Abstract
Herbal medicines have gained global importance due to their medicinal efficacy and cost-effectiveness. Unlike allopathic treatments, plant-based medicines offer better patient compliance with minimal side effects. This study aims to develop and evaluate the anti-acne activity of a hydrogel formulated using water extracts of Rubia cordifolia roots and Azadirachta indica leaves. Rubia cordifolia (Manjistha) is a vital Ayurvedic herb known for its blood purification properties and efficacy in treating skin disorders. It also supports kidney health and exhibits antibacterial, antioxidant, and anti-inflammatory activities beneficial for skin health. Azadirachta indica (Neem) is widely recognized for its antimicrobial and anti-inflammatory properties, making it effective in combating acne-causing bacteria. Physicochemical evaluations of the selected plants confirmed the presence of key phytochemicals, such as alkaloids, glycosides, phenols, and phytosterols. A hydrogel was formulated using Carbopol 934 (1.5% w/w) combined with aqueous extracts of Rubia cordifolia and Azadirachta indica at varying concentrations. In-vitro evaluation revealed that the herbal gel exhibited significant anti-acne activity, showing a superior zone of inhibition against bacterial cultures compared to standard amoxicillin discs. This study concludes that the formulated anti-acne herbal gel is a safe and effective alternative for managing acne vulgaris.
Keywords
Hydrogel; Acne; Anti-acne activity; Rubia cordifolia; Azadirachta indica.
Introduction
1. Herbal Medicines
Herbal medicines, also referred to as phytotherapeutic agents or phytopharmaceuticals, have been used for centuries as the primary method of treating various ailments. These medicines derive from plant sources and are often chosen for their natural origins and relatively fewer side effects compared to synthetic drugs. As a result, herbal medicine remains a widely accepted and commonly used treatment system in many parts of the world, especially in developing countries. According to the World Health Organization (WHO), approximately 80% of the global population relies on herbal medicines for health care needs.
Herbal treatments address a variety of conditions such as microbial infections, cardiovascular disorders, cancer, metabolic disorders, and more. The growing trend of using herbal medicines is partly attributed to the benefits they offer over conventional medications, such as enhanced bioavailability, improved solubility, sustained delivery, and reduced toxicity. This has led to an increasing demand for herbal-based remedies, with research continuously uncovering new advantages of plant-based formulations.
2.Herbal cosmetics
Herbal cosmetics (also known as natural cosmetics) refer to beauty and skin care products formulated with a blend of herbal extracts. These extracts may contain various active ingredients such as alkaloids, glycosides, flavonoids, vitamins, essential oils, and amino acids, which offer a range of skin benefits. The use of herbal extracts is particularly popular due to their antioxidant and anti-inflammatory properties. These properties make herbal cosmetics a preferred choice for many people seeking more natural alternatives to synthetic cosmetic formulations.
The benefits of herbal cosmetics often include skin rejuvenation, improved appearance, and protection against environmental damage. Furthermore, because herbal products generally have a gentler effect on the skin, they are seen as a safer option compared to synthetic products, which may cause irritation or adverse reactions in sensitive individuals.
3. Skin Cosmetics
Skin cosmetics are products formulated to enhance and protect the skin. These products play a critical role in maintaining skin health and can address a variety of skin concerns, from dryness to moisture retention, and from anti-aging to acne prevention. Common functions of skin cosmetics include:
- Cleansing: To remove impurities from the skin.
- Moisturizing: To prevent dryness and maintain skin hydration.
- UV Protection: To prevent sun damage and premature aging.
- Anti-aging: To reduce wrinkles and signs of aging.
- Whitening: To reduce dark spots and even out skin tone.
- Acne Treatment: To prevent and treat acne breakouts.
4. The Skin
The skin is the largest organ of the human body and serves as the outermost protective covering. It consists of three main layers:
- Epidermis: The outermost layer made of squamous epithelial cells, providing a protective barrier. The outermost part, the stratum corneum, is composed of flattened, keratinized cells. The epidermis includes the following sub-layers:
- Stratum corneum (outer protective layer),
- Stratum spinosum (prickly layer),
- Stratum granulosum (granular layer), and
- Stratum lucidum (clear layer, in thick skin like palms and soles).
- Dermis: Located beneath the epidermis, it consists mainly of collagen fibers that give the skin strength and elasticity. This layer also contains blood vessels, nerve endings, sweat glands, and hair follicles.
- Subcutaneous Tissue: The fat-rich layer beneath the dermis, also known as the hypodermis. It connects the skin to underlying structures, providing insulation, cushioning, and energy storage.
5.Acne and Herbal Extracts for Acne
Acne vulgaris (or simply acne) is an infectious disease and one of the most prevalent human diseases. Acne is characterized by different areas of scaly red skin (seborrhea), pinheads (papules), blackheads and whiteheads (comedones), large papules (nodules), and sometimes scarring (pimples). In acne, the skin changes, due to changes in pilosebaceous unit skin structures including hair follicles and their associated sebaceous glands. These changes usually require androgen stimulation. Acne vulgaris is usually due to an increase in body androgens, and occurs more often in adolescence during puberty, regardless of sex. Acne is usually seen on the face, upper part of the chest, and the back of subjects who possess greater number of oil glands.
