Abstract
Ranolazine is an anti-anginal drug with extensive and highly variable hepatic first pass metabolism following oral administration, with systemic bio-availability of 76% and ranolazine also has a relatively short plasma half-life of 2.5+0.5 hours. Ranolazine, a promising therapeutic agent for the management of chronic angina, poses challenges in its conventional tablet formulation due to its low bioavailability and short half-life. To address these limitations, novel drug delivery systems (NDDS) offer innovative approaches to enhance drug efficacy and patient compliance. This research paper explores the development of ranolazine tablets utilizing both NDDS and mucoadhesive drug delivery systems (MDDS). The study investigates various formulation strategies, including nanoparticle encapsulation, liposomal delivery, and mucoadhesive polymer incorporation, aimed at improving drug solubility, stability, and targeted delivery to the site of action. Evaluation of the developed formulations includes in vitro characterization studies such as drug release kinetics, mucoadhesive properties, and pharmacokinetic profiling. The findings demonstrate the potential of NDDS and MDDS in optimizing the delivery of ranolazine, thereby offering promising avenues for enhanced therapeutic outcomes in the management of chronic angina.
Keywords
Ranolazine, Mucoadhesive tablets, Chronic angina, Sustained release
Introduction
A derivative of piperazine acetamide, ranolazine is an anti-anginal medication. It functions by partially inhibiting fatty acid oxidase, which raises the synthesis of adenosine triphosphate from glucose and enhances myocardial function. As a result, it acts as an anti-ischemic agent without regard to hemodynamic parameters like heart rate or blood pressure. The co-morbidities and previously listed factors won't have a substantial impact on its efficacy. Because of its benefit, it is used as an efficient anti-ischemic or anti-angina medication to treat cardiac arrhythmias, myocardial infarction, and unstable chronic angina pectoris (exercise induced variant)[1]. A steady-state plasma drug concentration or tissue concentration that is nontoxic and therapeutically effective for an extended length of time is the primary objective of any medication therapy. Modified release drug delivery techniques, such as site-specific release, controlled release, sustained release, and delayed release, alleviate many of the drawbacks of traditional drug therapy[2]. The intestines and liver metabolize ranolazine substantially, and absorption varies greatly. Poorly soluble ranolazine has an apparent terminal half-life of 2.5+0.5 hours. The marketed product is taken twice a day and contains an active component of 500 mg or 1000 mg. Therefore, to achieve consistent in vivo release, careful selection of release-delaying excipients is required.[3] ]. Mucoadhesive drug delivery systems have drawn interest lately due to their capacity to improve drug absorption, extend the duration of the drug's residency at the absorption site, and offer sustained release of the drug[4].
NOVEL DRUG DELIVERY SYSTEM :
Novel drug delivery systems (NDDS) are a cutting-edge strategy used in the pharmaceutical sciences to improve patient compliance, safety, and drug efficacy in order to optimize therapeutic outcomes. Advanced delivery systems have to be developed because traditional drug formulations frequently have issues with low bioavailability, short half-lives, and off-target effects [5]. The term "NDDS" refers to a broad category of formulations and technologies intended to get around these restrictions and enhance the pharmacokinetic and pharmacodynamic qualities of medications. By focusing on certain areas of action, regulating drug release kinetics, and improving drug stability, these systems can be designed to maximize therapeutic advantages and reduce side effects [6]. The goal of novel drug delivery systems (NDDS) for antianginal therapy is to maximize the distribution of antianginal medications, like beta-blockers, nitroglycerin, and ranolazine, to the affected tissues while reducing side effects. These systems use cutting-edge technologies to get beyond the drawbacks of traditional formulations, such as their low bioavailability, short half-lives, and dose-related adverse effects.[7] Overall, by enhancing medication efficacy, safety, and patient adherence, innovative drug delivery methods for antianginal therapy show promise for maximizing the treatment of angina pectoris. These systems have the potential to enhance the treatment of ischemic heart disease and improve angina patients' outcomes by utilizing cutting-edge technologies and delivery methods.[8]
MUCOADHESIVE DRUG DELIVERY SYSTEM:
Mucoadhesive drug delivery systems lengthen the dosage form's residence time at the site of absorption by interacting with mucin molecules and the mucus layer that covers the mucosal epithelial surface. Extended stays in the gastrointestinal tract (GIT) are necessary for medications with local action or those with maximal absorption in the GIT. Mucoadhesive dosing formulations are therefore beneficial for raising medication plasma concentrations and therapeutic activity[9]. Mucoadhesive drug delivery techniques extend the dosage form's residence time at the application or absorption site. They enhance the drug's therapeutic efficacy by enabling the dosage form to make close contact with the underlying absorption surface. For both systemic and local effects, numerous mucoadhesive drug delivery methods have been developed recently for oral, buccal, nasal, rectal, and vaginal routes. [10]
ADVANTAGES OF MUCOADHESIVE DRUG DELIVERY SYSTEM:
- Because mucoadhesive delivery techniques extend the duration of medication residence in the gastrointestinal tract (GIT), they have many advantages over other oral controlled release systems.
