Abstract
The purpose of this research work is to improve the robustness of allopurinol with minor changes. These studies aimed to develop a allopurinol tablet formulation by wet granulation method. The studies of the tablets are pre-compression parameters like angle of repose, bulk density, taped density, Carr’s index, and Hausner ratio, and post compression parameters like appearance, thickness, hardness test, friability test, weight variation test, disintegration test, and stability studies. Finally, all parameters are within the limit. Allopurinol tablets containing higher concentration of povidone K-30 appears to be the best formulation. Hence, it may be summarized that the tablets prepared by wet granulation method might be a perfect and effective formulation to prevent the treatment of gout.
Keywords
Allopurinol, robustness, povidone K-30,Formulation, excipients, stability, drug, mixing,granulation, compression, punch, diluents, binders, disintegration. Lubricants, buffers, colour, capping, sticking, picking, talc,lactose, stearic acid, equipments, assay, friability, hardness,moisture, uniformity, dissolution, sifting, parameters, thickness,
Introduction
Allopurinol (1,5-Dihydro-4H-pyrazolo (3, 4-d) pyrimidin-4-one ) is structural isomer of hypoxanthine which is xanthine oxidase inhibitor, commonly used in the treatment of chronic gout associated with pathological conditions like leukemia, inflammation and in cancer medications. The drug is particularly useful in patients with recurrent renal deposition of urates, proliferative disease and malignancies [1-8].The aim of this research work is to improve the robustness of allopurinol with minor changes. These studies aimed to develop allopurinol tablet formulation by wet granulation method. A total of 4 formulations using various concentration of povidone K-30.
MATERIALS AND METHODS
Evaluation of the pharmaceutical powders
Bulk density determination
Weighed quantity of the powder (W) was taken in a graduated measuring cylinder and volume (V0) was measured and bulk density was calculated using the formula.
Bulk density (BD)= Weight of the powder/ Volume of powder
BD = W/V0 g/mL
Tapped density determination
Weighed quantity of powder was taken in a graduated cylinder and the volume was measured (V0). The graduated cylinder was fixed in the ‘Tapped Densiometer’ and tapped for 500, 750 and 1250 times until the difference in the volume after consecutive tappings was less than 2%.The final reading was denoted by (Vf).
Tapped density(TD)= Wg/ml
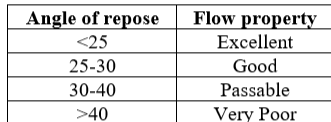
Angle of repose
Angle of Repose is defined as the maximum angle possible between the surface of a pile of the powder and the horizontal plane.
Table 1: Angle of repose and corresponding flow properties
Carr’s index:
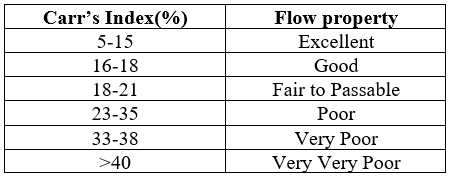
Carr’s index is also known as compressibility. It is indirectly related to the relative flow rate, cohesiveness and particle size. It is simple, fast and popular method of predicting powder flow characteristics.
Table 2: Carr’s index and corresponding flow properties
Hausner ratio
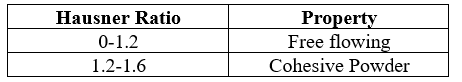
Hausner ratio indicates the flow properties of the powder and measured by the ratio of tapped density to bulk density.The relationship between Hauser’s ratio and flow property (9-12).
Table 3: Hausner ratio and corresponding flow properties
Assay
This test is a quantitative version of the identification test. Again, 10–20 tablets are ground and the active ingredient is dissolved or extracted in a suitable solvent using the standard procedure. The concentration of the extracted solution is determined using a specific and validated spectroscopic or chromatographic method against a solution of reference standard. These results are reported as percent of expected/labeled value. Uniformity of dosage unit, friability, hardness testing, disintegration test, dissolution were carried out using standard procedure and apparatus/ instrument (13-18).
Table 4: Formulation and Development of Core tablets

RESULTS
Evaluation of Pre-compression and Post-compression parameters of allopurinol tablets
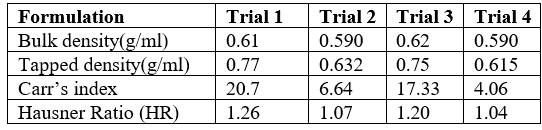
Pre - compression Parameters
Table 5: Pre-compression parameters of allopurinol tablets
Post- compression Parameters
Table 6: Post compression parameters
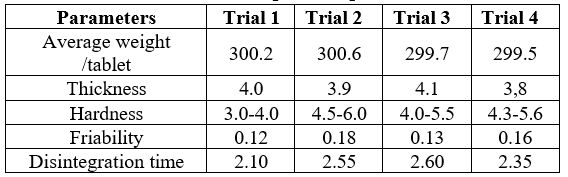
Hardness
The hardness of six tablets of each batch was checked by using Pfizer hardness tester The result showed that the hardness of the tablets was in the range of 3.0 to 5.6kg/cm2
Thickness
Thickness of the six tablets of each batch was measured by using Varnier Calipers. The results showed that the thickness of the tablets was in the range of 3.8 to 4.1mm respectively.
