Syzygium cumini, commonly known as Malabar plum, Java plum, black plum, jamun, jaman, jambul, or jambolan, is an evergreen tropical tree in the flowering plant family Myrtaceae, and favored for its fruit, timber, and ornamental value. Java plum leaves smell like turpentine. They are pink and soft when they are young, maturing to a glossy but leathery dark green with a yellow rib down the middle as they mature. The leaves make good livestock fodder. They are a good source of digestible carbohydrates, magnesium, phosphorus, and vitamin A.The fruit is an excellent source of vitamin C. In a preliminary study, Ndyomugyenyi (2008) demonstrated that the bioactive compounds in Java plum included sterols, triterpenes, coumarins, tannins, glycosides (cardiac and steroids), gallic acid ,alkaloids, reducing compounds, anthocyanin pigments, and saponins. Jambolan is rich in compounds containing anthocyanins, glucoside, ellagic acid, isoquercetin, kaemferol and myrecetin. The seeds are claimed to contain alkaloid, jambosine, and glycoside jambolin or antimellin, which halts the diastatic conversion of starch into sugar and seed extract has lowered blood pressure by 34.6% and this action is attributed to the ellagic acid content. The seeds have been reported to be rich in flavonoids, a well-known antioxidant, which accounts for the scavenging of free radicals and protective effect on antioxidant enzymes and also found to have high total phenolics with significant antioxidant activity and are fairly rich in protein and calcium. Java plums are rich in sugar, mineral salts, vitamins C, PP which fortifies the beneficial effects of vitamin C, anthocyanins and flavonoids. In this work extracted Java plum containing gallic acid by decoction method. For verification of that gallic acid we performed TLC in which we got RF value which is similar to gallic acid Rf value. From which we identified Gallic acid from java plum and our Extraction of Gallic Acid from Java Plum Leaves has been done.
Syzygium Cumini, Anthocyanins, Glucoside, Ellagic Acid, Isoquercetin
Syzygium cumini, commonly known as Malabar plum, Java plum, black plum, jamun, jaman, jambul, or jambolan, is an evergreen tropical tree in the flowering plant family Myrtaceae, and favored for its fruit, timber, and ornamental value. Java plum leaves smell like turpentine. They are pink and soft when they are young, maturing to a glossy but leathery dark green with a yellow rib down the middle as they mature. The leaves make good livestock fodder. They are a good source of digestible carbohydrates, magnesium, phosphorus, and vitamin A.The fruit is an excellent source of vitamin C. Java plum blossoms appear in clusters. The fruit also appears in clusters of 10 to 40 (although sometimes just a few). Their color changes from light green to magenta to deep purple as they ripen. Most Java plum fruit contain a single seed about an inch (25 mm) long, although sometimes fruit can contain as many as four or five seeds or no seeds at all. The flesh of the fruit leaf It is native to the Indian Subcontinent, adjoining regions of Southeast Asia, including Myanmar, Sri Lanka, and the Andaman Islands. It can reach heights of up to 30 metres (98 ft) and can live more than 100 years. A rapidly growing plant, it is considered an invasive species in many world regions.ves an astringent, puckery sensation in the mouth, but can be quite sweet. Leaves are like turpentine and have soothing aroma. Young pinkish leaves are changing into leathery, glossy and dark green with a yellow colored midrib. Leaves contain rich nutritional value, offers dense shade and used as foliage for animals. Flowers of this tree are very tiny, white colored and aromatic about 5mm in diameter. Flowering of this tree starts from March to April. Fruits are ovoid shaped, large berries, unripe fruit is green changes to pink and finally it changes to dark crimson after ripening. Its fruit has special property to color tongue purple and fruit is sweet, sour and astringent in taste.
Plant profile
Java plum leaves smell like turpentine. They are pink and soft when they are young, maturing to a glossy but leathery dark green with a yellow rib down the middle as they mature. The leaves make good livestock fodder. They are a good source of digestible carbohydrates, magnesium, phosphorus, and vitamin A. Syzygium cumini, Jambolan or otherwise known as Java Plum, is a mediumsized tropical and evergreen tree, about 10-30 m in height. The leaves are smooth, opposite, shiny, leathery and oval. The flowers are pink or nearly white. The fruits are oval, green to black when ripe, with dark purple flesh. It contains a large seed. The seeds and fruits are used in the treatment of diabetes. Seeds and bark are used against dysentery. Bark juice is used for treating wounds and enlargement of the spleen. Bark infusion is used to treat irregular menstruation, diarrhea, dysentery, children's thrush, etc. Fruits are used in the treatment of colic and diarrhea. Leaf infusion is used for diarrhea and diabetes. Fruits can be eaten raw or processed into desserts. It is juicy, purple, and olive-shaped. Jambolan also functions as a hedge in some areas and is interplanted with crops as a shade tree. The bark is a source of tannins and brown dye used in coloring and preserving fish nets. The branches are uses to whiten teeth. The wood is used in exterior joinery and carpentry, construction, boat building, plywood, agricultural implements, furniture, etc. Jambolan can tolerate waterlogged conditions and can withstand strong winds.
