Abstract
Pharmacological medicine regulation of cannabis has evolved significantly over the past few decades in response to increased awareness of the medicinal nature of the drug and the increasing clinical evidence base. From a long history of mainly restrictive use because of its abuse potential and psychoactivity, it has emerged that this substance has therapeutic potential, primarily in pain management, neurological diseases, and chronic diseases, including cancer and epilepsy. With these changes, most countries have amended their regulatory frameworks to permit the use of medical cannabis, albeit on different levels of legalization, regulation, and oversight. This paper will outline the regulatory development of cannabis as a pharmaceutical agent: changes in policy, scientific acceptance, and societal attitudes towards its use in medicine. It also discusses challenges to all parties in healthcare, including healthcare providers, patients, and policymakers-including safety, dosage control, and standardizing cannabis-based therapies. Continued regulatory development will be critical in unlocking the full breadth of medicinal cannabis potential for safe and effective adoption in healthcare settings around the world.
Keywords
Cannabis-derived pharmaceuticals, pharmaceutical regulation of cannabis, Cannabis drug approval, Cannbinoid based medicine.
Introduction
- Cannabis commonly known as; marijuana is a natural product derived from cannabis sativa plant.

1) Cannabis, often referred to as marijuana, is an annual that grows very well in a wide variety of indoor and outdoor environments. Cannabis is the most commonly used illicit drug in the United States, in part due to the effects of the psychoactive component delta-9tetrahydrocannabinol (THC). For decades, an increasing number of States have legalized allowing patients with particular medical conditions access to or cultivation of small amounts of cannabis.
2) The physio active properties of its active ingredients can abinoids have lead to its use for releasing and medicinal purpose for thousand of years. Medical cannabis/medical marijuana for patient with substance use disorder living in postacute & long term care setting because medical can I be refers to use of the un processed marijuana plant or its basic extract (example: cannabidiol, tetrahydrocannbillnessor other conditions)
4) A Cannabis is consumed widely through many methodologies, both medicinally and recreationally. Dried cannabis flower, commonly referred to as bud, was traditionally the most popular form of consumption but are gradually being edged out of market share newer, valueadded product forms such as high-potency extracts, vape cartridges, topicals, edibles, and be1verages . 5)Product Form and Route of Administration Each product form and the route offer a unique set of advantages and disadvantages, allowing the Consumer to shape according to their own experience with cannabis
- Medical Cannabis Regulation around the world:
2) International drug control treaties are not forbidden to allow cannabis for medical and scientific use on their territories (European Monitoring Centre for Drugs and Drug Addiction 2018a; World Health Organization 2014; European Monitoring Centre for Drugs and Drug Addiction 2018b; United Nations Ofce on Drugs and Crime 2013). For that, however, a set of strict control measures First and foremost of all, need to be set up agencies that will govern the controlled supply chain of medical cannabis and hence must report to the (INCB) narcotic control bboard.
- Define& objective of Regulation Of cannabis:
6) The regulation of cannabis is any law, policy, guideline, and standard that has to do with the cultivation, processing, distribution, possession, or use of cannabis products which include:
- Medicinal cannabis
- Recreational cannabis
- Cannabidiol (CBD) products
Industrial hemp
*Objectives of Regulation*
- Public health and safety
- Prevention of abuse and misuse
• Background of cannabis
The species Cannabis sativa L. (Cannabaceae) has been Cultivated by humankind since the emergence of the first Agricultural civilizations, being adapted to diverse uses, Including as a source of food, oil, and fibre, as well as for Medicinal, recreational, and religious purposes .6).The Cannabis sativa preparations-marijuana, hashish, and dagga-have been used in medicine for centuries. Other derivatives of Cannabis have been suggested for patients in whom it is desirable to stimulate feeding and to minimize emesis. The orexigenic effects of Cannabis have also been anecdotally reported as inducing the ‘munchies’ after smoking marijuana. Cannabis also contains polyphenols, flavonoids, chlorophylls, omega-3 fatty acids and other bioactive molecules. This section discusses the molecular structure and biosynthesis of various cannabinoids, their chemical or medicinal.