Weight variation test
Tablets of each batch were subjected to weight variation test, difference in weight and percent deviation was calculated for each tablet.
Friability
Tablets of each batch were evaluated for percentage friability. The average friabilityof all the formulations lies in the range of 0.12 to 0.18 % which was less than 1% as per official requirement of IP indicating a good mechanical resistance of tablets.
Invitro disintegration time
Tablets of each batch were evaluated for in-vitro disintegration time. The results showedthatthedisintegrationtimeofpreparedtabletswereintherangeof2.10to
2.55 mins.
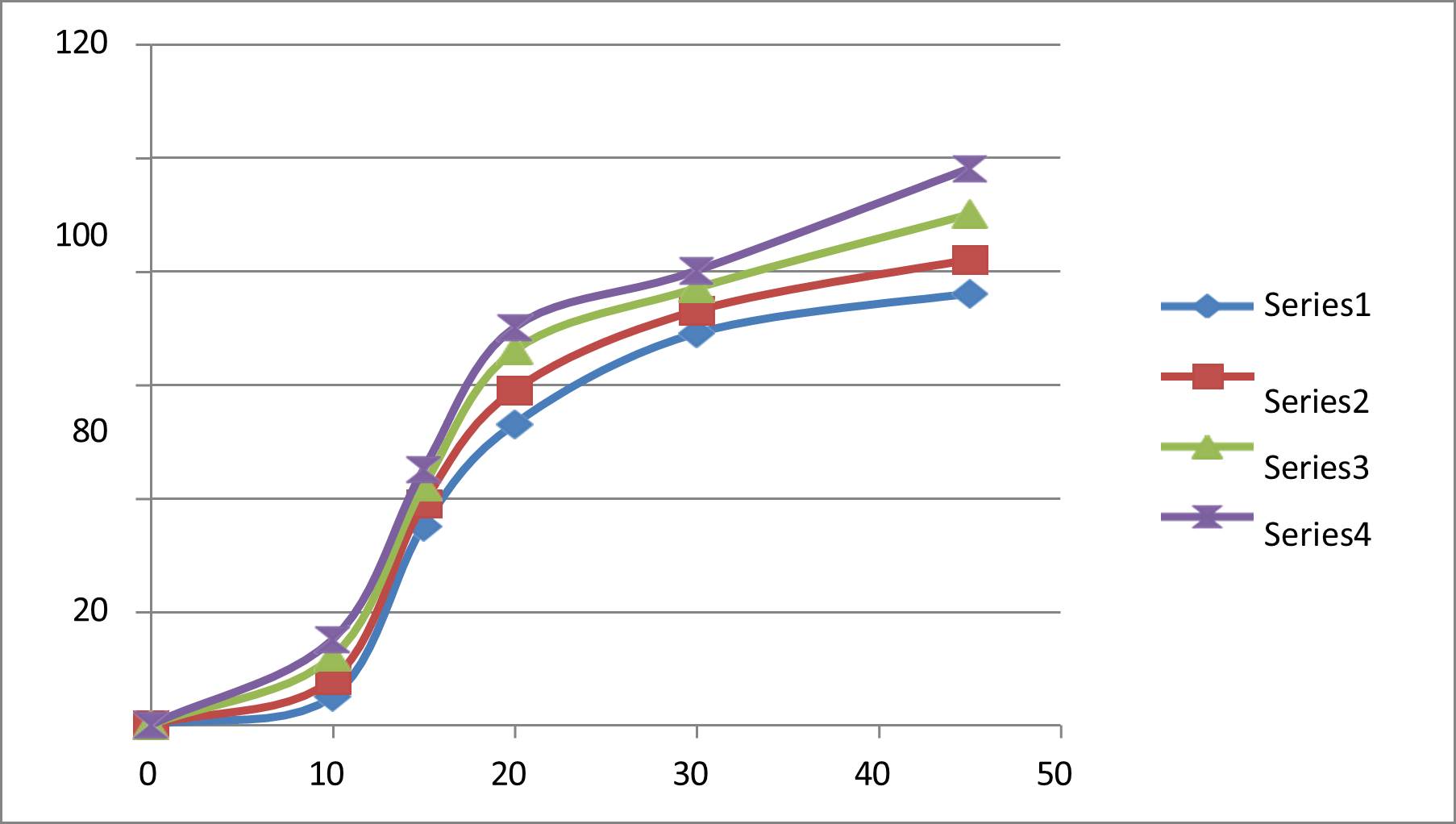
Invitro dissolution studies
The in-vitro dissolution studies were conducted in phosphate buffer pH 0.01N HCL. The Formulation F4 contains showed 98.15% of drug release within 45min.