Synonyms–
Syzygium cumini, commonly known as Malabar plum, Java plum, black plum, jamun, jaman, jambul, or jambolan, is an evergreen tropical tree in the flowering plant family Myrtaceae, and favored for its fruit, timber, and ornamental value.
Geographical source-
It is native to the Indian subcontinent, adjoining regions of Southeast Asia, including Myanmar, Sri Lanka, and the Andaman Islands. It can reach heights of up to 30 metres (98 ft) and can live more than 100 years.
It is an evergreen tropical tree in the flowering plant family Myrtaceae. Syzygium cumini is native to Bangladesh, India, Nepal, Pakistan, Sri Lanka, Malaysia, the Philippines, and Indonesia. Jamun is also found in Thailand, the Philippines, the West Indies, and many other tropical and subtropical countries (Morton, 1968). The fruit syrup is very useful for curing diarrhea. It is stomachic, carminative, and diuretic, apart from having cooling and digestive properties (Thaper, 1958).
Chemical constituent-
In a preliminary study, Ndyomugyenyi (2008) demonstrated that the bioactive compounds in Java plum included sterols, triterpenes, coumarins, tannins, glycosides (cardiac and steroids), gallic acid ,alkaloids, reducing compounds, anthocyanin pigments, and saponins.
Jambolan is rich in compounds containing anthocyanins, glucoside, ellagic acid, isoquercetin, kaemferol and myrecetin. The seeds are claimed to contain alkaloid, jambosine, and glycoside jambolin or antimellin, which halts the diastatic conversion of starch into sugar and seed extract has lowered blood pressure by 34.6% and this action is attributed to the ellagic acid content. The seeds have been reported to be rich in flavonoids, a well-known antioxidant, which accounts for the scavenging of free radicals and protective effect on antioxidant enzymes and also found to have high total phenolics with significant antioxidant activity and are fairly rich in protein and calcium. Java plums are rich in sugar, mineral salts, vitamins C, PP which fortifies the beneficial effects of vitamin C, anthocyanins and flavonoids.
Leaves-
The leaves are rich in acylated flavonol glycosides, gallic acid ,quercetin, myricetin, myricitin, myricetin 3-O-4-acetyl-Lrhamnopyranoside, triterpenoids, esterase, galloyl carboxylase, and tannin.

Fig.- 01 Leaves of Java Plum
Uses
- The Leaves Contain-
- Antioxidants And Have Antiulcer’
- Anti-Virus,
- Anti-Inflammatory Properties, While Helping Lower Blood Sugar Levels, Treating Constipation And Eliminating Allergies.
- Internal Diseases Treatment
Other Health Benefits of the Java Plum
- Fighting respiratory problems.
- Helping to manage weight.
- Boost immunity.
- Low-calorie count.
- Protects against anemia.
- Keeps the skin healthy.
- Improves gut health.
- Prevents nausea and vomiting.
Extraction

Fig.- 02 Extraction by decoction method
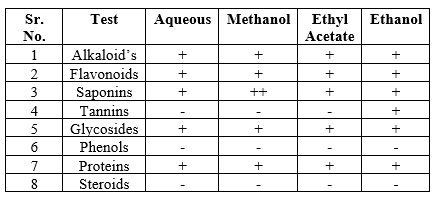
Dried leaf powder (50 g for each solvent) was soaked separately in 250 mL of each solvent namely: distilled water, ethanol, methanol, ethyl acetate, in a flask.
The five flasks were covered and then kept at room temperature for around 1 week. After that, the separate solutions were filtered by Whatman filter paper (no. 1). Each filtrate was collected in a round bottom flask and was subjected to evaporation to achieve a gummy appearance.
Then the gummy substance of each solvent was dried at room temperature. The powdered extracts were weighed and stored at 400C for further work.
From 50 g dried leaf powder, 7 g (14%) of extract was finally obtained from aqueous extract; 7.8 g (15.6%) of extract from ethanolic extract; 7.3 g (14.6%) from methanolic extract; 6.9 g (g (12.6%) from hexane extaction.
Analysis of Phytochemical.
Alkaloids
Dragendroff’s Test:
Filtrates were treated with Dragendroff’s reagent (solution of Potassium Bismuth Iodide). Formation of red precipitate indicates the presence of alkaloids.
Mayer’s Test:
To a few ml of plant sample extract, two drops of Mayer’s reagent are added along the sides of test tube. Appearance of white creamy precipitate indicates the presence of alkaloids [4].