Acne Grades:
- Grade 1 (Comedonal Acne): Characterized by blackheads (open comedones) and whiteheads (closed comedones), caused by blockages in hair follicles.
- Grade 2 (Inflammatory Acne): Small, red papules with surrounding redness and swelling.
- Grade 3 (Pustular Acne): Larger, pus-filled pustules.
- Grade 4 (Cystic Acne): Severe acne with nodules or cysts, which can lead to scarring.
6.Physiological Factors
Three main physiological factors contribute to the development of acne:
Overactive Sebaceous Glands: The sebaceous glands produce oil (sebum) to keep the skin lubricated. When these glands become overactive, excess sebum can accumulate and become trapped in hair follicles, causing blockages (comedones), which can lead to acne.
Abnormal Shedding of Skin Cells: The skin continuously renews itself by producing new cells and shedding old ones. In acne-prone skin, this process, called cell turnover, becomes irregular, leading to the accumulation of excess skin cells. These dead cells can clog pores, contributing to the formation of comedones (blackheads and whiteheads).
Bacterial Growth: The skin is home to many bacteria, including Propionibacterium acnes, which normally exist without causing harm. However, when pores become clogged with sebum and dead skin cells, these bacteria can multiply, leading to infection and inflammation, which results in pimples and other acne lesions.
7.MANJISTHA and NEEM as medicinal plants
Rubia cordifolia (Manjistha/Indian Madder) is an herbaceous branched climber or creeper of evergreen origin. It belongs to kingdom Plantae, class Eudicots and Rubiaceace family. Dried roots of Rubia cordifolia is used as a folk medicine in all over the world due to its pharmacological activities.
Azadirachta indica (Neem/ Indian Lilac) is one of two species in the genus Azadirachta. It is native to the northeast of Indian subcontinent, but is to neutralized and grown around the world in tropical and subtropical areas. Its fruits and seeds are the source of neem oil. It belongs to kingdom Plantae, class Eudicots and is a tree in the Mahogany family Meliaceae. All parts of neem tree- leaves, flowers, seeds, fruits, roots and bark have been used traditionally worldwide due to its pharmacological activities.
8.Hydrogel
Hydrogel are semi-solid preparations made by the combination of one or more hydrophilic polymer. They can hold more water in the structure so that they always keep moisture at the site of application and permit oxygen penetration. They act as scaffolds which provide structural integrity to tissue constructs, control drug and protein delivery to the tissues and provides barriers between tissue and material surfaces.
The main features of hydrogels include:
- Shape, stability, and softness similar to that of soft surrounding tissues.
- Chemical and biochemical stability.
- High permeability for water-soluble nutrients and metabolites across the biomaterial tissue - interface.
CARBOPOL(C934) BASED HYDROGEL
Carbopol is made up of carbomer, which is a polymer cross-linked together to get a microgel structure that helps to deliver the drug for the dermatological purpose. The Carbopol polymers are acrylic acid cross-linked with a poly alkenyl or divinyl glycol. These are anionic polymers that need neutralization to become jellified. Organic amines like triethylamine are added to neutralize these polymers in liquid.
Some advantages of Carbopol based hydrogel:
- Excellent rheological properties.
- Manifest high viscosity at low concentration.
- Compatibility with many active ingredients.
- Patient acceptability and good organoleptic characteristics.
MATERIALS AND METHODS
1.List of materials:
|
SL.NO
|
Botanical Name
|
Vernacular Name
|
Source/
Supplier
|
|
1
|
Rubia cordifolia Linn
|
Manjistha
|
Indoor ventures
|
|
2
|
Azadirachta indica
A.Juss
|
Neem
|
Indoor ventures
|
2.List of chemicals:
|
SL.NO
|
Materials/Solvents
|
Manufactures/Suppliers
|
|
1
|
Carbopol 934
|
Burgoyne Burbidges & Co,
Mumbai, India
|
|
2
|
Methyl paraben
|
Burgoyne Burbidges & Co,
Mumbai, India
|
|
3
|
Propyl paraben
|
Burgoyne Burbidges & Co,
Mumbai, India
|
|
4
|
Tri sodium EDTA
|
Medlise Chemicals, Kannur,
Kerala
|
|
5
|
Ethanol
|
Changshu Hongsheng Fine
Chemicals Co. Ltd
|
|
6
|
Propylene glycol
|
Medlise Chemicals, Kannur,
Kerala
|
|
7
|
Triethanolamine
|
Medlise Chemicals, Kannur,
Kerala
|
|
8
|
Distilled water
|
Universal agencies
|
3.List of equipments:
|
SL.NO
|
Equipments
|
Suppliers/ Manufactures
|
|
1
|
Digital weighing balance
|
SHIMADZU AY 220
|
|
2
|
Soxhlet apparatus
|
Medwin Diagnostics
|
|
3
|
Digital pH meter
|
Servo Enterprises
|
|
4
|
Desiccator
|
Universal agencies
|
|
5
|
Incubator
|
Medwin Diagnostics
|
|
6
|
Refrigerator
|
LG Cap 195 Ltr
|
4.Determination of physiochemical parameters
4.1. Ash values
Total Ash: About 2.0 g of powdered samples were accurately weighed and added to preweighed silica crucible and heated at a temperature of 500-600? in a muffle furnace till carbon-free, white colored ash was obtained. The silica crucible was then cooled in a desiccator, weighed and then calculate the percentage of total ash with reference to the air-dried drug.
