Abstract
Explores the therapeutic potential of selectively modulating serotonin receptor subtypes in neurological and metabolic disorders. Through literature review, computational modelling, and experimental validation, the roles of various serotonin (5-Hydroxy tryptamine) receptor subtypes (5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT5, 5-HT6, and 5-HT7) are investigated in conditions like depression, anxiety, migraine, obesity, and cognitive impairment. The methodology involves comprehensive literature review, molecular docking studies, in vitro and in vivo experiments, and clinical trials. Results reveal novel therapeutic targets among serotonin receptor subtypes, elucidate mechanisms of action, and present promising pharmacological interventions with improved efficacy and safety profiles. This research will be advances precision medicine in neurological and metabolic disorders by targeting specific serotonin receptor subtypes, contributing to improved patient outcomes and quality of life.
Keywords
Serotonin receptors, neurological disorders, metabolic disorders, therapeutic potential, selective modulation, precision medicine.
Introduction
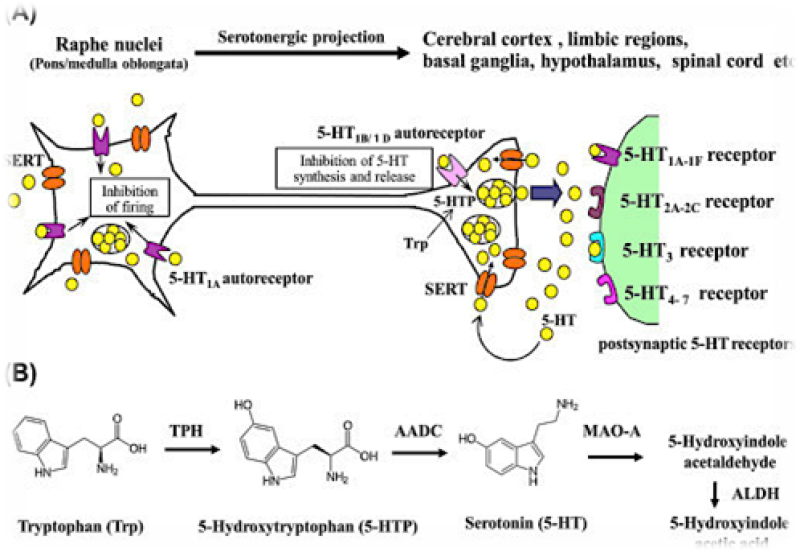
5-Hydroxy tryptamine(5-HT), also known as serotonin, is a neurotransmitter and hormone that plays a major role in neurologic and metabolic diseases. It is synthesized from tryptophan through the action of tryptophan hydroxylase (TPH)[1]. Serotonin is further transformed into melatonin, which is involved in regulating sleep patterns[2]. The physiological effects of serotonin have been studied in both animal models and human clinical trials, with potential therapeutic applications in the treatment of depression, anxiety, panic, sleep disorders, obesity, myoclonus, and serotonin syndrome[3].5-Hydroxy tryptamine (5-HT) is one of the most investigated and complex biogenic amines. The main 5-HT receptors are further classification as 5-HTI (5-HT1A, 5-HT1B, 5-HTID, 5-HTIE and 5-HT1F), 5-HT2 (5-HT2A, 5-HT2B and 5-HT2C), 5-HT3, 5-HT4, 5-HT5 (5-HT5A, 5-HT5B), 5-HT6 and 5-HT7 have been identified[4-6]. Specific drugs which are capable of either selectively stimulating or inhibiting these receptor subtypes are being designed. This has generated therapeutic potentials of 5-HT receptor modulators in a variety of disease conditions. Conditions where 5-HT receptor modulators have established their use with distinct efficacy and advantages include migraine, anxiety, psychosis, obesity and cancer therapy-induced vomiting by cytotoxic drugs[7-8].
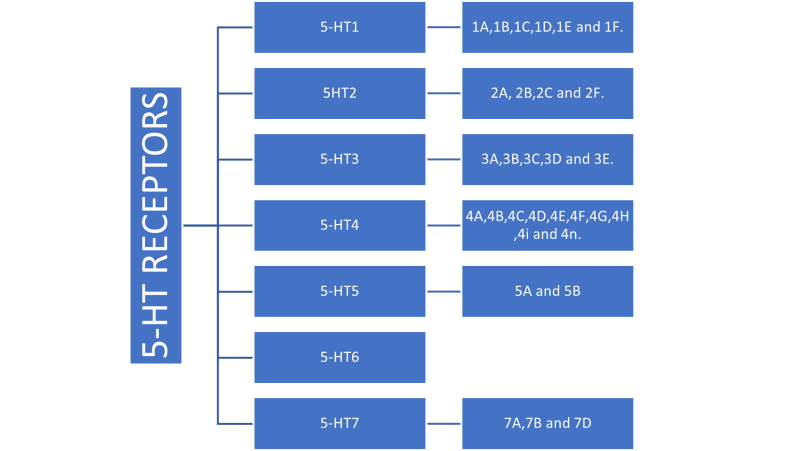
5-HT1
5-HT1 receptors are a class of serotonin receptors that play important roles in various physiological processes. They are involved in migraine pharmacotherapy, with selective agonists targeting these receptors being effective in aborting acute migraine attacks and inhibiting the release of calcitonin gene-related peptide (CGRP)[9]. The first selective 5-HT1 receptor agonist, sumatriptan, has been used for the treatment of migraines for nearly 30 years[10] . 5-HT1AR agonists are emerging as potential candidates for pain relief therapy, with promising results in preclinical studies[11] . Furthermore, pharmaceutical compositions combining flurbiprofen with 5-HT1 receptor agonists have been developed for the treatment or prevention of migraine headaches[12] . In terms of neuronal plasticity, the 5-HT1A receptor has been found to play a role in brain development and may be associated with various mental diseases[13] . Overall, 5-HT1 receptors have diverse functions and are being explored for their therapeutic potential in migraine treatment and pain relief, as well as their involvement in brain development and mental health.
There are some subtypes of 5-HT1
Figure No.01: Mechanism of action 5-HT1-7
5-H T1A
5-HT1B
5-HT1C
5-HT1D
5-HT1E
5-HT1F
5-H T1A:
The 5-Hydroxytryptamine receptor 1A (5-HT1A) gene has been associated with Type 1 diabetes (T1D) susceptibility[14].The HTR1A gene showed genome-wide significant linkage to T1D in Scandinavian families[15] .Markers tagging HTR1A and the ring finger protein 180 (RNF180) were associated with T1D in Swedish and Danish families[16]. However, this association was not confirmed in sporadic cases [17]. Both HTR1A and RNF180 genes were expressed at the mRNA level in human islets of Langerhans, and the 5-HT1A protein was present in isolated human islets of Langerhans and sections of human pancreas[18]. These findings suggest that HTR1A may play a role in T1D susceptibility by modulating the initial autoimmune attack or islet regeneration and insulin release . The 5-HT1A receptor was the first of the 5-HT family to be cloned (Kobilka et al., 1987; Fagin et al., 1988); it was also the first for which really selective ligands were described, such as 8-OH-DPAT (Gozlan et al., 1983; Middle miss and Fozard, 1983). This had a tremendous impact on the pharmacological characterization of 5-HT receptors in general and that of 5-HT1A in particular, including the distribution of 5-HT1A receptors in the brain.