Valser’s Test:
A few drops of Valser?s reagent is added to few ml of plant extract along the sides of test tube. A reddish- Brown precipitate confirms the test as positive.
Wagner’s Test:
A few drops of Wagner?s reagent are added to few ml of plant extract along the sides of test tube. A reddish- Brown precipitate confirms the test as positive.
Flavonoids
Alkaline Reagent Test:
Extracts were treated with few drops of sodium hydroxide solution. Formation of intense yellow colour, which becomes colorless on addition of dilute acid, indicates the presence of flavonoids.
Froth Test. The extract (50 mg) is diluted with distilled water and made up to 20 ml. The suspension is shaken in a graduated cylinder for 15 minutes. A two cm layer of foam indicates the presence of saponins
Tannins
Ferric Chloride Test:
The extract (50 mg) is dissolved in 5 ml of distilled water. To this few drops of neutral 5?rric chloride solution are added. A dark green colour indicates the presence of phenolic compound.
Glycosides For 50 mg of extract is hydrolysed with concentrated hydrochloric acid for 2 hours on a water bath, filtered and the hydrolysate is subjected to the following tests.
Legal’s Test:
50 mg of extract is dissolved in pyridine; sodium nitroprusside solution is added and made alkaline using 10% NaOH. Presence of glycoside is indicated by pink color. Phenols.
Lead Acetate Test:
The extract (50 mg) is dissolved in of distilled water and to this 3 ml of 10% lead acetate solution is added. A bulky white precipitate indicates the presence of phenolic compounds.
Proteins
The extract (100 mg) is dissolved in 10 ml of distilled water and filtered through Whatmann No. 1 filter paper and the filtrate is subjected to test for proteins.
Millon’s Test:
To 2 ml of filtrate few drops of Millon?s reagent are added. A white precipitate indicates the presence of proteins.
Carbohydrates
Molish’s Test. To 2 ml of plant sample extract, two drops of alcoholic solution of ?- naphthol are added. The mixture is shaken well and few drops of concentrated sulphuric acid is added slowly along the sides of test tube. A violet ring indicates the presence of carbohydrates.
Triterpenoids
Salkowski’s Test:
Extracts were treated with chloroform and filtered. The filtrates were treated with few drops of Conc. Sulphuric acid, shaken and allowed to stand. Appearance of golden yellow colour indicates the presence of triterpenes.
Phyto sterols
Libermann-
Burchard’s Test: Extracts were treated with chloroform and filtered. The filtrates were treated with few drops of acetic anhydride, boiled and cooled. Conc. Sulphuric acid was added.
a complex mixture. This separation process consists of two phases: a stationary phase and a mobile phase. The mobile phase consists of the mixture to be separated which percolates through the stationary phase. These two phases can be solid-liquid, liquid-liquid or gas-liquid.
Thin Layer Chromatography –
(TLC) is a solid-liquid form of chromatography where the stationary phase is a polar absorbent and the mobile phase can be a single solvent or combination of solvents.
Principle:
Thin Layer Chromatography (TLC) is a type of chromatography which is based upon the distribution of biomolecules between two immiscible phases. TLC was originally developed to separate lipid molecules and can be used to identify components in a sample, and for preparative purposes. In TLC the stationary phase is a polar absorbent, like finely ground alumina (Al2O3) or silica (SiO2) particles which are coated on a glass slide or plastic sheet to create a thin layer of the particular stationary phase. Silica contains some free – OH groups which form hydrogen bonds or other Vander-Waals interactions with the analyte components and as a result adsorption takes place. Sometimes a small amount of a binder such as plaster of Paris is mixed with the absorbent to facilitate the coating. The mixture to be separated is dissolved in a solvent and the solution is spotted at one end of the coated TLC plate next to the reference material.
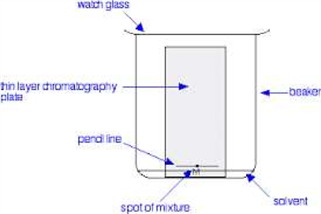
Fig. -03 Thin layer chromatoghraphy
In order to determine whether an unknown substance is the same as a substance of known structure, it is necessary to run the two substances side by side in the same chromatogram, preferably at the same concentration. The plate is placed with spotted end down in a covered jar containing a shallow layer of suitable solvent.
The solvent (mobile phase) is allowed to move up the plate by capillary action through the adsorbent at its own rate and as a result differential partitioning occurs between the components of the mixture dissolved in the solvent and the stationary adsorbent phase. The more strongly a given component of a mixture is adsorbed onto the stationary phase, the less time it will spend in the mobile phase and the more slowly it will migrate up the plate. When the solvent front has moved to within about 1 cm of the top end of the adsorbent, the plate should be removed from the developing chamber.If the components of the sample are coloured, they can be observed directly. If not, they can sometimes be visualized by shining ultraviolet light on the plate or by spraying the plate with a reagent (e.g. ninhydrin) that will react with one or more of the components of the sample.