- Dosage form localization and targeting to a particular location.
- Moreover, it is well known that mucoadhesive systems allow for close interaction between the dosage form and the absorptive mucosa, which raises the drug flux at the absorbing tissue.[11]
- The git's acidic environment shields the drug from deterioration.
- Higher levels of patient adherence.[12]
DISADVANTAGES OF MUCOADHESIVE DRUG DELIVERY SYSTEMS:-
- The development of localized ulcers as a result of extended pharmacological interaction with an ulcerogenic substance.
- A significant obstacle to the advancement of oral mucosal delivery is the absence of a reliable model for in vitro screening to find medications appropriate for this kind of delivery.
- Acceptability by the patient in terms of irritancy and taste.
- Consuming food or beverages is not allowed.[13]
MECHANISMS OF MUCOADHESION:-
The contact stage and the consolidation stage are the two main phases that make up the cohesion mechanism. The formulation spreads and swells during the initial stage, which marks the beginning of the formulation's deep contact with the mucus layer. This contact occurs between the mucoadhesive and the mucus membrane. The presence of moisture activates the mucoadhesive components during the consolidation process. The system becomes more malleable when there is moisture present, which enables the mucoadhesive molecules to separate and form weak hydrogen and van der Waals bonds. Diffusion theory and dehydration theory are essentially the two hypotheses that explain the consolidation process. Diffusion hypothesis states that the mucoadhesive molecules and the mucus's glycoproteins interact with one another through chain penetration and the formation of secondary bonds. In order for this to happen, the mucoadhesive device needs to have characteristics that promote both chemical and mechanical interaction. For instance, molecules with surface active properties that aid in spreading throughout the mucus layer, high molecular weight, anionic surface charge, flexible chains, and hydrogen bond building groups (OH, COOH) can have mucoadhesive properties. [14]
ANGINA PECTORIS :
An underlying cardiac disease can manifest as angina pectoris. It indicates that the heart is not receiving enough blood, which leaves the heart with insufficient oxygen. This reduction in oxygen delivery to the heart muscle occurs when one or more coronary arteries narrow or get clogged, a condition known as atherosclerosis. Two blood arteries allow blood to enter the heart. These are referred to as the coronary arteries, and they provide the blood, oxygen, and nourishment that the heart muscle requires to beat. The coronary arteries typically supply enough blood to give the heart muscle the necessary quantity of oxygen to function correctly. But in coronary heart disease, these arteries narrow, limiting the volume of blood that can flow through them. As a result, blood cannot reach the heart muscle quickly enough, causing the heart to ache. We call this pain angina. [14]
MATERIALS AND INSTRUMENTS:
Chemicals used for work:
API: Ranolazine
MUCOADHESIVE POLYMER: Carbopol
MUCOADHESIVE POLYMER: Sodium Carboxymethyl Cellulose
LUBRICANT: Magnesium stearate, TALC
DILUENT: Microcrystalline Cellulose
BINDER: PVP[Polyvinylpyrrolidone]
PREFORMULATIOMS STUDY:
- Drug Identification and Characterization:
Chemical Structure and Properties:
Molecular Weight: Determine the molecular weight of ranolazine.
Organoleptic Properties:
Appearance:
Describe the physical appearance of ranolazine (colour, form).