5-HT1B:
5-HT1B receptor is a serotonin receptor that is widely expressed in the central nervous system and has been implicated in various cognitive and psychiatric disorders. It is a potential drug target for the treatment of Alzheimer's disease (AD) and other serotonin-related disorders[19]. Genetic studies have shown that HTR1B polymorphisms are associated with mental and behavioural disorders, but the functional mechanisms underlying these associations are not well understood[20-21]. PET imaging studies using radioligands have been conducted to study the 5-HT1B receptor in the brain. The intrinsic activity of a PET radioligand for the 5-HT1B receptor may impact its ability to detect changes in endogenous serotonin levels[22]. Triptans, a class of drugs that target the 5-HT1B receptor, have been proven effective for acute treatment of migraine, but their use is contraindicated in patients with cardiovascular and cerebrovascular diseases[23]. The 5-HT1B receptor was initially rather loosely defined as non-5-HT1A; in other words, [3H]-5-HT sites which were not sensitive to 8-OH-DPAT (Middle miss and Fozard, 1983; Pezos and Palacios, 1985), had high affinity for some beta-blocking ligands such as [125I]-cyan pindolol in rodents (Hoyer et al., 1985b), but much less so in non-rodents and primates (Hoyer et al., 1986).
5-HT1C:
The 5-HT1C receptor, later known as the 5-HT2C receptor, was discovered and characterized over 30 years ago. It is a G-protein-coupled receptor (GPCR) that is involved in various clinical applications and has a complex gene structure. The 5-HT2C receptor has been extensively studied and found to have therapeutic potential in treating major depression, obesity, addiction, schizophrenia, epilepsy, and other disorders. It is affected by established antidepressants and antipsychotics and may play a role in antipsychotic-induced weight gain. Ligands that target the 5-HT2C receptor, such as antagonists and agonists, have been developed and tested for their therapeutic efficacy. The 5-HT2C receptor is also being investigated for its potential as a biomarker or predictor of treatment response in major depressive disorder[24-25].
5-HT1D:
The 5-HT1D receptor is involved in various physiological processes. It has been found that brain stem-derived serotonin inhibits afferent transmission in the monosynaptic stretch reflex exclusively via 5-HT1D receptors[26].In the context of hepatocellular carcinoma (HCC), Paeoniflorin (PF) has been shown to regulate HCC progression by downregulating 5-HT1D and blocking the Wnt/?-catenin pathway[27]. In diabetic rats, 5-HT1D receptors inhibit noradrenergic-induced vasoconstrictions via the nitric oxide pathway[28]. Additionally, in the mesenteric vasculature, 5-HT1D receptors mediate serotonergic-induced sympathoinhibition of noradrenergic outflow[29]. The role of 5-HT1D receptors in cardiac regulation and the influence of fluoxetine, an antidepressant that increases serotonin levels, remain unclear[30]. -HT1D receptor has been cloned, and functionally expressed in recombinant systems; ligands can label it specifically (Zgombick et al., 1995, 1996, 1997), although most of them will also label 5-HT1B receptors . 5-HT1D selective antagonists have been developed for migraine, with the hope of avoiding cardiovascular side effects, but with less success than anticipated (see, for example, PNU-142633; McCall et al., 2002).
5-HT1E:
5-HT1E is a receptor that is involved in signalling pathways related to cyclic AMP (cAMP) and extracellular-signal related kinases (ERK). Activation of 5-HT1E receptor by serotonin leads to the activation of both cAMP and ERK pathways in HEK293 cells, and this signalling is dependent on a G?i-linked cascade[31-32].The involvement of G?? and Gq in 5-HT1E activation is not observed[33]. Protein kinase A (PKA) inhibition only affects ERK signalling, not cAMP[34].Serotonin-stimulated ERK phosphorylation is solely dependent on G protein signalling and is not affected by the absence of ?-arresting[35]. Knockdown of 5-HT1E reduces the expression of genes related to cell cycle regulation and survival, leading to decreased cell survival . Additionally, 5-HT1E can regulate the expression of other genes such as Receptor activity modifying protein 1 (RAMP1) and Nuclear receptor 1 (NR4A1) . The presence of 5-HT1E receptors in the hippocampus suggests a possible role in memory regulation. The 5-ht1E receptor was first detected in human brain membranes ( using radioligand binding studies that found that 5-HT, in the presence of blocking agents for other 5-HT1 subtypes that were known at that time (5-HT1A, 5-HT1B, 5-HT1C/2C), demonstrated a biphasic competition curve to 5-CT (Leonhardt et al., 1989; Miller and Teitler, 1992; Bruinvels et al., 1993; Barone et al., 1994).
5-HT1F:
5-HT1F receptor agonist that has shown potential therapeutic effects in the treatment of various conditions. It has been found to stimulate mitochondrial biogenesis and accelerate renal recovery in acute kidney injury (AKI) models[36]. A Lasmiditan has been shown to induce mitochondrial biogenesis and suppress neuroinflammation in the spinal cord, leading to the amelioration of mechanical allodynia in neuropathic pain[37]. In preclinical models of migraine and cluster headache, Lasmiditan has demonstrated the ability to reduce trigeminus vascular activation and alleviate cranial autonomic manifestation. The 5-HT1F receptor could be described as a pure product of molecular biology: It was identified by homology screening starting from the existing known 5-HT1 receptor sequences (Adham et al., 1996, 1997). The receptor is still relatively poorly characterized; it is pharmacologically and structurally close to 5-ht1E, but has been the subject of intense research (Adham et al., 1994b; Phoebus et al., 1996) since Sumatriptan, the 5-HT1B/1D agonist, also has affinity for 5-HT1F receptors.
The 5-HT2 receptors belong to the superfamily of G-protein coupled receptors, and consists of three subtypes: 5-HT2A, 5-HT2B and 5-HT2C(formerly known as the 5-HT2, 5-HT2F and 5-HT1C, respectively) receptors with related molecular structure, amino acid sequence and signalling properties (Hoyer et al. 1994; Leysen, 2004).
There are four 5-HT2 receptors :
5-HT2A
5-HT2B
5-HT2C
5-HT2F
The 5-HT2A and 5-HT2B receptors are both involved in various physiological processes. Activation of the 5-HT2A receptor has been linked to atypical functioning of organs other than the lungs in asthma, as well as psychoactive effects and hallucinogenic properties of certain drugs[38-39]. On the other hand, the 5-HT2B receptor has been associated with adverse effects, including cardiac valvopathy, and is important for embryonic development[40]. The 5-HT2B receptor has also been found to play a role in the modulation of respiratory interneurons and motoneurons, suggesting its involvement in respiratory activity . Additionally, the 5-HT2B receptor has been shown to be a target for the development of no hallucinogenic psychedelic analogy with therapeutic effects . Overall, both the 5-HT2A and 5-HT2B receptors have distinct roles and implications in various physiological and pharmacological processes. the 5-HT2C receptor is a serotonin receptor that has been implicated in social behaviour and the regulation of body weight. It plays a complex role in different social contexts in zebrafish, influencing social investigation, social novelty, and mirror-induced aggressive displays[41-42].In the context of obesity, 5-HT2C receptors have been targeted by agonists and positive allosteric modulators (PAMs) as potential anti obesity medications. 5-HT2F receptor was explored using a variety of structurally different compounds in a radioligand binding assay. In addition, the 5-HT2F receptor was shown to stimulate production of inositol 1,4,5-trisphosphate in the transformed cells. Based on the affinities of the compounds tested[43].