• Therapeutic uses of cannabis
The therapeutic or medicinal activity of cannabis or cannabidiol can be broadly found in treatment of various disease, disorder, syndromes,and health condition like epilepsy, Parkinson disease,post traumatic stress disorder (PTSD),skin disease,canser or their subsequent effect like appetite loss, chronic pain and nausea . Two pharmaceutical cannabinoid products are approved by Health Prescriptionse is available for Canada, Sativex (pure THC/CBD extract) and nabilone (a synthetic derivative of THC). CB1 agonist). The following are the specific Health Canada labelled indications for these Two are for adults with moderate to severe spasticity. 11) cannabis is a complex, annual, herbaceous plant with over 560 compounds in multiple chemical classes, including cannabinoids, terpenes, and sugars. There are more than 120 known phytocannabinoids, or naturally occurring cannabinoids, produced by the plant, with ?9-tetrahydrocannabinol (?9-THC) identified as the major psychoactive compound (ElSohly et al., 2021, ElSohly and Slade, 2005, Fischedick et al., 2010). Cannabidiol (CBD) is another major phytocannabinoid, which again depends on genetics of the plant. Examples of the minor cannabinoids are cannabinol (CBN), cannabigerol (CBG), cannabichromene (CBC), cannabidivarin (CBDV), ?8-tetrahydrocannabinol (?8-THC), ?10-tetrahydrocannabinol (?10-THC), and hexahydrocannabinol (HHC). Not all plants will have minor cannabinoids, as the chemotype of the plant and environment during growing determine what minor cannabinoids are produced. Chemotherapy and multiple sclerosis induced nausea and, Vomiting correspondingly. Thus, access for chronic pain considered off label use.
Historical overview of the medicinal use of cannabis: the genus of the hemp plant (family Cannabaceae) commonly referred to as marijuana t was classified first in 1753 and comprises two species, Cannabis sativa and Cannabis indica, and one subspecies, Cannabis ruderalis. Strains are subvarieties of the species. Hemp is a variety of C sativa. The first uses of it were documented in China and then returned by Irish physician W.B.
O’Shaughnessy in 1843. The evolution of vaping products in the United States made significant and innovative leaps forward with the emerging legalization of cannabis and the advent of discreet vaping.
Key milestones in cannbies research and legislation :
Research milestones:
964: THC isolation and synthesis
1975: cannabinoid Reseptors discovered
1992: Endocannabinoid system identified
1996: cannabinoids show neuroprotective effect.
2013: CBD’S potential for epilepsy treatment
US legislation :
1937: Marijuana tax Act(prohibition)
1970: controlled substance Act(schedule I)
1996: California proposition 21’s
International legislation:
1961: single convention on narcotic drugs.
(global prohibition)
2013: Uruguay’s cannbies legalization
Medical cannabis Approval:
1992: Marinol (jynthetic THC)
2018: Epidiolex (CBD for epilepsy)
2020: sativex(or mucosal spray for MS)
• Regulatory framework
It is a narcotic drug (1961) Cannabis is defined as flowering or fruiting tops of the cannabis plant resin from which the resin has not been extracted and The esin itself cannbies
areMarijuana,THC(tetrahydrocannabinol),CBD(cannabidiol)
• Key terms of cannabis
1.Cannbies: includes all parts of plant
2.Marijuana: typically refers to dried flower or leaves
3.Hemp: Cannabis with <0>
4.THC: primary physio active compound.