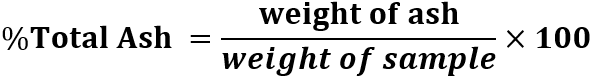
Acid-insoluble Ash: The total ash obtained in powdered sample were taken in a beaker and boiled for 5 minutes with 25 ml of hydrochloric acid. The contents of the beaker were filtered through Whatman filter paper no.41. The residues were then washed with hot water, till washings were free from chlorides. Then the filter paper, along with residues of plant powder were placed in a silica crucible and ignited in a muffle furnace, at 550? for 1 hour. The crucible was cooled and weighed to a constant weight. The percentage of acid insoluble ash was then calculated with reference to the air-dried drug.
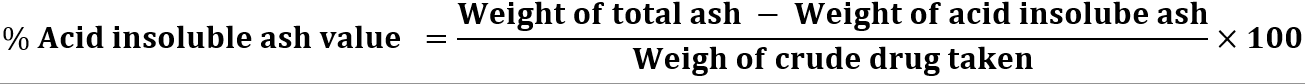
Water soluble Ash value: The total ash obtained in powdered sample were taken in a beaker and boiled for 5 minutes with 25 ml of water. The contents of the beaker were filtered through Whatman filter paper no. 41. The residues were then washed with hot water. Then the filter papers, along with the residues of plant powder were placed in a silica crucible and ignited in a muffle furnace for 15 minutes at a temperature not exceeding 450?. The crucible was cooled and weighted to a constant weight. The percentage of water soluble ash was then calculated with reference to the air-dried drug.
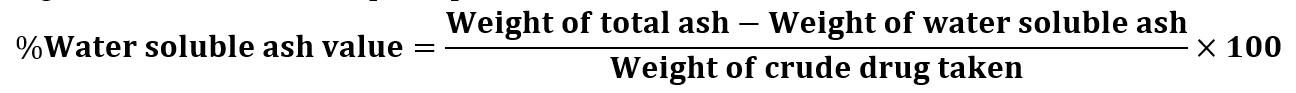
4.2. Determination of moisture content
Loss on drying method: Weigh accurately 3 g powdered drug and transferred it in to a tarred glass bottle. Then the crude drug was heated at 105? in an oven till constant weight was obtained. Percentage moisture content of the sample was calculated.
4.3. Extractive value
Water soluble extractive value: 5 g of coarse air-dried drug sample were taken with 100 ml of chloroform water in a stoppered flask for 24 hours. It was shaken frequently during first 6 hours and filtered. Then evaporate 25 ml of the water extract to dryness in a tarred flat-bottomed Petri dish and dried at 105? and weighed. The percentage water soluble extractive value was calculated with reference to the air-dried drug.
Alcohol soluble extractive value: 5 g of previously weighed air-dried drug were taken in a stoppered flask and 100 ml of 95% ethanol was added to it. It was shaken frequently during first 6 hours and filtered .25 ml of filtrate was evaporated to dryness in a tarred flat-bottomed Petri dish, dried at 105? and weighed. The percentage of ethanol soluble extractive value was calculated with reference to air-dried drug.
5.Preparation of plant extracts
The collected plant leaves are carefully washed under water and was dried at shade for 10-15 days. These shade dried plant material were then homogenized to a coarse powder using an electronic blender and then stored at air tight container until further use. Various organic solvent such as ethanol, chloroform, water and petroleum ether were used for extraction. 10 gm of homogenized coarse powder of sample were soaked in different conical flasks containing 10 ml of petroleum ether, chloroform, ethanol and water. Then it is allowed to stand 30 minutes with occasional shaking, finally each sample extract was filtered through sterilized WhatmanNo:1 filter paper. This filtrate is used to detect various biologically active constituents present in various solvents extract.
6.Extraction of plant material
The collected plant roots of Rubia cordifolia and leaves of Azadirachta indica were shade dried and coarsely powdered. The drug is extracted by continuous hot extraction (soxhlation) using water in Soxhlet apparatus. The process last for 2 days until the solvent present in the siphon tube becomes colorless. Water retained within the extract can be recovered by distillation process and it was then air dried and concentrated. The concentrated extract of the drugs was then incorporated in to the formulation.