5-HT3 are five subtypes :
5-HT3a
5-HT3b
5-HT3c
5-HT3d
5-HT3e
The 5-HT3 receptor is a pentameric ligand-gated ion channel that plays a role in various physiological processes and is a therapeutic target for psychiatric and neurological disorders. Humans have five different subunits (A-E) that can form heteromeric receptors[44].These receptors are found not only on the cell membrane but also in intracellular and cell-free mitochondria, where they can impact mitochondrial function[45]. The ligand binding region of the 5HT3A subunit is conserved across different species, suggesting evolutionary significance[46]. The intracellular domain of the 5-HT3A subunit interacts with the resistance to inhibitors of choline esterase (RIC-3) protein, and specific amino acid residues are critical for this interaction[47].The distribution of 5-HT3 receptors is wider than the nervous system, and there is potential for targeting these receptors in conditions involving metabolic or inflammatory disorders[48].Additionally, a 5-HT3 receptor modulator containing heterocyclic nitrogen has been developed and shows promise for treating diseases related to the 5-HT3 receptor . The 5-HT3A receptor is a member of the pentameric ligand-gated ion channel family and plays a role in neurotransmission and psychiatric disorders. Multiple cryo-electron microscopy structures have been determined for the 5-HT3 receptor, but hydrophobic collapse of the transmembrane pores in molecular dynamics simulations has been observed. The 5-HT3B gene encodes for the 5-HT3B subunit of the serotonin receptor type 3 (5-HT3), which is involved in chemotherapy-induced nausea and vomiting during antiemetic therapy with 5-HT3 receptor antagonists. Mutations in the H3.3B gene have been associated with certain human cancers, including colorectal cancer (CRC). Novel genes encoding 5-HT(3C), 5-HT(3D), and 5-HT(3E) have recently been described but the functional importance of these proteins is unknown. In the present study, in silico analysis (confirmed by partial cloning) indicated that 5-HT(3C), 5-HT(3D), and 5-HT(3E) are not human-specific as previously reported: they are conserved in multiple mammalian species but are absent in rodents. Expression profiles of the novel human genes indicated high levels in the gastrointestinal tract but also in the brain, Dorsal Root Ganglion (DRG) and other tissues. Following the demonstration that these subunits are expressed at the cell membrane, the functional properties of the recombinant human subunits were investigated using patch clamp electrophysiology. 5-HT(3C), 5-HT(3D), and 5-HT(3E) were all non-functional when expressed alone[49]. All other subunit subtypes must heterotetrametric with 5-HT3A subunits to form functional channels. Additionally, there has not currently been any pharmacological difference found between the heteromeric 5-HT3AC, 5-HT3AD, 5-HT3AE, and the homomeric 5-HT3A receptor. These subunits may be the same (homo pentameries 5-HT3A receptors) or different (heterotetrametric receptors, usually comprising of 5-HT3A and 5-HT3B receptor subunits), with the latter having a number of distinct properties.Thompson AJ, Lummis SC. 5-HT3 receptors. Curr Pharm Des. 2006.
5-HT4:
5-HT4 receptors are a promising target for the treatment of depression, cognitive impairment, and gastrointestinal (GI) motility disorders. Activation of 5-HT4 receptors has been associated with improved learning and memory in depression[50]and pro-cognitive effects in animal models[51].In the GI tract, 5-HT4 receptor agonists stimulate propulsive motility, making them potential treatments for constipation[52-53] . These agonists act locally in the gut mucosa, minimizing access to systemic 5-HT4 receptors and reducing unwanted side effects[54].The structure-activity relationship of 5-HT4 receptor agonists has been studied, leading to the design of novel partial agonists with potential biological activity . The presence of 5-HT4 receptors in the epithelial layer of the human intestines supports the concept that mucosal 5-HT4 receptors could be a safe and effective therapeutic target for constipation . Overall, 5-HT4 receptors hold promise for the treatment of depression, cognitive disorders, and GI motility disorders. There are 10 subtypes of 5-HT4-
5-HT4a
5-HT4b
5-HT4c
5-HT4d
5-HT4e
5=HT4f
5-HT4g
5-HT4h
5-HT5i
5-HT4n
5-HTa:
The 5-HT4a receptor is a member of the serotonin receptor family and is commonly expressed in various systems in the body, including the central nervous system, gastrointestinal system, cardiovascular system, and urinary system[55].Activation of the 5-HT4a receptor has been shown to protect against respiratory depression caused by opioids, suggesting its potential role in the fine-tuned recovery from opioid-induced respiratory depression[56]. Differential internalization has been observed between the two splice variants of the 5-HT4 receptor, 5-HT4a and 5-HT4b, with only the 5-HT4b receptor undergoing time-dependent internalization upon agonist stimulation[57] .The 5-HT4a receptor has been found to be palmitoylated, and stimulation with agonist increases the turnover rate of receptor-bound palmitate, suggesting a functional role for palmitoylation/palmitoylation in 5-HT4a receptor signaling.
5-HTb:
The h5-HT4b receptor undergoes time-dependent internalization upon agonist stimulation, while the h5-HT4a receptor does not[58]. The 5-HT4b receptor is co expressed with the 5-HT4a receptor in various tissues, including the cardiac atrium and ventricle[59]. The pharmacological properties of the 5-HT4a and 5-HT4b receptors are similar, and their binding affinities and agonist potencies correlate with their effects in human atrium[60]. In early Alzheimer's disease patients, there is an upregulation of cerebral 5-HT4 receptor binding, which is positively correlated with amyloid-? burden and negatively correlated with cognitive function[61]. The 5-HT4 receptor may play a role in memory and learning, and its activation has been suggested to modulate acetylcholine release and reduce amyloid-? accumulation[62].
5-HTc
The C terminus of 5-HT4c has a high number of putative phosphorylation sites[63]. The four splice variants of the 5-HT4 receptor, including 5-HT4c, have an identical pharmacological profile and ability to stimulate adenylyl cyclase activity in the presence of 5-HT.The 5-HT4 receptor is known to be involved in learning and memory[64].Activation of the 5-HT4 receptor stimulates the extracellular signal-regulated kinase (ERK) pathway, which is independent of protein kinase A (PKA) .The 5-HT4 receptor may use the ERK pathway, in addition to the cAMP/PKA signaling pathway, to control memory processes .
5-HT4d and 5-HT4e.
The 5-HT4 receptor has different splice variants, including 5-HT4(e) and 5-HT4(d). The 5-HT4(e) receptor is found in the brain and heart atrium, while the 5-HT4(d) receptor is stably expressed in CHO cells. The binding profile of 5-HT4 ligands for the 5-HT4(e) receptor is consistent with other isoforms, but the potency of agonists in functional assays is inversely correlated with their affinities in binding assays[65] The 5-HT4(d) receptor has a high affinity for 5-HT4 ligands and is more efficiently coupled to its effector than the 5-HT4(e) receptor . The C-terminal tails of 5-HT4 receptor isoforms may influence their functional properties, as seen with the different agonist efficacies of renzapride at the 5-HT4(d) and 5-HT4(e) receptor .The 5-HT4(d) receptor also displays constitutive activity and some ligands with antagonist properties act as partial agonists[66].
5-HTf
5-HTg
5-HTh
5-HTi
5-HTn
The 5-HT4 receptor is a subtype of the 5-hydroxytryptamine (5-HT) receptor family that promotes cyclic AMP formation. It is coded by a complex gene and has multiple variants, including 5-HT4n[67].
The 5-HT4 receptors couple to the stimulatory Gs’ protein and include 4 isoforms (5-HT4a, 5-HT4b, 5-HT4e, 5-HT4f) in mouse (Hernandez & Janson’s, 2010) and at least 10 different splice variants (5-HT4a, 5-HT4b, 5-HT4c, 5-HT4d, 5-HT4e, 5-HT4f, 5-HT4g, 5-HT4hb, 5-HT4i, and 5-HT4n) in human (Bockaert,Claeysen,Compan, & Dumuis, 2004) the 5-HT4 receptor had been well.