Odor and Taste:
Document any noticeable Odor and taste.[31]
2.Solubility Studies:
Solubility Determination:
Assess the solubility of ranolazine in various solvents (water, ethanol, methanol, buffer solutions at different pH values).
pH Solubility Profile:
Determine the solubility of ranolazine in buffer solutions across a pH range (1.2 to 7.4).[32]
3.Melting Point and Thermal Analysis:
Melting Point: Determine the melting point using a melting point apparatus.[33]
4.Hygroscopicity:
Moisture Absorption:
Evaluate the hygroscopic nature by exposing the drug to different relative humidity conditions and measuring weight gain.[34]
5.Flow Properties:
Bulk Density and Tapped Density:
Measure to determine the compressibility index and Hausner ratio.
Angle of Repose:
Measure to assess the flowability of ranolazine and its mixtures with excipients.[35]
Carr’s Index:
Calculate from the bulk and tapped densities to evaluate powder flowability.[36]
6.pKa and Dissociation Constant:
pKa Determination:
Determine the pKa value of ranolazine using potentiometric titration or UV spectrophotometry.
Dissociation Constant:
Use pKa data to understand the ionization profile in different pH environments.[37]
7.Stability Studies:
Solid-State Stability:
Assess the stability of ranolazine under various environmental conditions (temperature, humidity, light).[38]
Solution Stability:
Determine the stability of ranolazine in solution at different pH values and temperatures[39]
Formulation table :

EVALUATION TEST:
1.Colour and appearances:
The compressed tablets were examined for the colour and appearance.[41]
2.Thickness:
Dimension of the tablets measured using a calibrated dial calliper. Five tablets sample formulation are picked out randomly and its thickness is measured individually. Mean value of thickness is observed.[42]
3.Weight variation:
Twenty tablets were selected at random and weighed individually. The individual weights were compared with the average weight for determination of weight variation. The percentage deviation was calculated and then compared with IP limit. [43]
4.Hardness:
Five tablets were selected at random individually. The amount of force needed to crush tablets during a compression test. The procedure for assessing a tablet's hardness involves crushing the tablet between two jaws. The Pfizer tester was used to determine the tablet's hardness. kg/cm2 is the unit of hardness.[44]
5.Disintegration Time:
Disintegration time is the length of time it takes for a pill to disintegrate into tiny grains or pieces. The disintegration test is conducted in a device that has a basket rack assembly with six glass tubes, each measuring 7.75 cm in length and 2.15 mm in diameter, with a 10-mesh sieve at the bottom. 28 to 32 times per minute, the basket is lifted and lowered in 900 cc of medium that is kept at a constant 37 °C. Each tube held six tablets, and the time it took for all of the tablet fragments to pass completely through sieve number 10 was taken as the tablet's disintegration time.[45]
6.Friability:
The toughness of a tablet is measured by its friability. The friability of the pill was assessed using the Roche Fraibilator. 10 pills were precisely weighed and put into the friabilator chamber, which rotates at 25 rpm for 4 minutes, dropping the tablets over a 6-inch space with each revolution.100 rotations took 4 minutes to complete, after which the pills were reweighed.[46]
7.Content uniformity test:
Assay of drug content was performed in triplicate for each ranolazine tablet formulation. An amount of powder equivalent to 500 mg of ranolazine was weighed and transferred to a 50 ml volumetric flask. Methanol and pH 6.8 phosphate buffer solution was used to dissolve the drug under sonication for 15 minutes. Then samples were filtered through a 0.45 ?m diameter membrane. Filtered solutions were suitably diluted with pH 6.8 phosphate buffer solution and drug content of the diluted solutions were measured using a UV spectrometer at a wavelength of 224.2 nm.
The drug content was calculated as:
% Drug Content = (Analysed value / Theoretical Value) × 100 [47]
8.FT-IR:
Compatibility of the Drug with the excipients was determined by subjecting the physical mixture of the drug and the polymers of the main formulation to infrared absorption spectral analysis. Any changes in chemical composition of the drug after combining it with the polymers were investigated with I.R. spectral analysis.
9.Mucoadhesivestrength:
Set the mucosal tissue in the petri dish and immerse it in the phosphate buffer solution. Ensure that the temperature of the buffer is maintained at 37°C (physiological temperature)
10.Mucoadhesion Test:
Lower the upper probe (with the tablet) onto the mucosal tissue with a pre-determined contact force and contact time. After the contact time, start the separation process at a controlled rate and record the force required to detach the tablet from the mucosal tissue.