7.Formulation of Anti-acne gel
|
SL NO.
|
Ingredients
|
F1
|
F2
|
F3
|
|
1
|
Water extract of
Rubia cordifolia
|
2.5%
|
1.5%
|
1%
|
|
2
|
Water extract of
Azadirachta indica
|
2.5%
|
1.5%
|
1%
|
|
3
|
Carbopol 934 (g)
|
1.5
|
1.5
|
1.5
|
|
4
|
Propylene glycol
|
5
|
5
|
5
|
|
5
|
Ethanol (ml)
|
3
|
3
|
3
|
|
6
|
Disodium EDTA (g)
|
0.03
|
0.03
|
0.03
|
|
7
|
Methyl paraben (g)
|
0.2
|
0.2
|
0.2
|
|
8
|
Propyl paraben (g)
|
0.02
|
0.02
|
0.02
|
|
9
|
Tri ethanolamine
|
q.s
|
q.s
|
q.s
|
|
10
|
Distilled water
|
Up to 100
|
Up to 100
|
Up to 100
|
8.Procedure
Water required for this formulation was divided in to two parts. In one part, weighed the amount of Carbopol 934 was slowly sprinkled with vigorous mechanical stirring and waited for an air bubble to separate. The exact amount of extracts were dissolved by using ethanol, to this add propylene glycol and mix it properly. To 5 ml of distilled water taken in a beaker add required quantity of methylparaben and propylparaben.
This was dissolved by heating in a water bath and disodium EDTA was added. Finally, all the ingredients were mixed properly with Carbopol solution and volume is made up to 100 ml by adding remaining distilled water. Then tri ethanolamine was added dropwise with continuous stirring to obtain of required skin Ph (6.8-7) and to maintain required gel consistency.
9.Evaluations of hydrogel
A. Physical evaluation
Physical parameters such as color and appearance were evaluated.
B.Homogeneity
The developed gel was tested for homogeneity by visual inspection after the gel have been set in the container for their appearance and presence of any aggregates.
C.pH
The pH of gel formulation was determined by using digital pH meter. About 2.5 gm of the gel was accurately weighed and dispersed in 25 ml of distilled water and stored for two hours. The measurement of pH of formulation was carried out in triplicate and the average values are represented. The pH of dispersions was measured by using pH meter.
D.Spreadability
Formulation 0.5 gm were placed within a circle of 1 cm diameter pre-marked on a glass plate over which a second glass plate was placed. A weight of 5 gm was placed on upper glass plate for 5 minutes. The increase in the diameter due to spreadability of the formulation was noted.
E.Viscosity
The viscosity of the gel was determined by using Brookfield viscometer at 10 rpm. About 200 g of the gel was taken in a beaker and spindle was dipped in it for about 5 minutes and then the reading was recorded.
F.Swelling index
To determine the swelling index of the prepared gel, 1 gm of gel was transferred on porous aluminum foil and then placed in a 50 ml of beaker containing 10 ml 0.1N NaOH. Then the sample were removed from beaker at different time intervals and put it in a dry place for some time after it reweighed. Swelling index was calculated by using the formula:
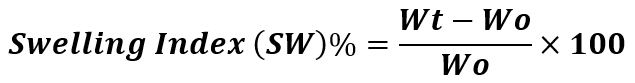
G.Extrudability
The prepared gel formulation was filled in standard capped collapsible aluminum tube and sealed by crimping to the end. The weight of tube was recorded and the tube was placed between two glass slides and were clamped. 50 gm hydrogel was placed over the slides and then the cap was removed. The amount gel extruded was collected and weighed. The percentage of extruded gel was calculated as
- When it is greater than 90% then extrudability is excellent.
- When it is greater than 80% then extrudability is good.
- When it is 70% then it is fair.
H.Stability study
The stability study was performed as per ICH guidelines. Prepared formulations were stored at different temperatures and humidity conditions like ambient temperature (R.T), refrigerator temperature (8±1?) and condition of accelerated stability testing (45? ± 2?/75%±5%RH) for a period of three months.
I.In-vitro anti-acne activity evaluation
Agar well diffusion method:
Anti-acne activity of the developed hydrogel was determined by agar plate diffusion method. This method involves:
- Preparation of pre-inoculum:
The bacteria lactobacillus is prepared from curd. The curd is filtered and the broth is incubated at 25? for 24hrs.
- Preparation of pour plates:
A sabouraud dextrose agar (150 ml) is autoclaved and poured to the already autoclaved plate and cooled to room temperature and allowed to solidify. The culture was spread on the agar surface aseptically by using sterilized cotton.
- Making wells on agar plates:
Wells of 6mm in diameter were made aseptically on the agar plate by using a sterilized well digger. The gel samples (100 ml) were aseptically added in to the well by using a micropipette. The petri plates are kept in a refrigerator and after 1 hour the plates were incubated in inverted condition at 37? for 48 hours.
- Measurement of the zone of inhibition:
After 48 hours, the plates were observed for the presence of inhibition of bacterial growth and it was indicated in the form of a clear zone of inhibition around each well containing different samples. The size of the inhibitory zone was measured in ‘mm’. The zone of inhibition obtained for the developed herbal anti-acne gel was compared with the standard. Amoxicillin disc was used as a standard.
RESULTS AND DISCUSSION
1.Plant collection and authentication
The plants Rubia cordifolia and Azadirachta inidca were collected from Kasaragod district, Kerala (India) in the month of March 2023 and were authenticated by Dr. Harikrishnan. E, Assistant professor, Department of Botany, Payyanur college, Payyanur, Kannur, Kerala.