5-HT5 subtypes:
5-HT5A
5-HT5B
The 5-HT5A receptor plays a critical role in cognitive processes, but further studies are needed to determine its specific aspects and functions[58]. 5-HTP, a precursor to serotonin, has significant physiological effects and is involved in the treatment of various neurological and metabolic diseases[69]. A high-performance liquid chromatographic method has been developed for the analysis of 5-HT in cell extracts and culture medium, allowing for the investigation of drug/condition-response relationships in vitro[70]. Increased levels of 5-HT and its metabolite have been observed in mutant mice and patients with cerebellar atrophy, and pharmacotherapy with 5-HT precursors has shown promise in improving motor coordination .Compounds that bind to 5-HT5 receptors have been developed and may have potential therapeutic applications in neurodegenerative and neuropsychiatric disorders[71]. The 5-HT5A receptor is a member of the 5-HT receptor family and signals through the Gi/o protein. It is involved in nervous system regulation and is a potential target for the treatment of various neurological disorders, including psychosis, depression, schizophrenia, and neuropathic pain[72]. However, the 5-HT5A receptor is the least understood serotonin receptor, and there is a lack of selective agonists and antagonists for this receptor .Recent studies have shown that activation of the 5-HT5A receptor in parvalbumin interneurons in the hippocampal dentate gyrus plays a role in the delayed behavioural response to antidepressants[73] .Additionally, the peripheral 5-HT5A receptors have been found to inhibit cardiac sympathetic neurotransmission, and their role in type 1 diabetes-related cardiopathies has been investigated[74].These findings provide insights into the function and potential therapeutic targeting of the 5-HT5A receptor in various neurological and cardiovascular conditions.
5-HT5B:
The 5-ht5b receptor subtype has been studied in the context of Rett syndrome, where it was found to be dysregulated[75].Unlike other cell surface receptors, 5-ht5b is located in endosomes and interacts with 5-HT1A receptors, reducing their surface expression .This interaction is mediated by specific trans-membrane domains and suggests a regulatory role for 5-ht5b in the activity of other 5-HT receptors[76]. The 5-HT5 receptor family consists of two members: 5-HT5a and 5-HT5b receptors. The 5-HT5a receptor has been identified in rodents and humans, whereas the 5-HT5b receptor is expressed only in rodents (Nelson, 2004). The roles and signal transduction pathways triggered by 5-HT5a and 5-HT5b receptors in the fatal brain have yet to be identified.
5-HT6:
The 5-HT6 receptor is a serotonin receptor subtype that is primarily expressed in the central nervous system. It plays a role in neurodevelopmental processes and is involved in cognitive processes. Pharmacological inhibition of the receptor has shown pro-cognitive effects in animal models of cognitive impairment. Several 5-HT6 receptor antagonists have been evaluated in clinical studies for the treatment of cognitive deficits associated with Alzheimer's disease (AD) and other neurological disorders. However, the outcomes of these studies have been largely disappointing. There is also potential for 5-HT6 receptor antagonists to be used in the management of neuropsychiatric symptoms in dementia. Understanding the structural basis and signaling pathways of the receptor can aid in the design of more effective medications for neuropsychiatric disorders[77-78]. Serotonin receptor 5-HT6 is involved in cognition and Alzheimer’s disease (AD) development. However, the mechanism of 5-HT6 in AD pathology is not clear. 5-HT6 receptor mRNA has not been found in the peripheral tissue, suggesting that compounds acting at this receptor may have limited peripheral side effects (Hannon & Hoyer, 2008). Given the distribution of 5-HT6 receptors in brain, particularly in areas associated with learning and memory, much research has focused on the role of this receptor in cognitive function. 5-HT6 receptors have been identified in areas of the rat and human brain associated with learning and memory: hippocampus, CA1, CA3, dentate gyrus, olfactory tubercles, cerebral cortex, nucleus accumbent, and striatum.[ T.P. Blackburn] The notion that several atypical antipsychotic agents such as clozapine, quetiapine, and olanzapine possess high affinity for the 5-HT6 receptor.
5-HT7 subtypes:
5-HT7A
5-HT7B
5-HT7D
The 5-HT7 receptor is a serotonin receptor that is primarily found in the nervous system and gastrointestinal tract. It plays a role in regulating mood, cognition, digestion, and vasoconstriction. In skeletal muscle microcirculation, the 5-HT7 receptor mediates arteriolar dilation in response to serotonin[79]. The receptor is also implicated in neurodegenerative diseases and has potential therapeutic implications . However, there are challenges in studying the 5-HT7 receptor, as current antibodies lack specificity in distinguishing between wild-type and knockout tissues[80]. Further research is needed to understand the signaling pathways and cellular mechanisms involved in the activation of the 5-HT7 receptor and to develop more specific antibodies for its detection. The 5-HT7 receptor plays a significant physiological role in the regulation of REM sleep, diurnal rhythm, pain, thermoregulation, mood and hippocampus-dependent cognitive processes (Roberts and Hedlund, 2012). The exact function of 5-HT7 receptors in memory and cognition is still unclear. 5-HT7 receptors are metabotropic receptors and highly expressed in specific thalamic nuclei as well as limbic regions (Hannon and Hoyer, 2008). In humans, the presence of three subtypes (5-HT7A, 5-HT7B, and 5-HT7D) has been observed (Heidmann et al., 1997). To date, no radioligands are readily available for human PET studies of this receptor. However, promising attempts for ligand development have been made (Hansen et al., 2014; Kumar and Mann, 2014; Deau et al., 2015).
5-HT7A:
Serotonin receptors 5-HT1A and 5-HT7 are involved in the development of various psychopathologies. Some data indicate that there is an interplay between 5-HT1A 5-HT7 receptors that could be implicated in the regulation of their function. This work analyzed the effects of chronic 5-HT7 activation on the functional activity of 5-HT7 and 5-HT1A receptors, on the corresponding protein levels, and on the expression of genes encoding 5-HT7 and 5-HT1A receptors in the mouse brain[81].
5-HT7B:
The 5-HT7 receptor has multiple isoforms, including 5-HT7(a), 5-HT7(b), and 5-HT7(d)[82] .The 5-HT7(b) isoform has been identified in human placental cDNA and shows high affinity for ligands such as 5-carboxyamidotryptamine (5-CT) and 5-hydroxytryptamine (5-HT)[83] .It couples positively to adenylyl cyclase and can stimulate cAMP production[84].
5-HT7D:
5-HT7D receptor are isoforms which exhibits distinct trafficking patterns and internalization compared to other isoforms[85]. Activation of the 5-HT7 receptor promotes NMDA receptor activity and enhances NMDA-evoked peak currents[86]. The serotonergic system, regulated by the 5-HT7 receptor, plays a role in blood glucose regulation . Additionally, the 5-HT7 receptor is involved in the pathophysiology of various disorders and can be targeted for antidepressant therapy and context of irritable bowel syndrome (IBS), the 5-HT7 receptor is implicated in the pathogenesis of both IBS with diarrhoea (IBS-D) and IBS with constipation (IBS-C), with increased expression in the brain and colon.
RESULT :
The therapeutic potential of targeting serotonin receptors for the treatment of both neurological and metabolic disorders is an active area of research, with pharmacotherapeutic interventions showing promising results. Understanding the intricate interplay between serotonin signaling and neurological/metabolic pathways is essential for the development of effective therapeutic strategies. This review provides a comprehensive overview of serotonin’s involvement in neurological and metabolic disorders, highlighting its significance as a potential target for the management of these conditions.
CONCLUSION:
serotonin is a multifunctional neurotransmitter and hormone that exerts profound effects on both neurological and metabolic systems. Serotonin represents a promising avenue for the development of innovative treatments for neurological and metabolic disorders. By unravelling the complexities of serotonin biology and harnessing its therapeutic potential, we may pave the way for improved outcomes and enhanced quality of life .
REFERENCES:
- 1.Massimo, E., Maffei. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology.. International Journal of Molecular Sciences, (2020). doi: 10.3390/IJMS22010181.
- Duyen, N., K., Pham., Andrew, R., Chadeayne., James, A., Golen., David, R., Manke. 5-Meth-oxy-N,N-di-n-propyl-tryptamine (5-MeO-DPT): freebase and fumarate.. Acta Crystallographica Section E: Crystallographic Communications, (2021). doi: 10.1107/S2056989021003753.