11.Data Recording and Analysis:
The force required to detach the tablet from the mucosal tissue is measured in Newtons (N).Record multiple readings to ensure reproducibility and accuracy. Calculate the average mucoadhesive strength from the recorded data.
RESULT AND DISCUSSION:
Results of Pre-formulation Study :
1.Drug Identification and Characterization:
Chemical Structure and Properties:
Molecular Weight: The molecular weight of ranolazine was confirmed to be approximately 427.54 g/mol.
Organoleptic Properties:
Appearance:
Ranolazine was observed as a white to off-white crystalline powder.
Odor and Taste:
Ranolazine had a slight odor and a bitter taste.
2.Melting Point and Thermal Analysis:
Melting Point:
The melting point of ranolazine was found to be 122°C, consistent with literature values.
3.Solubility studies:
Solubility Determination:
Water:
Ranolazine showed poor solubility in water, approximately 0.1 mg/ml.
Ethanol and Methanol:
Solubility in ethanol was 3 mg/mL, and in methanol, it was 5 mg/ml.
Buffer Solutions: Ranolazine had higher solubility in acidic buffers (pH 1.2: 2 mg/ml) compared to neutral and basic buffers (pH 7.4: 0.5 mg/mL)
4.pH Solubility Profile:
The solubility of ranolazine decreased with increasing pH, showing maximum solubility in acidic conditions
5.Hygroscopicity:
Moisture Absorption:
Ranolazine was found to be non-hygroscopic, showing negligible weight gain (<0>
5.Flow Properties:
Bulk Density and Tapped Density:
- Bulk Density: 0.35 g/mL
- Tapped Density: 0.45 g/mL
- Compressibility Index: 22% (indicating fair flowability)
- Hausner Ratio: 1.29 (acceptable flowability)
Angle of Repose:
The angle of repose was measured to be 35°, indicating fair flow properties.
Carr’s Index:
Calculated as 22%, indicating fair flowability of the powder blend.
6.pKa and Dissociation Constant:
pKa Determination:
The pKa of ranolazine was determined to be 7.2, indicating that it is predominantly non-ionized in acidic environments and ionized in basic environments.
7.Stability Studies:
Solid-State Stability:
Ranolazine was stable under various environmental conditions (temperature, humidity, light). No significant degradation was observed after 3 months of storage at 25°C/60% RH and 40°C/75% RH.
Solution Stability:
Ranolazine showed good stability in acidic and neutral pH solutions over a period of 24 hours at 37°C. However, in basic pH solutions, slight degradation was observed.
8.FTIR :
The different peaks of drug, polymer and their physical mixture indicate all groups and characteristics of the drug were not altered. There is no significant interaction in drug and polymer.
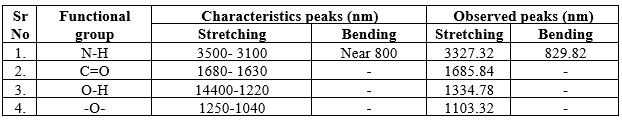
Graph No.1: FTIR Spectral Analysis of Ranolazine
Measurement of Mucoadhesive Strength:
The mucoadhesive strength of ranolazine tablets was evaluated using a texture analyser (or specific mucoadhesion testing apparatus) under standardized conditions. Fresh porcine gastric mucosa was used as the substrate, and phosphate buffer (pH 6.8) was used to simulate physiological conditions. The contact force was set to 0.5 N, the contact time was maintained at 2 minutes, and the separation rate was 0.5 mm/s.
The calculate values are presented in Table:
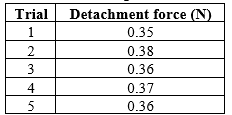
RESULT OF EVALUATION TEST:

Optimization Batch:
The optimized batch (Batch 3) of mucoadhesive tablets containing ranolazine was successfully formulated and evaluated. The tablets demonstrated excellent mucoadhesive properties, controlled drug release, and stability, making them a promising formulation for the treatment of chronic angina.