2.Physico-chemical parameters
|
SL. NO
|
Test
|
Rubia cordifolia
|
Azadirachta indica
|
|
1
|
Total Ash (%w/w)
|
6.58±0.03%
|
5.82±0.02%
|
|
2
|
Acid insoluble Ash (%w/w)
|
0.95±0.02%
|
3.23±0.03%
|
|
3
|
Water soluble Ash (%w/w)
|
5.5±0.03%
|
4.25±0.02%
|
|
4
|
Water soluble extractive
value (%w/w)
|
20.12±0.04%
(NLT 19.6 %)
|
11.25±0.04%
(NLT 10.3%)
|
|
5
|
Alcohol soluble extractive
value (%w/w)
|
18.82±0.04%
(NLT16.4%)
|
7.69±0.03%
(NLT 5.8%)
|
|
6
|
Moisture content (%w/w)
|
9.80±0.04%
(NMT 10.4%)
|
9.72±0.03%
(NMT 10.6%)
|
3.Preliminary phytochemical screening
Phytochemicals present in petroleum ether, chloroform, ethanol and aqueous extract of plant Rubia cordifolia
|
SL.NO
|
Compounds
|
Petroleum ether
|
Chloroform
|
Ethanol
|
Aqueous
extract
|
|
1
|
Alkaloids
|
++
|
-
|
-
|
+++
|
|
2
|
Glycosides
|
++
|
-
|
+
|
+
|
|
3
|
Flavonoid
|
-
|
+
|
+
|
-
|
|
4
|
Carbohydrate
|
+
|
+
|
-
|
+
|
|
5
|
Saponin
|
-
|
-
|
-
|
-
|
|
6
|
Phytosterol
|
++
|
-
|
-
|
+++
|
|
7
|
Tannins
|
-
|
+
|
-
|
+
|
|
8
|
Protein and Amino
acid
|
+++
|
-
|
+
|
++
|
|
9
|
Total
|
10
|
3
|
3
|
11
|
(+++) = Intensively present, (+) = Present, (-) = Absent
Phytochemicals present in petroleum ether, chloroform, ethanol and aqueous extract of plant Azadirachta indica
|
SL.NO
|
Compounds
|
Petroleum ether
|
Chloroform
|
Ethanol
|
Aqueous
extract
|
|
1
|
Alkaloids
|
-
|
+
|
+
|
++
|
|
2
|
Glycosides
|
++
|
-
|
+
|
-
|
|
3
|
Flavonoids
|
-
|
-
|
-
|
+
|
|
4
|
Carbohydrate
|
++
|
-
|
-
|
-
|
|
5
|
Saponin
|
-
|
-
|
-
|
+
|
|
6
|
Phytosterol
|
-
|
+
|
+
|
+++
|
|
7
|
Tannins
|
-
|
-
|
-
|
++
|
|
8
|
Proteins and amino
acid
|
-
|
-
|
-
|
-
|
|
9
|
Total
|
4
|
2
|
3
|
9
|
(+++) = Intensively present, (+) = Present, (-) = Absent
4.Soxhlet extraction of plant materials
The extraction of dried roots of Rubia cordifolia and dried leaves of Azadirachta indica were carried out by continuous hot Soxhlet extraction process by using water as solvent. The extracts obtained were collected and concentrated which was then weighed and kept in a desiccator until it was used for further studies.
Percentage yield of the water extracts
|
SL.NO
|
Plant
|
Solvent
|
Weight of dry powder
(g)
|
Weight of dry extract
(g)
|
Percentage yield (%w/w)
|
|
1
|
Rubia cordifolia
|
Water
|
100g
|
5.12
|
5.12%
|
|
2
|
Azadirachta indica
|
Water
|
100g
|
5.62
|
5.62%
|
5.Formulation of hydrogel
In the present study hydrogel were prepared by using polymer (Carbopol 934).
6.Evaluation of hydrogel
6.1. Physical evaluation
The physical parameters such as color and appearance were checked and results showed that the developed hydrogel were greenish in color, translucent in appearance and showed good homogeneity without lumps.
|
SL.NO
|
Formulation
|
Color
|
Appearance
|
|
1
|
Control
|
White
|
Clear and transparent
|
|
2
|
F1
|
Brown
|
Clear and transparent
|
6.2. Homogeneity
|
SL.NO
|
Formulation
|
Homogeneity
|
|
1
|
Control
|
Homogenous
|
|
2
|
F1
|
Homogenous
|
6.3. pH
The pH of the prepared formulation was measured using digital pH meter.
|
SL.NO
|
Formulation
|
pH
|
|
1
|
Control
|
7.04±0.01
|
|
2
|
F1
|
6.58±0.02
|
6.4. Spreadability
The spreading diameter of the prepared hydrogel (in cm) was found to be 3.70±0.05 which indicates good spreadability to test hydrogel. The result obtained indicated that the formulated hydrogel can be applied easily.
|
SL.NO
|
Formulation
|
Spreadability (cm)
|
|
1
|
Control
|
3.58±0.07
|
|
2
|
F1
|
3.70±0.05
|
6.5. Viscosity
The viscosity of the gel was measured by using Brookfield viscometer with spindle No. 7 at 10 rpm at room temperature.