- Xiuying, Yan., Ping, Xiang., Yunli, Zhao., Zhiguo, Yu., Hui, Yan. Determination of 5-MeO-DIPT in Human Urine Using Gas Chromatography Coupled with High-Resolution Orbitrap Mass Spectrometry. Journal of Analytical Toxicology, (2020). doi: 10.1093/JAT/BKAA00.
- Tsvetkova,, Milena,, . Serotonin receptors. (2023). doi: 10.1016/b978-0-323-85492-4.00050-8.
- Jelena, V., Nikoli?., Katarina, Vukojevi?., Violeta, Soljic., Josip, Miskovic., Martina, Orlovic, Vlaho., Mirna, Saraga-Babi?., Natalija, Filipovi?. Expression Patterns of Serotonin Receptors 5-HT1A, 5-HT2A, and 5-HT3A during Human Fetal Lung Development. International Journal of Molecular Sciences, (2023). doi: 10.3390/ijms24032965.
- Romina, Gonzalez-Pons., Kiera, McRae., Janice, M., Thompson., Stephanie, W., Watts. The 5-HT7 receptor restrains 5-HT-induced 5-HT2A mediated contraction in the isolated abdominal vena cava. Journal of Cardiovascular Pharmacology, (2021). doi: 10.1097/FJC.0000000000001057.
- Yuxiao, Chen., Jie-qi, Sun., Hongyan, Bi. Efficacy and safety of negative allosteric modulators of 5-hydroxytryptamine 2A receptors in the treatment of Alzheimer's disease psychosis: A systematic review and meta-analysis.. Advances in Clinical and Experimental Medicine, (2023). doi: 10.17219/acem/161159.
- Radomir, Juza., Radomir, Juza., Premysl, Vlcek., Premysl, Vlcek., Eva, Mezeiova., Kamil, Musilek., Ondrej, Soukup., Jan, Korabecny. Recent advances with 5?HT3 modulators for neuropsychiatric and gastrointestinal disorders. Medicinal Research Reviews, (2020). doi: 10.1002/MED.21666.
- Jacob, C., A., Edvinsson., Aida, Maddahi., I., M., Christiansen., Philip, V., Reducha., Karin, Warfvinge., Majid, Sheykhzade., Lars, Edvinsson., Kristian, Agmund, Haanes. Lasmiditan and 5-Hydroxytryptamine in the rat trigeminal system; expression, release and interactions with 5-HT1 receptors. Journal of Headache and Pain, (2022). doi: 10.1186/s10194-022-01394-z.
- Pasquale, Linciano., Claudia, Sorbi., Antonella, Comitato., Anna, Lesniak., Magdalena, Bujalska-Zadro?ny., Agata, Paw?owska., Anna, Bielenica., Jolanta, Orzelska-Górka., Ewa, K?dzierska., Grazyna, Biala., Simone, Ronsisvalle., Silvia, Limoncella., Livio, Casarini., Elena, Cichero., Paola, Fossa., Grzegorz, Sata?a., Andrzej, J., Bojarski., Livio, Brasili., Rita, Bardoni., Silvia, Franchini. Identification of a Potent and Selective 5-HT1A Receptor Agonist with In Vitro and In Vivo Antinociceptive Activity.. ACS Chemical Neuroscience, (2020). doi: 10.1021/ACSCHEMNEURO.0C00289.
- Paulina, S., Rojas., Jenny, L., Fiedler. What Do We Really Know About 5-HT1A Receptor Signaling in Neuronal Cells?. Frontiers in Cellular Neuroscience, (2016). doi: 10.3389/FNCEL.2016.00272.
- Lili, Xu., Shanglin, Zhou., Kunqian, Yu., Bo, Gao., Hualiang, Jiang., Xuechu, Zhen., Wei, Fu. Molecular Modeling of the 3D Structure of 5-HT1AR: Discovery of Novel 5-HT1AR Agonists via Dynamic Pharmacophore-Based Virtual Screening. Journal of Chemical Information and Modeling, (2013). doi: 10.1021/CI400481P.
- Markus, Ritter., Husameldin, El-Nour., Mari, Anne, Hedblad., Joseph, H., Butterfield., Olof, Beck., Niclaus, Stephanson., Mikael, Holst., R., Giscombe., Efrain, C., Azmitia., Klas, Nordlind. Serotonin and its 5-HT1 receptor in human mastocytosis.. Immunopharmacology and Immunotoxicology, (2012). doi: 10.3109/08923973.2011.651222.
- Renato, N., Zangiacomo., Guilherme, Lopes, Pinheiro, Martins., Publio, Viana., Natally, Horvat., Marco, A., Arap., William, C., Nahas., Miguel, Srougi., Giovanni, Guido, Cerri., Marcos, Roberto, de, Menezes. Percutaneous thermoablation of small renal masses (T1a) in surgical candidate patients: oncologic outcomes. European Radiology, (2021). doi: 10.1007/S00330-020-07496-Z.
- Ying-Kit, Cheung., S, C, S, Cheng., Y, Ke., Y, Xie. Human immunogenic T cell epitopes in nucleoprotein of human influenza A (H5N1) virus.. Hong Kong Medical Journal, (2012).
- Gen, Tsujio., Shinichiro, Kashiwagi., Yuka, Asano., Wataru, Goto., Koji, Takada., Tamami, Morisaki., Satoru, Noda., Tsutomu, Takashima., Naoyoshi, Onoda., Masahiko, Ohsawa., Kosei, Hirakawa., Masaichi, Ohira. A Case of T1a Breast Cancer with Axillary Lymph Node Metastasis. Gan to kagaku ryoho. Cancer & chemotherapy, (2017).
- Samina, Asad., Pernilla, Nikamo., Alexandra, Gyllenberg., Hedvig, Bennet., Ola, Hansson., Ola, Hansson., Nils, Wierup., Nils, Wierup., Annelie, Carlsson., Gun, Forsander., Sten-Anders, Ivarsson., Helena, Elding, Larsson., Åke, Lernmark., Bengt, Lindblad., Johnny, Ludvigsson., Claude, Marcus., Kjersti, S., Rønningen., Jan, Nerup., Flemming, Pociot., Holger, Luthman., Malin, Fex., Ingrid, Kockum. HTR1A a novel type 1 diabetes susceptibility gene on chromosome 5p13-q13.. PLOS ONE, (2012). doi: 10.1371/JOURNAL.PONE.0035439.
- Yang, Yang., Lijing, Zhang., Jiaojiao, Yu., Zhaobin, Ma., Li, Moxiang., Jin, Wang., Hu, Pengcheng., Jia, Zou., Xueying, Liu., Ying, Liu., Su, An., Cheng, Xiang., Xiao-Xi, Guo., Qian, Hao., Tian-Rui, Xu. A Novel 5-HT1B Receptor Agonist of Herbal Compounds and One of the Therapeutic Uses for Alzheimer's Disease.. Frontiers in Pharmacology, (2021). doi: 10.3389/FPHAR.2021.735876.
- Xi, Xia., Mei, Ding., Jin-feng, Xuan., Jia-xin, Xing., Jun, Yao., Xue, Wu., Bao-jie, Wang. Functional polymorphisms and transcriptional analysis in the 5' region of the human serotonin receptor 1B gene (HTR1B) and their associations with psychiatric disorders. BMC Psychiatry, (2020). doi: 10.1186/S12888-020-02906-4.
- Yusha, Liu., Alec, W, Gibson., Marjorie, R, Levinstein., Atom, J., Lesiak., Shao-En, Ong., John, F., Neumaier. 5-HT1B Receptor-Mediated Activation of ERK1/2 Requires Both G?i/o and ?-Arrestin Proteins.. ACS Chemical Neuroscience, (2019). doi: 10.1021/ACSCHEMNEURO.8B00596.
- Mikael, Tiger., Katarina, Varnäs., Yoshiro, Okubo., Johan, Lundberg. The 5-HT1B receptor - a potential target for antidepressant treatment. Psychopharmacology, (2018). doi: 10.1007/S00213-018-4872-1.