Cannabis-derived pharmaceuticals are medications that utilize active compounds from cannabis (cannabinoids) to treat various medical conditions. Here are some examples:
*Investigational Cannabis-Derived Pharmaceuticals: *
- Cannabidiol (CBD) for: - Anxiety disorder, Sleep disorders, Inflammatory diseases, Cancer pain
- Tetrahydrocannabinol (THC) for: Pain management, Muscle spasticity, Glaucoma
- Cannabigerol (CBG) for: Inflammatory bowel disease, Pain management
- Cannabidivarin (CBDV) for: Epilepsy, Autism spectrum disorder
• FDA approved for cannbies:
20)The agency has yet to approve any marketing application for a drug containing cannabis to treat any disease or condition. FDA has approved one cannabis-derived and three cannabisrelated drug products. The approved products are available only with a prescription from a licensed healthcare provider.
21)FDA has approved Epidiolex, which contains a purified form of the drug substance CBD, for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients 1 years of age and older. Additionally, FDA has approved Epidiolex for the treatment of seizures associated with tuberous sclerosis complex in patients 1 year of age or older. This means FDA has determined that this particular drug product is safe and effective for its intended use.
24)The agency also approved Marinol and Sydnor’s for therapeutic uses in the United States, including to treat anorexia associated with weight loss in AIDS patients. Marinol and Syndros contain the active ingredient dronabinol, which is a synthetic delta-9-tetrahydrocannabinol (THC), considered the psychoactive component of cannabis. Another FDA-approved drug is Cesamet, that contains the active ingredient nabilone, which is chemically related to THC but synthetically derived.
- The FDA supports the conduct of that research by:
40)Providing information on the process needed to conduct clinical research using cannabis. Providing information on the specific requirements needed to develop a drug that is derived from a plant such as cannabis. In December 2016, the FDA updated its Guidance for Industry: Botanical Drug Development, which provides sponsors with guidance on submitting IND applications for botanical drug products.
Providing specific support for investigators interested in conducting clinical research using cannabis and its constituents as part of the IND process through meetings and regular interactions throughout the drug development process.
General support to investigators in understanding and following the procedures for conducting clinical research through the FDA Center for Drug Evaluation and Research.
- Why is Marijuana illegal in India? Can it be legalized?
32)Indians have a very strong mythical and medicinal relationship with Marijuana. Its been here for centuries as part of our festivities and culture. In 1986, Govt of India under pressure from the medicinal lobby of the US, gave in to create stringent narcotic laws that made the sale, production, and transportation of illegal in the country. This led to for or against arguments about legalizing this drug ever since. The law has proved incapable of really making a difference and has only shifted a source of state income to international drug cartels.
34)According to estimates approximately 60,000 kgs of hash and 40,000 kgs of opium are prepared in Himachal Pradesh. The seized amount is only 500 kgs annually. The government can increase the state’s income by legalizing this activity, and it can also support the economy of hill states having less water and the high demand for this drug can be met at a legal level which is reducing the illegal crimes associated with this industry.
• Product development and approval:
Formulation Of Cannabis:
17) formulations can be synthetic, phytopharmaceutical (cannabinoids), or natural plant based (Sativex-like drugs)19).The regulations differ based on the origin of the molecule used in the formulation. The Central Licensing Authority approves synthetic and phytopharmaceuticals agents, while the Ministry of AYUSH approves plant-based products. The rules, conduct of regulatory framework, and capabilities differ for both these central authorities. The culture of Phase 1/2/3 clinical trials is new for Ayurveda, Siddha, and Unani, reflecting in the conduct and rigor of data safety monitoring requirements as compared with their allopathy drug counterparts.
18)These drugs’ production and distribution fall under stringent controls and watch of federal and state authorities based on the origin of the raw material (imported vs. locally grown), transport of the drug between states (needs the nod of individual state authorities), and storage of the drug before dispensation (controlled and overseen by local state FDA and police authorities). This labyrinthian bureaucracy puts a tremendous burden on the PI and the IECs for discovery of and compliance to the nuanced interpretation of these evolving laws.