|
SL.NO
|
Formulation
|
Viscosity (cp)
|
|
1
|
Control
|
16526±0.58
|
|
2
|
F1
|
16901±0.64
|
6.6. Swelling Index
|
SL.NO
|
Time (hr)
|
Swelling index,
Control (%)
|
Swelling index,
F1(%)
|
|
1
|
1
|
4.41±0.02
|
4.23±0.001
|
|
2
|
2
|
8.52±0.10
|
7.63±0.002
|
|
3
|
3
|
10.32±0.14
|
12.59±0.001
|
|
4
|
4
|
21.36±0.28
|
17.14±0.002
|
|
5
|
5
|
28.32±0.20
|
21.3±0.001
|
|
6
|
6
|
33.14±0.08
|
38.59±0.002
|
|
7
|
7
|
40.32±0.26
|
44.54±0.005
|
|
8
|
8
|
52.6±0.20
|
58.14±0.002
|
|
9
|
9
|
55.87±0.18
|
63.52±0.002
|
6.7. Extrudability
|
SL.NO
|
Formulation
|
Extrudability
|
|
1
|
Control
|
Good
|
|
2
|
F1
|
Good
|
7.Stability study of hydrogel
|
SL.NO
|
Evaluation Parameters
|
At 0 days
|
At 30 days
|
|
1
|
Appearance
|
Brown
|
No change
|
|
2
|
Viscosity
|
16901±0.64
|
16900±0.6
|
|
3
|
Extradurability
|
Good
|
Good
|
|
4
|
pH
|
6.58±0.02
|
6.57±0.02
|
8.In-vitro anti- acne activity
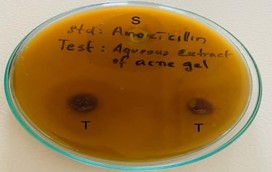
The anti-acne activity of the developed formulation was done by using agar well diffusion method. The anti-bacterial activity was measured in terms of zone of inhibition. The zone of inhibition obtained for various formulations was compared with the standard Amoxicillin disc. All the developed formulations showed anti- bacterial activity against Lactobacillus but F1 produced a better zone of inhibition of about 7mm which was near to the zone of inhibition produced by standard Amoxicillin disc (10mm).
Measurement of zone of inhibition
|
SL.NO
|
Test Samples
|
Zone of inhibition (mm)
|
|
1
|
Control
|
10
|
|
2
|
F1
|
7
|
CONCLUSION
The aim of study is to formulate the herbal hydrogel using root extract of Rubia cordifolia and leaf extract of Azadirachta indica. for anti-acne activity. Physicochemical and phytochemical investigations of the roots of Rubia cordifolia and leaves of Azadirachta indica have been reported here in this thesis work.
The preliminary phytochemical screening of plant shows that the water extracts shows the presence of major constituents such as glycosides, saponins, flavonoids may contribute to anti-inflammatory activity which is beneficial in the treatment of acne. Phytoconstituents present in plants were extracted by water using Soxhlet apparatus. Hydrogel containing herbal extract were prepared and the revealed that Carbopol 934 as a polymer showed good compatibility for hydrogel formulation. From results revealed that the prepared hydrogel formulation is good in appearance, homogeneity, and easily spreadable. Higher the value of viscosity greater the consistency of prepared hydrogel.
The in-vitro anti-acne evaluation was done by using agar well diffusion method and anti-bacterial activity was measured in terms of zone of inhibition. The formulation(F1) produced a better zone of inhibition of about 7mm against Lactobacillus bacteria which was near to the zone of inhibition produced by standard Amoxicillin disc (10mm).
However, in the present work herbal formulations reported to have more significant advantages over synthetic formulations. Hence, we conclude that herbal gel for acne is effective, safe and ease of manufacturing and in the economic point of view they are cheap when compared to chemical based gel.
ACKNOWLEDGEMENT
We earnestly reverse the Almighty for this boundless love and blessings, which accompanied in all journey of our life. We therefore take this opportunity to express our deepest gratitude to those who have assisted us in the completion of this thesis work.
First of all, we greatly thankful to Kerala University of Health Sciences, Thrissur for giving us an opportunity to do our graduation under them.We are grateful to the Management of Rajiv Gandhi Institute of Pharmaceutical Sciences and Research, Trikaripur for supporting us throughout our dissertation period.
With great respect we thank Dr. M. Paridhavi, Principal, Rajiv Gandhi Institute of Pharmaceutical Sciences and Research, for his academic supports.
We would like to greatfully thank Prof. Dr Arun Kumar K.V, Vice Principal, Rajiv Gandhi Institute of Pharmaceutical Sciences and Research, Trikaripur for providing sincere and valuable guidelines also we would like to express our sincere gratitude for being our guide and providing continuous support for our thesis and related research for his patience, motivation, understanding and his immense knowledge and friendship during our studies. We would like to express our sincere gratitude towards Dr. Harikrishnan.E, Assistant Professor, Department of Botany Payyannur for his patience during the time of collection and authentication of plant material. We would like to express our heartfelt gratitude towards all the respected teaching and non-teaching staff members of our college for providing timely help.