- Cheng, Wei, Lu., Tzu, Yu, Lin., Shu, Kuei, Huang., Su-Jane, Wang., Su-Jane, Wang. 5-HT1B receptor agonist CGS12066 presynaptically inhibits glutamate release in rat hippocampus.. Progress in Neuro-psychopharmacology & Biological Psychiatry, (2018). doi: 10.1016/J.PNPBP.2018.05.019.
- P., R., Patil., M., A., Chaudhari., P, V, Sapkale., Surajj, Sarode., Rageeb, Md., Usman. A review on functional comparison of 5-ht1a and 5-ht2c receptors. Journal of Drug Delivery and Therapeutics, (2014). doi: 10.22270/JDDT.V4I5.975.
- Natalie, Hesselgrave., Ramin, V., Parsey., Ramin, V., Parsey. Imaging the serotonin 1A receptor using [11C]WAY100635 in healthy controls and major depression. Philosophical Transactions of the Royal Society B, (2013). doi: 10.1098/RSTB.2012.0004.
- Ana, M., Lucas-Osma., Yaqing, Li., Katie, Murray., Shihao, Lin., Sophie, Black., Marilee, J., Stephens., Andrew, H., Ahn., Charles, J., Heckman., Keith, K., Fenrich., Karim, Fouad., David, J., Bennett. 5-HT1D receptors inhibit the monosynaptic stretch reflex by modulating C-fiber activity.. Journal of Neurophysiology, (2019). doi: 10.1152/JN.00805.2018.
- Yang, Zhou., Xun, Liu., Yahan, Gao., Rulan, Tan., Zhiyuan, Wu., Qixin, Zhong., Feng, Zeng. Paeoniflorin Affects Hepatocellular Carcinoma Progression by Inhibiting Wnt/?-Catenin Pathway through Downregulation of 5-HT1D.. Current Pharmaceutical Biotechnology, (2021). doi: 10.2174/1389201021666201009153808.
- Juan, Francisco, Fernández-González., José, Ángel, García-Pedraza., José, Luis, Ordóñez., Anaïs, Clara, Terol-Úbeda., María, Luisa, Martín., Asunción, Morán., Mónica, García-Domingo. Renal Sympathetic Hyperactivity in Diabetes Is Modulated by 5-HT1D Receptor Activation via NO Pathway. International Journal of Molecular Sciences, (2023). doi: 10.3390/ijms24021378.
- José, Ángel, García-Pedraza., José, Ángel, García-Pedraza., Mónica, García-Domingo., Mónica, García-Domingo., Miriam, Gómez-Roso., Miriam, Gómez-Roso., Alicia, Rodríguez-Barbero., Alicia, Rodríguez-Barbero., M.L., Martín., M.L., Martín., Asunción, Morán., Asunción, Morán. 5-HT modulates the rat mesenteric vasopressor outflow by 5-HT1D sympatholytic receptors.. Clinical and Experimental Pharmacology and Physiology, (2017). doi: 10.1111/1440-1681.12841.
- Cristina, López., Miriam, Gómez-Roso., José, Ángel, García-Pedraza., María, Luisa, Martín., Asunción, Morán., Mónica, García-Domingo. Fluoxetine oral treatment discloses 5-HT1D receptor as vagoinhibitor of the cardiac cholinergic neurotransmission in rat.. Canadian Journal of Physiology and Pharmacology, (2019). doi: 10.1139/CJPP-2018-0390.
- Vinay, Kumar, Sharma., Kiersten, Campbell., Xu-Yu, Yang., Ryan, K., Dale., Y., Peng, Loh. Characterization of serotonin?5?HTR1E signaling pathways and its role in cell survival. The FASEB Journal, (2023). doi: 10.1096/fj.202300128R.
- Y., Peng, Loh. Characterization of serotonin?5?HTR1E signaling pathways and its role in cell survival. The FASEB Journal, (2023). doi: 10.1096/fj.202300128r.
- Vinay, Kumar, Sharma., Xu-Yu, Yang., Soo-Kyung, Kim., A, R, Maafi., Lan, Xiao., A., Inoue., William, A., Goddard., Y, P, Loh. 5HTR1E receptor interacts with Neurotrophic factor??1 and serotonin to activate two distinct signaling pathways. The FASEB Journal, (2022). doi: 10.1096/fasebj.2022.36.s1.l7465.
- Alfredo, Meneses. Chapter 6 – 5-HT1E/1F Receptor. (2014). doi: 10.1016/B978-0-12-800836-2.00006-4.
- MT, Klein., M, Teitler. Distribution of 5?ht1E receptors in the mammalian brain and cerebral vasculature: an immunohistochemical and pharmacological study. British Journal of Pharmacology, (2012). doi: 10.1111/J.1476-5381.2012.01868.X.
- P., Raj., Kevin, Hurtado., Jaroslav, Janda., Rick, G., Schnellmann. 5-HT1F Agonist Lasmiditan Induces Mitochondrial Biogenesis And Alters Successful And Failed Repair Genes In A Mouse Model Of Acute Kidney Injury. (2023). doi: 10.1124/jpet.122.244110
- 5-HT1F Receptor Agonist Ameliorates Mechanical Allodynia in Neuropathic Pain via Induction of Mitochondrial Biogenesis and Suppression of Neuroinflammation. Frontiers in Pharmacology, (2022). doi: 10.3389/fphar.2022.834570.
- Role of 5-HT2 Receptors Family in the Allergy-Induced Increased Aorta Contractile Responses to 5-HT. Physiological Research, (2023). doi: 10.33549/physiolres.934968.
- Prithvi, Hemanth., Pallavi, Nistala., Vy, Thuy, Nguyen., Jose, M., Eltit., Richard, A., Glennon., Malgorzata, Dukat. Binding and functional structure-activity similarities of 4-substituted 2,5-dimethoxyphenyl isopropylamine analogues at 5-HT2A and 5-HT2B serotonin receptors. Frontiers in Pharmacology, (2023). doi: 10.3389/fphar.2023.1101290.
- 5HT 2 Rezeptor vermittelte Kontrolle des neonatalen medullären respiratorischen Netzwerks des Säugers. (2022). doi: 10.53846/goediss-113.
- Kai, Bai. Roles of the 5-HT2C receptor on zebrafish sociality. Progress in Neuro-psychopharmacology & Biological Psychiatry, (2023). doi: 10.1016/j.pnpbp.2023.110769.
- Jianping, Chen., Erik, J., Garcia., Christina, R., Merritt., Joshua, C., Zamora., Andrew, A., Bolinger., Konrad, Pazdrak., Susan, Stafford., Randy, C., Mifflin., Eric, A., Wold., Christopher, P., Wild., Haiying, Chen., Noelle, C., Anastasio., Kathryn, A., Cunningham., Jian, Zhou. Discovery of Novel Oleamide Analogues as Brain-Penetrant Positive Allosteric Serotonin 5-HT2C Receptor and Dual 5-HT2C/5-HT2A Receptor Modulators.. Journal of Medicinal Chemistry, (2023). doi: 10.1021/acs.jmedchem.3c00908.
- David, B., Wainscott., Marlene, L., Cohen., K, W, Schenck., J, E, Audia., J, S, Nissen., Melvyn, Baez., J, D, Kursar., V, L, Lucaites., D, L, Nelson. Pharmacological characteristics of the newly cloned rat 5-hydroxytryptamine2F receptor. Molecular Pharmacology, (1993).;43(3):419-426.
- Santosh, T, R, B, Rao., Ilona, Turek., Julian, Ratcliffe., Cassandra, Cianciarulo., Christine, Kettle., D., R., Whelan., Helen, Irving. 5-HT3 Receptors on Mitochondria Influence Mitochondrial Function. International Journal of Molecular Sciences, (2023). doi: 10.3390/ijms24098301.