Sometimes the spots can be visualized by allowing the plate to stand for a few minutes in a closed container in which the atmosphere is saturated with iodine vapor. The ninhydrin reaction is used to detect the presence of amino acids. Amino acids contain a free amino and carboxyl group which reacts together with ninhydrin to produce a characteristic blue colour (or occasionally pale yellow). In this reaction first an amino group is attached to the first or alpha carbon of the amino acid’s carbon chain and then the nitrogen atom of the amino group reacts with ninhydrin to give a blue-purple product known as Ruhemann’s purple . Some amino acids (e.g. proline, secondary amine) give yellow-orange colour. Thin layer chromatography, or TLC, is a method for analyzing mixtures by separating the compounds in the mixture. TLC can be used to help determine the number of components in a mixture, the identity of compounds, and the purity of a compound. By observing the appearance of a product or the disappearance of a reactant, it can also be used to monitor the progress of a reaction. TLC is a sensitive technique - microgram (0.000001 g) quantities can be analyzed by TLC - and it takes little time for an analysis (about 5-10 minutes).
TLC consists of three steps
- Spotting, - Development, - Visualization.
Photographs of each step are shown on the course website. First the sample to be analyzed is dissolved in a volatile (easily evaporated) solvent to produce a very dilute (about 1%) solution.
Spotting
consists of using a micro pipet to transfer a small amount of this dilute solution to one end of a TLC plate, in this case a thin layer of powdered silica gel that has been coated onto a plastic sheet. The spotting solvent quickly evaporates and leaves behind a small spot of the material.
Development
consists of placing the bottom of the TLC plate into a shallow pool of a development solvent, which then travels up the plate by capillary action. As the solvent travels up the plate, it moves over the original spot. A competition is set up between the silica gel plate and the development solvent for the spotted material. The very polar silica gel tries to hold the spot in its original place and the solvent tries to move the spot along with it as it travels up the plate. The outcome depends upon a balance among three polarities - that of the plate, the development solvent and the spot material. If the development solvent is polar enough, the spot will move some distance from its original location. Different components in the original spot, having different polarities, will move different distances from the original spot location and show up as separate spots.
Visualization-
When the solvent has traveled almost to the top of the plate, the plate is removed, the solvent front marked with a pencil, and the solvent allowed to evaporate. Visualization of colored compounds is simple – the spots can be directly observed after development. Because most compounds are colorless however, a visualization method is needed. The silica gel on the TLC plate is impregnated with a fluorescent material that glows under ultraviolet (UV) light. A spot will interfere with the fluorescence and appear as a dark spot on a glowing background. While under the UV light, the spots can be outlined with a pencil to mark their locations. A second method of visualization is accomplished by placing the plate into iodine vapors for a few minutes. Most organic compounds will form a dark-colored complex with iodine. It is good practice to use at least two visualization techniques in case a compound does not show up with one particular method.
Rf value
is used to quantify the movement of the materials along the plate. Rf is equal to the distance traveled by the substance divided by the distance traveled by the solvent. Its value is always between zero and one. A TLC analysis might be summarized something like, "Using a silica gel plate and ethyl acetate as the development solvent, unknown mixture X showed three spots having Rf's of 0.12, 0.25, and 0.87". Comparing these Rf’s with the Rf’s of known compounds might enable a tentative identification to be made. Note that observing three spots means only that there are at least three components in the mixture.
Some components may have such similar polarities that they appear under one spot after development.
The Rf (retardation factor) value is the ratio of the distance moved by the solute to the distance moved by the solvent
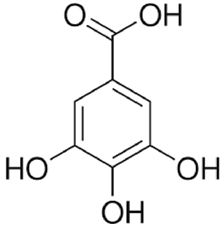
Fig-04 Gallic acid

Fig-05 Tlc plate
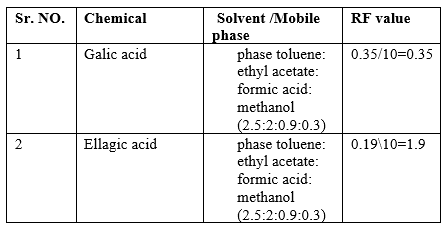
RF value of galic acid =3.5/10
= 0.35
Mobile phase use
Ethyl acetate, formic acid, glacial acetic acid, and water in varying ratios was tried. The mobile phase toluene: ethyl acetate: formic acid: methanol (2.5:2:0.9:0.3)


 Sarika Sahu*
Sarika Sahu*
 Chumman Sahu
Chumman Sahu





 10.5281/zenodo.11000691
10.5281/zenodo.11000691