity (Table 2).
Solubility check of drug
Solubility check is also a key parameter in drug development as it determines the ability of a drug to dissolve in various solvents. This information is crucial for designing dosage forms that ensure optimal absorption and bioavailability of the drug. In conclusion, organoleptic characterization, melting point determination, and solubility check are indispensable tools in pharmaceutical research that aid in ensuring the quality and effectiveness of drugs. These techniques play a vital role in formulating safe and efficient medications for patient use (Table 3).
Characterization of Active Pharmaceutical Ingredients (Omeprazole)
Calibration curve of Omeprazole by using UV Spectroscopy
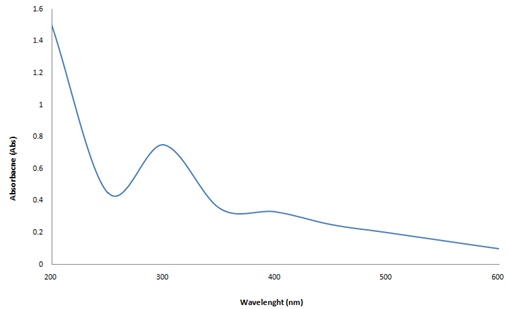
The scan of Omeprazole solution in UV region (200-400) was performed to check the wave length of maximum absorption (? max). The ?max was found to be at 283 nm (Figure 1). Hence calibration curve of Omeprazole was constructed at 283 nm in pH 6.8 phosphate buffers by taking concentration on x-axis and absorbance on y-axis (Figure 1). The calibration curve showed the regression coefficient of 0.999 and found to be linear in the concentration range of 2 to 10 µg/ml (Table 4; Figure 2).
FTIR Spectra for Omeprazole and Omeprazole-HPMC compatibility studies
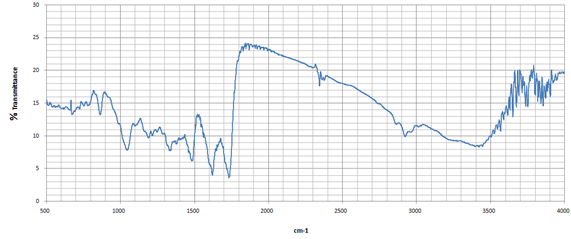
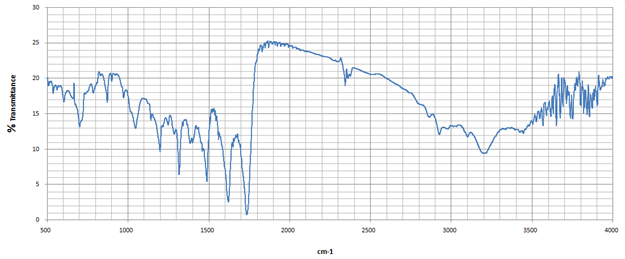
The FTIR spectrum of omeprazole shows prominent peaks indicating sulfoxide, aromatic rings, and other functional moieties. The strong absorption band around 1,700 cm-1 indicates carbonyl stretching vibrations, while bands around 1,600 cm-1 correspond to aromatic C=C stretching vibrations. N-H bending vibrations can be detected around 1,550 cm-1, reflecting interactions within the heterocyclic ring system (Figure 3a; Table 5). FTIR spectroscopy is used to assess drug-excipient combinations, identifying molecular interactions through characteristic absorption bands. In the case of omeprazole and HPMC, FTIR analysis can reveal potential hydrogen bonding or chemical structure changes. Researchers can compare spectral data before and after mixing to determine new peaks or significant shifts. FTIR analysis provides insight into the physical state of omeprazole when combined with HPMC, indicating their chemical stability under different conditions. This helps maintain the intended therapeutic effects while minimizing adverse reactions. Understanding the compatibility between omeprazole and HPMC through FTIR analysis will ultimately contribute to optimizing formulations for improved patient outcomes (Figure 3b; Table 5).
Precompression Evaluation of powder blended characteristics of tablet formulation for Floating Tablets containing Omeprazole
Tablets are a common drug delivery system that delivers active pharmaceutical ingredients. The powder blending process is crucial in Floating Tablets formulation, as it affects the drug release profile and dosage performance. Factors like particle size distribution, flow properties, blend uniformity, and compressibility must be carefully assessed to achieve optimal drug release kinetics. A narrow particle size distribution ensures uniform blending and prevents segregation during compression, leading to a more consistent drug release profile. Poor flow properties can result in uneven distribution of active ingredients, causing variability in drug release rates. Evaluating powder blended characteristics is essential for achieving consistent and reliable matrix tablet formulations. The product has a Carr Index of 11.2 to 15.7, indicating free flow, and Hausner grain ratio of 1.02 - 1.18 (Table 6).
Postcompression Evaluation of powder blended characteristics of tablet formulation for the Floating Tablets containing Omeprazole
Postcompression evaluation is a crucial step in the pharmaceutical industry to ensure the quality and efficacy of tablet formulations. It involves assessing parameters such as hardness, friability, disintegration time, and drug release profile to determine the overall performance of the tablet formulation. Hardness reflects the mechanical strength of a tablet, while friability measures the tendency of a tablet to break or chip under stress. A low friability value indicates good tablet integrity. Disintegration time determines how quickly a tablet breaks down into smaller particles for drug absorption. The drug release profile is the most crucial parameter, as it directly impacts the therapeutic efficacy of a tablet formulation. By carefully assessing these parameters, pharmaceutical companies can produce high-quality tablets that meet regulatory standards and deliver optimal clinical outcomes. The physical examination results of the formula are within limits and meet standard requirements (Table 7).