DISSOLUTION PROFILE
Table 7: Dissolution profile

CONCLUSION
The improvement robustness of this product allopurinol 300mg was successfully formulated. Systemic formulation development was proceeded with exhaustive drug excipient compatibility study to finalize the excipient. Allopurinol tablets containing higher concentration of povidone K-30 appears to be the best formulation. The Pre-compression parameters of all formulations showed good flow properties and these can be used for tablet manufacture. The post compression parameters of all formulations were determined and the values were found to be within the pharmacopoeial limits. Wet granulation method is the best-suited method for formulating of allopurinol tablets. Thus it may be concluded that the development of allopurinol can be successfully prepared and evaluated and its popularity in the future.
REFERENCES
- Singh A, Gupta A.,& Chandra (2020).Application of Quality by Design (QbD) Approach in Developing Robust Allopurinol Formulations. Journal of Pharmaceutical Sciences, 15(3), 345-358.
- Patel, B., Shah, S., & Desai, P. (2019). Application of Response Surface Methodology (RSM) for Optimization of Allopurinol Tablet Formulation. Pharmaceutical Technology, 10(2), 123-135.
- Kumar, R., Sharma, V., & Singh, S. (2018). Strategies for Enhancing the Stability of Allopurinol, A Review Journal of Pharmaceutical Sciences and Research, 6(4), 187-198.
- Sharma, R., Jain, S., & Gupta, A. (2017). Development and Evaluation of Sustained Release Allopurinol Formulations Using Natural Polymers. Drug Delivery, 5(3), 215-228.
- Jain N, Patel R & Singh A. (2016).Strategies for Enhancing the Solubility of Allopurinol, A Comprehensive Review. Journal of Pharmaceutical Innovation, 4(2), 87-102.
- Singh S, Gupta A & Sharma R.(2015).Development and Evaluation of Allopurinol Capsules: A Quality by Design (QbD) Approach. International Journal of Pharmaceutical Sciences and Research, 7(3), 132-145.
- Rao, S., Reddy, M., & Kumar, S. (2014). Development of Liposomal Allopurinol Formulations for Improved Bioavailability.Journal of Drug Delivery Science and Technology, 24(4), 345-358.
- Gupta,A.,Sharma,R.,&Patel,S.(2013).Strategies for Enhancing the Bioavailability of Allopurinol: A Comprehensive Review. Journal of Pharmaceutical Sciences and Research, 5(2), 87-102.
- Kumar, S., Patel, R., & Singh, A. (2012). Development and Evaluation of Allopurinol Suspensions: A Quality by Design (QbD)Approach.International Journal of Pharmaceutics, 45(3), 278-291.
- Xu, J., Wang, W., Zhang, W., & Chen, J. (2021). Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases: Mechanisms and Pharmacotherapies. Frontiers in Cardiovascular Medicine, 8, 698612
- Sharma, S., Kumar, D., &Soni, G. (2011). Statistical design for optimization of allopurinol for mulations: Identification of critical material attributes and process parameters. Journal of Pharmaceutical Sciences, 100(8), 3200-3212.
- Cai, H., Harrison, D. G. (2000). Endothelial Dysfunction in Cardiovascular Diseases: The Role of Oxidant Stress.Circulation Research, 87(10),840-844
- Machlin,L.J.,Bendich,A.,Free radical tissue damage: protective role of antioxidant nutrients. The FASEB Journal, 1987.
- Bredemeier M., Lopes L.M., Eisenreich M.A., Hickmann S, Bongiorno G.K., Dantas, L.S., & Guaragna, J.C.V. (2018). Efficacy of xanthine oxidase inhibitors inpreventing cardiovascular events: asystematic review and meta-analysis. Cardiology in Review, 26(1), 33-38.
- Butler, R., Morris, A.D., Belch, J.J., Hill, A., Struthers, A.D. (2018). Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension, 35(6), 746-751.
- Xu, J., Wang, W., Zhang, W., & Chen, J. (2021). Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases: Mechanisms and Pharmacotherapies. Frontiers in Cardiovascular Medicine, 8, 698612.
- Sharma, S., Kumar, D., &Soni, G. (2011). Statistical design for optimization of allopurinol formulations: Identification of critical material attributes and process parameters. Journal of Pharmaceutical Sciences, 100(8), 3200-3212.
- Cai, H., Harrison, D. G. (2000). Endothelial Dysfunction in Cardiovascular Diseases: The Role of Oxidant Stress. Circulation Research,87(10),840-844.


 Pavithran P.* 1
Pavithran P.* 1
 Vasanthan A 2
Vasanthan A 2
 Senthilkumar K. L. 3
Senthilkumar K. L. 3








 10.5281/zenodo.11388241
10.5281/zenodo.11388241