REFERENCES
- Manta Saxeena, Jyothi Saxeena, Rajeev Nema, Dharmendra Singh and Abhishek Gupta. Phytochemistry of medicinal plant. Journal of Pharmacognosy and Phytochemistry. 2013; 1(6): 168-182.
- P.Garodia, H.Ichikawa, N.Malani, G.Sethi, and B.P.Aggarwal. From ancient medicine to modern medicine: Ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol. 2007;1(5):25-37.
- Fabio Firenzuoli and Luigi Gori.Herbal medicine today.Clinical and research issues evidence.2007;4(1):37-40.
- Goyal M,Samsal D and Nagori BP. Ayurveda and ancient science of healing. First edition. Intech publishers.2012;12-14.
- Cardini F, Wade C, Regalia AL, Gui S, Li W, Raschetti R, Kronenberg F. Clinical research in traditional medicine: priorities and methods. Compl Ther Med. 2006; 14:282–287.
- S.Bale, K.Harding and D. Leeper. An introduction to wounds. Am. J. Surg.1999;5(1):5- 12
- J.Li, J.Chen, and R.Kirsener. Pathophysiology of Acute Wound Healing, Journal of Drugs and Medicine.2007;25(1):9-18.
- Pandey S, Meshya N and Viral D. Herbs play an important Role in the Field of Cosmetics. International Journal of Pharm. Tech Research. 2010; 2:632-639.
- Jain A, Basal E. Inhibition of Propionibacterium acnes-induced mediators of inflammation by Indian herbs. Phytomedicine.2003;10: 34-38.
- Kamboj VP.Herbal medicine.Current science.2000;78(1):35-51.
- Y.W.Chien, Novel Drug Delivery Systems: Transdermal Drug Delivery. Informa Healthcare 2003;15(2): 301-380.
- Hunt MJ, Barnetson RS. A comparative study of gluconolactone versus benzoyl peroxide in the treatment of acne. Australas J Dermatol. 1992;33(3):131–141.
- Peck GL, Downing DT, Pandya M, Prolonged remissions of cystic and conglobate acne with 13-cis-retinoic acid. N Engl J Med.1979;300(7):329-336.
- Bettoli V,Zauli S, Virgili A. Is hormonal treatment still an option in ace today? Br J Dermatol. 2015;172 (5):205-220.
- Rao GM, Rao CV, Shirwaikar A. Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia linn. J Ethnopharmacol. 2006; 103:484–90.
- Baldwin HE, Berson DS. Guidelines of care for the management of acne vulgaris.
- J Am Acad Dermatol. 2016;74(5):945-973.
- Alexeyev OA, Morris T, Zouboulis CC, Patrick S. Why we continue to use the name Propionibacterium acnes. Br J Dermatol. 2018;179(5):12-27.
- Motosko CC, Pomeranz MK, Hazen A, Acne: a side-effect of masculinizing hormonal therapy in transgender patients. Br J Dermatol. 2019;180(1):26-30.
- Özcelik S Kulac ?, Yaz?c? M, Öcal E. Distribution of childhood skin diseases according to age and gender. Turk Pediatri Ars. 2018;53(2):105-112.
- Lalla JK, Nandedkar SY, Paranjape MH, Talreja NB. Clinical trials of ayurvedic formulations in the treatment of acne vulgaris. J Ethnopharmacol. 2001;78(1):99–102.
- Capitanio B, Sinagra JL. Underestimated clinical features of post adolescent acne.
- J Am Acad Dermatol.2010;63:782-789.
- Iftikhar U, Choudhry N. Serum levels of androgens in acne & their role in acne severity. Pak J Med Sci. 2019;35(1):52-59.
- Drabu S, Khatri S, Babu S. Neem: Healer of all ailments. Res J Pharm Biol Chem Sci. 2012;3(1):120–126.
- Dréno B. What is new in the pathophysiology of acne, an overview. J Eur Acad Dermatol Venereol. 2017;31(2): 25-30.
- Dakappa SS, Adhikari R, Timilsina SS, Sajjekhan S. A review on the medicinal plant Rubia cordifolia. (Rubiaceae). J Drug Deliv Ther.2013;3(2):162-168.
- Deguchi Y, Miyazaki K. Anti-hyperglycemic and anti-hyperlipidemic effects of neem leaf extract. Nutr Metab Lond. 2010;5(6): 7-9.
- Laily N, Kusumaningtyas. The potency of neem Azadirachta indica (L.) leaves as a functional immunostimulatory ingredient. Procedia Chem.2015; 14:301-307.
- Chen, H.Y, Yen, G.C. Antioxidant activity and free radical-scavenging capacity of extracts from majistha (Rubia cordifolia.) roots. Food Chem. 2007;101: 686-694.
- Ashraf, Mahmood. Chemical composition, antioxidant, antitumor, anticancer and cytotoxic effects of Azadirachta indica leaf extracts. Pharm. Biol. 2016; 54:171-181.
- Jiang, L, Wang, Y. Antitumor effect of neem leaves on lung cancer: A network pharmacology study. Arab. J. Chem.2020;13: 773-797.