In vitro drug release studies:
Among all formulations, F1 formulation containing highest concentration sublimating agent showed enhanced drug release in 12 minutes compared to other formulations. It might be due to high water absorption ratio with lowest disintegration time by combined effect of two variables (A&B). t50% and t90% of all formulations were in the range of 5.36 min to 12.3min and 11.34 to 22.46 min respectively (Table 8; Figure 4).
Stability Studies
As per the data, it was concluded that tablet dosage form was stable enough till 6 months under the accelerated conditions as per the ICH (Table 5).
CONCLUSIONS
Omeprazole is a proton pump inhibitor (PPI) used to treat gastrointestinal conditions like gastroesophageal reflux disease (GERD) and peptic ulcers. It works by decreasing stomach acid production, providing relief from symptoms like heartburn and indigestion. However, it has potential side effects like headaches, nausea, and diarrhea. Long-term use of PPIs can increase the risk of health issues like bone fractures and nutrient deficiencies. The focus on floating systems has led to the reduction in synthetic polymers and the use of natural polymers with desirable properties such as biocompatibility and biodegradation. This study aimed to formulate a floating drug delivery system for Omeprazole, which was tested using wet granulation techniques. The results showed that all formulations had good flow properties, possibly due to the presence of gas generating agents NaHCO3 and citric acid. Buoyancy of the tablets was governed by swelling of hydrocolloid particles on the surface when they come in contact with the gastric fluid and presence of void space or porosity in the dry center of the tablet. In vitro drug release studies revealed that the formulated tablet F1 showed the highest percentage of cumulative drug release at the end of 12th minutes, possibly due to the presence of HPMC K100. Stability studies conducted according to ICH guidelines for 6 months showed that HPMC with Omeprazole as floating tablets have comparable floating and drug release characteristics, suggesting fair clinical efficacy.
CONFLICTS OF INTEREST
None
REFERENCES
- Obeagu EI. Effects of Long-Term Omeprazole Use on Red Blood Cells: A Review. Elite Journal of Medica Science. 2024;2(1):44-52.
- Prasad VG M, McFarland LV, Thacker HP, Puri R, Lawate PS. Efficacy and Safety of Omeprazole for the Treatment of Acid Peptic Disorders: A Systematic Review and Meta?Analysis. International Journal of Clinical Practice. 2024;2024(1):9990554.
- Zhang O, Xu Z. Research Progress on Omeprazole in the Treatment of Peptic Ulcers. MEDS Clinical Medicine. 2024 May 10;5(3):15-22.
- Brummer Y, Cui W, Wang Q. Extraction, purification and physicochemical characterization of fenugreek gum. Food Hydrocoll. 2003;17(3):229–36.
- Razavi SMA, Mortazavi SA, Matia?Merino L, Hosseini?Parvar SH, Motamedzadegan A, Khanipour E. Optimisation study of gum extraction from Basil seeds (Ocimum basilicum L.). Int J food Sci Technol. 2009;44(9):1755–62.
- Gashaw S, Getachew A, Mola F. Characterization of Acid Hydrolyzed Taro Boloso?I (Colocasia esculenta Cultivar) Starch as a Diluent in Direct Compression of Tablets. Adv Pharmacol Pharm Sci. 2024;2024(1):6560070.
- Bekaert B, Janssen PHM, Fathollahi S, Vanderroost D, Roelofs T, Dickhoff BHJ, et al. Batch vs. continuous direct compression–a comparison of material processability and final tablet quality. Int J Pharm X. 2024;7:100226.
- Pandey SK, Pudasaini J, Parajuli N, Singh RE, Shah KP, Adhikari A, et al. Formulation and evaluation of floating tablet of nimesulide by direct compression method. Magna Sci Adv Res Rev. 2024;10(1):153–61.
- Sharma G, Dhall M. PREPARATION AND EVALUATION OF MELOXICAM CHEWABLE TABLETS FOR BETTER ORAL DELIVERY. Indian Drugs. 2024;61(2).
- Rodriguez-Saavedra LR, Alva-Plasencia PM, Curo-Vallejos YF, Saavedra-Suárez SF, Chávez-Abanto LA, Caballero-Aquiño OE, et al. Dissolution kinetics of propranolol hydrochloride 40 mg tablets under biowaiver conditions. J Pharm Pharmacogn Res. 2024;12(5):814–21.
- Noman MA, Alburyhi MM, El-Shaibany A, Alwesabi NA. Preformulation and Characterization Studies of Pandanus Odoratissimus L Extract Active Ingredient in Treatment of Nocturnal Enuresis. World J Pharm Pharm Sci. 2024;13(2):1603–20.
- van der Haven DLH, Jensen R, Mikoroni M, Zafar U, Elliott JA, Fragkopoulos IS. Tablet ejection: A systematic comparison between force, static friction, and kinetic friction. Int J Pharm. 2024;124369.
- Thokal VG, Dhole Y, Deshmukh SP. Novel approaches in the formulation and evaluation of Ashwagandha tablets. GSC Biol Pharm Sci. 2024;27(2):297–301.
- Subhramanya ABK, Nayak P, Ramalingappa H, Hemanna HK, Shetty P. ICH Q1 a Stability Testing for New Dosage Form. 2024; May 10;5(3):15-22.













 Anand Dhangi*
Anand Dhangi*














 10.5281/zenodo.13946445
10.5281/zenodo.13946445