Medicinal Cannabis:
40,45) Cannabis is an exceptional case of conventional medicine manufacturing practices because: There are many plant-based medicines or APIs and supplements, but they reach the end consumer/patient in the form of extracts. No other plant, such as cannabis, exists in the original form of a flower, except for the traditional forms. (Herbal preparations can be omitted, as they have been gradually lost from the pharmacopoeia and are not included in the definition of medicines with curative effect)
30)Cannabis is a very potent API with numerous combinations of components
(cannabinoids, terpenes) as well as their multiple therapeutic applications, with some of them showing side effects or psychotropic effects and thus potential safety implications. The main characteristics of cannabis as an API can be given by genetics, but during plant growth, it can significantly modify its potency (concentration).
The fact that cannabis has become a separate professional profile outside the pharma production sector has led to inconsistencies in the application of GMP regulations in this process.
• Application of GMP regulations
Now let’s have a look at some specific aspects of the application of these GMP regulations in Cannabis production, as based on our experience and our product and process risk analyses for cannabis.
46)Dried flower of Cannabis “direct use” as a medicinal product.
As table 1 of the application of the GMP regulations to the manufacture of active substances of plant origin would place us on the third row:
45) Based on the guidelines in the table, GMP regulations will be complied with once physical processing and packaging taking place, which includes drying and shaving as the last processing steps before dispensing to the patients. Since GMP regulations only apply to the final processing steps, GACP regulation during the cultivation steps becomes really important due to the following aspects:
30)Here, all the active ingredients are developed in an uncontrolled environment (based on
Pharma standards) for more than 10 weeks, which is an unusual case. If we draw a parallel to the API formulation, GMPs are applicable at the stage where the active molecule manifests. Not here, the active molecule emerges and is produced way prior to when we enter into the GMP stage.
51,50)Environmental conditions (temperature, humidity and light) as well as cultivation processes and strategies (density, irrigation, fertilisers, …) considerably influence the composition of the different APIs and the yield of the crop.
Any of the above parameters out of control may invalidate the product completely. Knowing that GMPs are not mandatory, the application of GACP becomes even more important.
Supplier evaluation, personnel training, facility maintenance are some of the very strict processes even though they are not GMPControlling the various parameters that constitute the crop cultivation phase is extremely important to ensure the quality, stability and crop yield.
Having said all this above, maintaining these parameters again incurs a higher cost of investment and operation, so a balance needs to be found:.
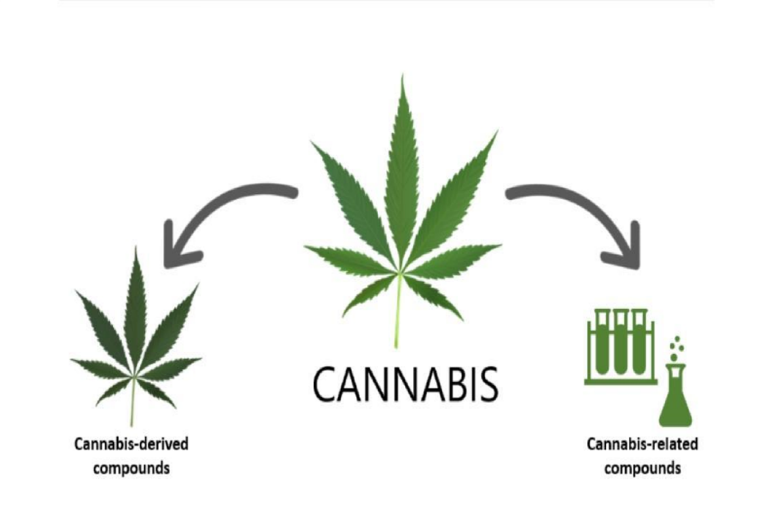
Fig: medical cannabis ethnics
Cannabis:
Cannabis sativa L. is a plant that contains more than 80 different naturally occurring compounds called “cannabinoids”
Two of the most well-known cannabinoids:
Cannabidiol (CBD)
Tetrahydrocannabinol (THC)
Plants are grown to produce different concentrations of cannabinoids – THC or CBDT These plants variations are known as cultivars
Cannabis_ drived compound’s:
Compounds that occur naturally in the plant – such as CBD and THC These compounds are taken directly from the plant Can be used to make drug products.