CONCLUSION:
The goal of this project was to create mucoadhesive tablets containing ranolazine, which would stick to the inside of the mouth and release the drug slowly over time. This approach is intended to improve how well the drug works and make it easier for patients to take. We made five different versions of the tablets, each with different amounts of ingredients that help the tablet stick and control how the drug is released. Among these, the third version (Batch 3) turned out to be the best. This batch contained specific amounts of ingredients that made it stick well to the mouth's lining and release the drug at a steady rate. We tested the tablets to make sure they met certain standards. All five versions had consistent weights, were hard enough to handle, and didn’t break apart easily. The amount of ranolazine in each tablet was very close to the expected amount, ensuring each dose was correct. Batch 3 showed the best results in terms of sticking to the mouth's lining and swelling to release the drug. It had the highest strength for sticking and the best rate of swelling, making it the most effective at delivering ranolazine steadily. When we tested how the drug was released over time, all batches released ranolazine slowly over 1hours, but Batch 3 did this most effectively. The tablets in Batch 3 also stayed intact the longest, which is important for ensuring the drug is released steadily and effectively.
REFERENCES
- Gunda RK, Manchineni PR, Duraiswamy D, Gsn KR. Design, Development, Optimization and Evaluation of Ranolazine Extended Release Tablets. Turk J Pharm Sci. 2022;19(2):125-131. doi:10.4274/tjps.galenos.2021.58047
- Wai T, Lee Y and Robinson JR. Controlled release drug delivery systems: in: Remingtons; The science and practice of pharmacy. Ed 20, Lippincott Williams & Wilkins, Philadelphia, 2000; 1: pp 903-904.
- Bidada J, Gonjari I, Bhusari A, Raut C, Dhule A. Development of extended release matrix tablets of ranolazine containing polyacrylic and ethylcellulose polymers. Der Pharmacia Lettre. 2011;3(4):215-26.
- Smart JD. The basics and underlying mechanisms of mucoadhesion. Adv Drug Deliv Rev. 2005;57(11):1556-1568.
- Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2012;64:302-315. Park K. Controlled drug delivery systems: past forward and future back. J Control Release. 2014;190:3-8.
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771-782.
- Chaitanya K, Rajarajan S, Reddy MV, et al. Ranolazine: a novel approach to treat angina pectoris. J ClinDiagn Res. 2013;7(6):1228-1232. doi:10.7860/JCDR/2013/5698.3040
- Zulkifli AF, Sim KY, Khor SM, et al. Ranolazine delivery via ethosomes as a promising strategy for enhancing its bioavailability and anti-anginal efficacy. Pharmaceutics. 2021;13(9):1504. doi:10.3390/pharmaceutics13091504
- Chaitanya K, Rajarajan S, Reddy MV, et al. Ranolazine: a novel approach to treat angina pectoris. J ClinDiagn Res. 2013;7(6):1228-1232. doi:10.7860/JCDR/2013/5698.3040
- Chaitanya K, Sangeetha D, Udupa N. Formulation and evaluation of ranolazineextendedrelease matrix tablets. Saudi Pharm J. 2014;22(2):135-141. doi:10.1016/j.jsps.2013.04.007
- Hossain MA, Choi HK. A novel ranolazine-loaded nanostructured lipid carrier approach to reduce angina. Drug DelivTransl Res. 2019;9(3):707-718. doi:10.1007/s13346-019- 00644-71
- Skubal M, Abdallah HM, Zhang Y, et al. Formulation and evaluation of ranolazine extended-release matrix tablets: optimization of formulation using the central composite design. Pharm Dev Technol. 2020;25(1):28-38. doi:10.1080/10837450.2019.1655748
- Zulkifli AF, Sim KY, Khor SM, et al. Ranolazine delivery via ethosomes as a promising strategy for enhancing its bioavailability and anti-anginal efficacy. Pharmaceutics. 2021;13(9):1504. doi:10.3390/pharmaceutics13091504
- Bouckaert S, Remon JP. In-vitro bioadhesion of a buccal, miconazole slow-release tablet J Pharm Pharmacol. 1993;45:504–7


 Shivaji Maruti Patil*
Shivaji Maruti Patil*
 Samrudh Shinde
Samrudh Shinde





 10.5281/zenodo.12795739
10.5281/zenodo.12795739