- Santosh, T, R, B, Rao., Ilona, Turek., Helen, Irving. Phylogenetic analyses of 5-hydroxytryptamine 3 (5-HT3) receptors in Metazoa. PLOS ONE, (2023). doi: 10.1371/journal.pone.0281507.
- Hoa, Quynh, Do., Michaela, Jansen. Duplicated binding site for RIC-3 in 5-Hydroxytryptamine Receptors Subtype 3. bioRxiv, (2022). doi: 10.1101/2022.02.17.480943.
- Helen, Irving., Ilona, Turek., Christine, Kettle., Nor, Syafinaz, Yaakob. Tapping into 5-HT3 Receptors to Modify Metabolic and Immune Responses.. International Journal of Molecular Sciences, (2021). doi: 10.3390/IJMS222111910.
- Xie, Dejian., Chen, Fang., Li, Xiaoxia., Liu, Jiachuan., Zhang, Wei., Huang, Jinkun. 5-HT3 receptor modulator containing heterocyclic nitrogen as well as preparation method and application thereof. (2021).
- Holbrook JD, Gill CH, Zebda N, Spencer JP, Leyland R, Rance KH, Trinh H, Balmer G, Kelly FM, Yusaf SP, Courtenay N, Luck J, Rhodes A, Modha S, Moore SE, Sanger GJ, Gunthorpe MJ. Characterisation of 5-HT3C, 5-HT3D and 5-HT3E receptor subunits: evolution, distribution and function. J Neurochem. 2009 Jan;108(2):384-96. doi: 10.1111/j.1471-4159.2008.05775.x. Epub 2008 Nov 29. PMID: 19012743..
- Kristin, Köhler-Forsberg., Vibeke, H., Dam., Brice, Ozenne., Anjali, Sankar., Vincent, Beliveau., Elizabeth, Landman., Søren, Larsen., Asbjørn, Seenithamby, Poulsen., Cheng-Teng, Ip., Anders, Jørgensen., Michal, Meyer., Dea, S., Stenbæk., Hans, Eiberg., Jacob, Madsen., Claus, Svarer., Martin, Balslev, Jørgensen., Vibe, G., Frokjaer., Gitte, M., Knudsen. Serotonin 4 Receptor Brain Binding in Major Depressive Disorder and Association With Memory Dysfunction.. JAMA Psychiatry, (2023). doi: 10.1001/jamapsychiatry.2022.4539.
- Susannah, E., Murphy., Susannah, E., Murphy., Angharad, N, de, Cates., Angharad, N, de, Cates., Amy, Gillespie., Amy, Gillespie., Beata, R., Godlewska., Beata, R., Godlewska., Jessica, C., Scaife., Jessica, C., Scaife., Lucy, Wright., Lucy, Wright., Philip, J., Cowen., Philip, J., Cowen., Catherine, J., Harmer., Catherine, J., Harmer. Translating the promise of 5HT4 receptor agonists for the treatment of depression.. Psychological Medicine, (2021). doi: 10.1017/S0033291720000604.
- James, J., Galligan. Colonic 5-HT4 receptors are targets for novel prokinetic drugs.. Neurogastroenterology and Motility, (2021). doi: 10.1111/NMO.14125.
- Alejandro, Castro-Alvarez., Emigdio, Chavez-Angel., Ronald, Nelson. Understanding the Molecular Basis of 5-HT4 Receptor Partial Agonists through 3D-QSAR Studies. International Journal of Molecular Sciences, (2021). doi: 10.3390/IJMS22073602.
- John, R., Konen., John, R., Konen., Melody, M., Haag., Daria, Guseva., Molly, C., Hurd., Alisha, A., Linton., Brigitte, Lavoie., Colleen, B., Kerrigan., Colleen, B., Kerrigan., Emily, J., Joyce., Stephan, C., Bischoff., Steve, Swann., Luana, Griffin., Jun, Matsukawa., Matthew, D., Falk., Tony, Gibson., Grant, W., Hennig., Jill, Wykosky., Gary, M., Mawe. Prokinetic actions of luminally acting 5-HT4 receptor agonists.. Neurogastroenterology and Motility, (2021). doi: 10.1111/NMO.14026.
- Rafig, Gurbanov., Hazel, Karadag. HTR4 (5-hydroxytryptamine receptor 4). Atlas of genetics and cytogenetics in oncology and haematology, (2020). doi: 10.4267/2042/70748.
- Till, Manzke., Ulf, Guenther., Evgeni, Ponimaskin., Miriam, Haller., Mathias, Dutschmann., Stephan, W., Schwarzacher., Diethelm, W., Richter. 5-HT4(a) Receptors Avert Opioid-Induced Breathing Depression Without Loss of Analgesia. Science, (2003). doi: 10.1126/SCIENCE.1084674.
- Sébastien, Dilly., Jean-François, Liégeois. Structural Insights into 5-HT1A/D4 Selectivity of WAY-100635 Analogues: Molecular Modeling, Synthesis, and in Vitro Binding.. Journal of Chemical Information and Modeling, (2016). doi: 10.1021/ACS.JCIM.5B00753.
- Stephane, Y., Younes. Les récepteurs 5-HT4b adoptent différentes conformations ligand-spécifique ayant des propriétés de signalisation et de régulation distinctes. (2012).
- Armelle, Pindon., Geert, Van, Hecke., Katty, Josson., Paul, Van, Gompel., Anne, Simone, Josephine, Lesage., Josée, E., Leysen., Mirek, R., Jurzak. Internalization of human 5-HT4a and 5-HT4b receptors is splice variant dependent.. Bioscience Reports, (2004). doi: 10.1007/S10540-005-2582-5
- Trond, Bach., Trygve, Syversveen., Ane, Marit, Kvingedal., Kurt, A., Krobert., Trond, Brattelid., Alberto, J., Kaumann., Finn, Olav, Levy. 5HT4(a) and 5-HT4(b) receptors have nearly identical pharmacology and are both expressed in human atrium and ventricle.. Naunyn-schmiedebergs Archives of Pharmacology, (2001). doi: 10.1007/S002100000299.
- Bard., Branchek., Weinshank. Dna encoding a human serotonin receptor (5-ht4b) and uses thereof. (2003).
- Karine, Madsen., Wolf-Julian, Neumann., Klaus, K., Holst., Klaus, K., Holst., Lisbeth, Marner., Mette, T., Haahr., Szabolcs, Lehel., Gitte, M., Knudsen., Steen, G., Hasselbalch. Cerebral serotonin 4 receptors and amyloid-? in early Alzheimer's disease.. Journal of Alzheimer's Disease, (2011). doi: 10.3233/JAD-2011-110056.
- Olivier, Blondel., Monique, Gastineau., Monique, Gastineau., Yamina, Dahmoune., Yamina, Dahmoune., Michel, Langlois., Michel, Langlois., Rodolphe, Fischmeister., Rodolphe, Fischmeister. Cloning, Expression, and Pharmacology of Four Human 5-Hydroxytryptamine4 Receptor Isoforms Produced by Alternative Splicing in the Carboxyl Terminus. Journal of Neurochemistry, (2002). doi: 10.1046/J.1471-4159.1998.70062252.X.
- Gael, Barthet., Bérénice, Framery., Florence, Gaven., Lucie, P., Pellissier., Eric, Reiter., Eric, Reiter., Sylvie, Claeysen., Joël, Bockaert., Aline, Dumuis. 5-Hydroxytryptamine4 Receptor Activation of the Extracellular Signal-regulated Kinase Pathway Depends on Src Activation but Not on G Protein or ?-Arrestin Signaling. Molecular Biology of the Cell, (2007). doi: 10.1091/MBC.E06-12-1080.