- Chen, H.Y., Yen, G.C. Antioxidant activity and free radical-scavenging capacity of extracts from manjistha (Rubia cordofolia) root. Food Chem. 2007; 10:686-694.
- Farag, R.S, Abdel-Latif, Tawfeek. Phytochemical screening and antioxidant activity of some medicinal plants. Biotechnol. Rep. 2020; 28:36-40.
- L.M. Wahl, S.M.Wahl, R.F.Diegelman. Wound Healing: Biochemical and clinical Aspects, Journal of Herbal medicines.1992;2(5):40- 62.
- T Mohammad Islam, Rodriguez-Hornedo. Rheological Characterization of Topical Carbomer Gel Neutralized to Different Ph. Inj JPharm.2004;21(7):1192-1199.
- Mr. S. Gopalakrishnan, Pharmacognostic, Phytochemical and invitro platelet aggregation inhibitory activity of Rubia cordifolia Linn. Food chem.2008;14(5):28-30.
- Li H, Xiao, Zhang J, Wang H. Research progress on chemical constituents and pharmacological effects of Rubia cordifolia L. Chin. Tradit. Herb Drugs.2006; 39 (6):1433–1436.
- Qiao Y. F, Zhu T. R. Studies on antibacterial constituents from the roots of Rubia cordifolia L. Acta. Pharm. Sin. 2009; 25 (11): 834–839.
- Singh R., Geetanjali G. Isolation and synthesis of anthraquinones and related compounds of Rubia cordifolia. J. Serbian. Chem. Soc. 2009;70 (7): 937–942.
- Siril E. A. Pharmacognostic studies on Indian madder (Rubia cordifolia L.). J. Pharm. Phytochem. 2003; 1 (5): 52-55.
- Barbalho SM, Goulart RA, Brunnati. Rubia cordifolia (manjistha): A plant of multipurpose medicinal applications. Med Aromat Plants. 2012;1(4):1-6.
- Wang S. X, Zhu T. R. Studies on anthraquinones from the roots of Rubia cordifolia L. Acta Pharm. Sin. 2010; 27 (10): 743–747.
- Mosaddek A. S. M, Rashid M. A comparative study of the anti-inflammatory effect of aqueous extract of neem leaf and dexamethasone. Bangladesh Journal of Pharmacology. 2008;3(1):44–47.
- CK.Kokate, Practical pharmacognosy, 4th edition, Vallabh Prakashan, Delhi. 2001;108-111.
- Himesh Soni and Akhlesh Kumar Singhai. A Recent Update of Botanicals for Wound Healing Activity. International Research Journal of Pharmacy.2012;3(7):1- 7.
- Ilango K, Maharajan G, Narasimhan S. Anti-inflammatory activities of Azadirachta indica leaf extract. Natural Product Research. 2013;27(16):1463–1467.
- Bhanwra S. Effect of Azadirachta indica (neem) leaf aqueous extract on paracetamol induced liver damage in rats. Indian Journal of Physiology and Pharmacology. 2000;44(1):64–68.
- B. V Sumalatha, Devprakash, G.P. Senthil Kumar and Thamizh Mani. Research Journal of Pharmaceutical Biological and Chemical Sciences.2012;3(3):105-110.
- Sabde S, Bodiwala HS, Karmase A. Journal of Natural Medicine. 2011; 65(4):662- 669.
- Shokeen P, Bala M, Tandon V. Evaluation of the activity of 16 medicinal plants against Malassezia furfur. Int J Antimicrob Agents. 2009;33(1):86–91.
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999; 12:564–582.


 Mufeeda Mohammad Ali*
Mufeeda Mohammad Ali*
 Haneena Swafiya A
Haneena Swafiya A
 Hasanath P
Hasanath P
 Marjhan rafeeq
Marjhan rafeeq
 Sharafa BK
Sharafa BK
 Dr Arun Kumar KV
Dr Arun Kumar KV
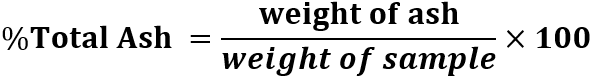
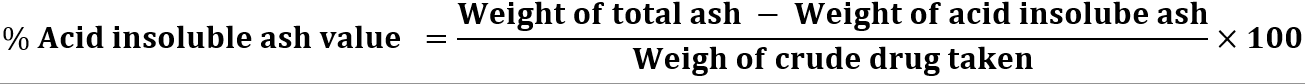
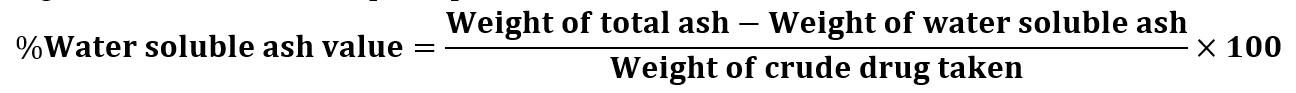
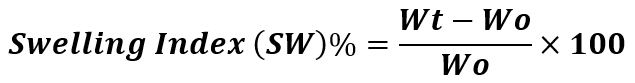

 10.5281/zenodo.14538539
10.5281/zenodo.14538539