Example: highly-purified CBD taken directly from the plant
Cannabis-related compounds:
These synthetic are created in a laboratory Can be used to manufacture drug products Some synthetic compounds may occur in the plant and some do not.
Examples: synthetically-derived (also naturally occurring) dronabinol and nabilone is not naturally occurring
Adopt a highly visible and salient cannabis product symbol:
The regulations (§ 40412(a)) require a warning symbol denoting the presence of cannabis. In initially proposed medical regulations
• Labelling and packaging
48) The role of packaging in the use of medicinal cannabis flower All medicines have unique and specific storage requirements – to keep them safe and maintain both quality and integrity until patient consumption.
52)These storage requirements will include recommendations on materials, temperatures, safety considerations and more, ultimately outlining how to best mitigate risks to the medicine’s effectiveness.
34)For instance, packaging that is inadequate may cause contamination or degradation from exposure to oxygen or other substances, and loss of potency. With medicinal cannabis, air-tight protection, avoidance of UV light, and extreme temperatures are all considerations in ensuring quality.
- some key considerations must be designed and developed into products, including jars and bags: consumption advice, tamper-evident and child-resistant demands, and marketing restrictions.Principal Requirements for Medicinal Cannabis Flower Packaging To meet both the product quality and legal requirements for medical cannabis flower packaging,– to avoid product degradation. Exposure to oxygen can dry out the flower, which negatively affects its potency and efficacy. This also avoids contamination through leakage.
- Temperature – extreme temperatures can affect the potency of the medicine and may have the drying effect. The packaging should be able to provide sufficient temperature regulation to keep the product fresh and its integrity intact.
- Humidity – too much or too little moisture can affect medicinally grown cannabis flower in either encouraging mold growth or through drying of the product. Packaging must provide a protective barrier to the medicine for suitable humidity control.
- UV light -UV light is harmful to medicinal cannabis products; thus, it degrades the product and reduces its efficacy. Packaging, therefore must be endowed with UV light protection in its outer barrier.
- Child resistance – with all prescription medicines, child-resistant packaging is paramount. Packaging jars or bags must be designed to prevent children’s access, and thereby accidental ingestion of the product, while still easy to access for adults.
- Labelling – packaged products must also comply with the rules of labelling, which include dosage and administration instructions, expiry dates, storage conditions and warnings. Controls are also in place over what cannot be placed on medicinal cannabis flower labels-such as claims about the product’s medical benefits if not derived from strong evidence.
Information that must be includeding following
List of ingredients:
This is the same as for food. However, the method of extraction for the cannabis extract as an ingredient should be specified. For non-edibles, the solvent for extraction may be non-food grade, such as butane. Solvents for extract bound for edibles must be food-grade, and supercritical carbon dioxide is used by the cannabis industry, but not exclusively. Ethanol and water extraction are also used
• Market Access & Distribution
38)The market and distribution of cannabis-based pharmaceutical products are rapidly growing due to the increasing legalization of such products, breakthroughs in medical studies, and growing demand for alternative treatments. Here is the overview of the key aspects of the market and distribution of cannabis-based pharmaceuticals:
Market of Cannabis-Based Pharmaceutical Products
46)The global market for cannabis pharmaceuticals is rapidly growing because of the increasing acceptance of cannabis in medical treatments and changes in regulations. Some of the key points include:
? Market Segmentation:
Therapeutic Areas: The market is divided based on the medical conditions that cannabis products are used to cure, that include:
- Chronic Pain: Cannabis-based products, especially those containing THC or CBD are commonly used to treat chronic pain which includes conditions such as neuropathic pain and pain from cancer or conditions like fibromyalgi
- Neurological Disorders: There is Epidiolex (CBD), which is already approved for the treatment of two rare types of epilepsy: Dravet syndrome and Lennox-Gastaut syndrome. Additional investigations on the use of cannabis to treat multiple sclerosis and Parkinson’s disease are ongoing.