- Jeanne, Mialet., Isabelle, Berque-Bestel., Isabelle, Berque-Bestel., Pierre, Eftekhari., Monique, Gastineau., Mireille, Giner., Mireille, Giner., Yamina, Dahmoune., Yamina, Dahmoune., Patrick, Donzeau-Gouge., Johan, Hoebeke., Michel, Langlois., Michel, Langlois., Sames, Sicsic., Sames, Sicsic., Rodolphe, Fischmeister., Frank, Lezoualc'h. Isolation of the serotoninergic 5?HT4(e) receptor from human heart and comparative analysis of its pharmacological profile in C6?glial and CHO cell lines. British Journal of Pharmacology, (2000). doi: 10.1038/SJ.BJP.0703101.
- Joël, Bockaert., Sylvie, Claeysen., Valérie, Compan., Aline, Dumuis. 5-HT4 receptors.. Current Drug Targets - Cns & Neurological Disorders, (2004). doi: 10.2174/1568007043482615.
- Geoffrey, Edward, Gymer., Kiyoshi, Kawamura., Sachiko, Mihara., Mikio, Morita., Seiji, Nukui., Alan, Stobie., Uchida, Chikara. Oxo or oxy-pyridine compounds as 5-HT4 receptor modulators. (2003).
- Alfredo, Meneses. Chapter 10 – 5-HT5 Receptor. (2014). doi: 10.1016/B978-0-12-800836-2.00010-6.
- Massimo, E., Maffei. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology.. International Journal of Molecular Sciences, (2020). doi: 10.3390/IJMS22010181.
- Qiangqiang, He., Maoru, Li., Xuechun, Wang., Zhenjiang, Xia., Yuzhi, Du., Yan, Li., Wei, Lixin., Jing, Shang. A simple, efficient and rapid HPLC–UV method for the detection of 5-HT in RIN-14B cell extract and cell culture medium. (2019). doi: 10.1186/S13065-019-0591-X.
- Duyen, N., K., Pham., Andrew, R., Chadeayne., James, A., Golen., David, R., Manke. 5-Meth-oxy-N,N-di-n-propyl-tryptamine (5-MeO-DPT): freebase and fumarate.. Acta Crystallographica Section E: Crystallographic Communications, (2021). doi: 10.1107/S2056989021003753.
- Yangxia, Tan., Peiyu, Xu., Sijie, Huang., Gong, Yang., Fulai, Zhou., Xinheng, He., Honglei, Ma., H., Eric, Xu., Yi, Jiang. Structural insights into the ligand binding and Gi coupling of serotonin receptor 5-HT5A. Cell discovery, (2022). doi: 10.1038/s41421-022-00412-3.
- Anat, Levit, Kaplan., Ryan, T., Strachan., Joao, M., Braz., Veronica, B., Craik., Samuel, T., Slocum., Thomas, J., Mangano., Vanessa, Amabo., Henry, R., O’Donnell., P., Lak., Allan, I., Basbaum., Bryan, L., Roth., Brian, K., Shoichet. Structure-Based Design of a Chemical Probe Set for the 5-HT5A Serotonin Receptor.. Journal of Medicinal Chemistry, (2022). doi: 10.1021/acs.jmedchem.1c02031.
- Yotam, Sagi., Lucian, Medrihan., Katia, George., Miles, Barney., Kathryn, A., McCabe., Paul, Greengard. (2020). Emergence of 5-HT5A signaling in parvalbumin neurons mediates delayed antidepressant action.. Molecular Psychiatry, Available from: 10.1038/S41380-019-0379-3.
- Yang, Yang., Lijing, Zhang., Jiaojiao, Yu., Zhaobin, Ma., Li, Moxiang., Jin, Wang., Hu, Pengcheng., Jia, Zou., Xueying, Liu., Ying, Liu., Su, An., Cheng, Xiang., Xiao-Xi, Guo., Qian, Hao., Tian-Rui, Xu. A Novel 5-HT1B Receptor Agonist of Herbal Compounds and One of the Therapeutic Uses for Alzheimer's Disease.. Frontiers in Pharmacology, (2021). doi: 10.3389/FPHAR.2021.735876.
- Anton, Lindberg. Development of novel 5-HT1B PET radioligands. (2019).
- Séverine, Chaumont-Dubel., Sonya, Galant., Matthieu, Prieur., Tristan, Bouschet., Joël, Bockaert., Philippe, Marin. Impact of 5-HT6 Receptor Subcellular Localization on Its Signaling and Its Pathophysiological Roles. Cells, (2023). doi: 10.3390/cells12030426.
- Licong, He., Qiaoyu, Zhao., Jianzhong, Qi., Yifan, Wang., Wenyu, Han., Zhangcheng, Chen., Yao, Cong., Sheng, Wang. Structural insights into constitutive activity of 5-HT6 receptor.. Proceedings of the National Academy of Sciences, (2023). doi: 10.1073/pnas.2209917120.
- Serotonin 5-HT7 receptor slows down the Gs protein: a single molecule perspective. Molecular Biology of the Cell, (2023). doi: 10.1091/mbc.e23-03-0117.
- Alejandro, Quintero-Villegas., Sergio, I., Valdés-Ferrer. Central nervous system effects of 5-HT7 receptors: a potential target for neurodegenerative diseases. Molecular Medicine, (2022). doi: 10.1186/s10020-022-00497-2.
- E., M., Kondaurova., Darya, Bazovkina., Vladimir, S., Naumenko. [5-HT1A/5-HT7 receptor interplay: Chronic activation of 5-HT7 receptors decreases the functional activity of 5-HT1A receptor and its ?ontent in the mouse brain].. Molecular Biology, (2017).;51(1):136-142. doi: 10.1134/S0026893316060108.
- María, J., Ramírez., E, García-Garayoa., G., Romero., Antonio, Monge., J, Roca., J., Del, Río., Berta, Lasheras. VB20B7, a novel 5-HT-ergic agent with gastrokinetic activity. I. Interaction with 5-HT3 and 5-HT4 receptors.. Journal of Pharmacy and Pharmacology, (2011). doi: 10.1111/J.2042-7158.1997.TB06753.X.
- Jeffrey, R., Jasper., Alan, Kosaka., Z., P., To., David, J., Chang., Richard, M., Eglen. Cloning, expression and pharmacology of a truncated splice variant of the human 5?HT7 receptor (h5?HT7(b)). British Journal of Pharmacology, (1997). doi: 10.1038/SJ.BJP.0701336.
- Clinton, E., Canal., Daniel, Felsing., Wanying, Zhu., Yue, Liu., Tania, C., Córdova-Sintjago., Raymond, G., Booth. Development of novel serotonin 7-targeting compounds based on the 2-dimethylaminotetralin scaffold (1059.13). The FASEB Journal, (2014). doi: 10.1096/FASEBJ.28.1_SUPPLEMENT.1059.13.
- Soo-Hyun, Park., Yun-Beom, Sim., Sung-Su, Kim., Jae-Ryeong, Lee., Naveen, Sharma., Hong-Won, Suh. Effects of 5,7-dihroxytryptamine administered supraspinally or spinally on the blood glucose level in D-glucose-fed and immobilization stress models. Animal Cells and Systems, (2016). doi: 10.1080/19768354.2016.1221854.
- Maryam, S., Vasefi., Kai, Yang., Jerry, Li., Jeff, S., Kruk., John, J., Heikkila., Michael, F., Jackson., John, F., MacDonald., Michael, A., Beazely. Acute 5-HT7 receptor activation increases NMDA-evoked currents and differentially alters NMDA receptor subunit phosphorylation and trafficking in hippocampal neurons. Molecular Brain, (2013). doi: 10.1186/1756-6606-6-24.


 Dil Prasad Subba*
Dil Prasad Subba*
 Kumar Satyender
Kumar Satyender


 10.5281/zenodo.10800578
10.5281/zenodo.10800578