- Mental Health: Cannabis, particularly CBD, is being studied in its potential to address anxiety, depression, PTSD, and other psychological and neurological diseases.
- Nausea and Vomiting: Cannabis is administered to also suppress nausea and vomiting resulting from chemotherapy.
- Product Types: Cannabis pharmaceutical products may include:
- Oils and Tinctures: These are typically the most common type of cannabis medication available, offering precise dosing.
- Capsules and Tablets: Oral products that provide controlled doses, widely used in pharmaceutical applications.
- Topical: Creams and balms applied to the skin for localized relief, particularly in the treatment of pain and inflammation
- Injectables: In some jurisdictions, cannabis-based injectable formulations are being developed specifically for certain medical uses.
49) Market Distribution: The pharmaceutical market of cannabis is spreading to the entire world. Certain countries such as North America and Europe are widely developed with progressive legalization policies. Major markets include:
North America: The United States and Canada have legitimized medical cannabis. The U.S. market will be largely opened with prospects across the states, which are progressing to approve cannabis for medical purposes and the FDA is allowing more cannabis-based drugs in the United States.
Europe: Germany, UK, and Italy are particularly expanding the medical cannabis programs. Germany happens to be one of the largest markets for cannabis medicine in Europe.
Asia and Latin America: The markets in these regions take time to grow, but medical cannabis is fast gaining popularity, starting from countries such as Israel and Brazil
Market Trends
Increased Research & Development: With more clinical trials conducted and increasing medical literature being published on the medical benefits of cannabis, more evidence keeps coming out for use in some specific medical conditions, thereby expanding the market and increasing investor interest.
Consumer Demand: Changing public perceptions about cannabis and the increasing desire to move away from prescription medication makes the demand for cannabis medicine stronger, especially in pain management, mental health, and neurological diseases.
Insurance & Reimbursement: In some regions, as medical cannabis products become more mainstream, insurance covers certain cannabis medications, opening access for many patients.
Distribution of Cannabis Pharmaceutical Products
The distribution of cannabis-based pharmaceutical products is complex due to challenges facing the legal and regulatory necessities, but steadily improving with changes in the cannabis law.
Key aspects of distribution include:
45)Regulatory Landscape and Distribution Channels
Controlled Distribution: Cannabis pharmaceuticals are highly controlled. Their distribution is often limited to authorized distributors, pharmacies, and medical facilities. In many countries, prescriptions may be required and distribution limited to licensed medical dispensaries or pharmacies.
Licensing Requirements: Pharmaceutical companies must obtain licenses for manufacturing, distributing, and selling cannabis products. These licenses are granted based on strict regulatory criteria to ensure product safety, quality, and compliance with health standards.
Online Sales: In some regions, like Canada and the U.S., cannabis pharmaceuticals can be sold through licensed online dispensaries or pharmacies, which helps expand access for patients, especially those in rural areas.
- Post marketing surveillance
37)-marketing surveillance in the case of cannabis-based pharmaceutical products is, therefore, very important to ensure that treatments remain safe and effective for the general population. PMS would help ensure that cannabis therapies are used with confidence in medical practice by closely monitoring adverse events, gathering real-world evidence, and responding to emerging safety signals. However, the dynamic nature of cannabis medicine necessitates continuous adjustments in monitoring activities and mutual coordination between service providers, regulatory authorities, and researchers.
International Consideration
45)International Coordination and Harmonization
Harmonization of Regulations: Even though a lot of progress has been made, international harmonization of regulations regarding cannabis still presents the greatest challenge. Countries have different attitudes towards cannabis with their regulatory frameworks reflecting diverse levels of scientific evidence, public acceptance, and political will. Accordingly, there are inconsistencies in approval processes in regulation, standard for the quality of the product, and the type of conditions for which cannabis can be prescribed.
32)International Trade and Export: As the medical cannabis market takes on more significance, some countries begin focusing on becoming global exporters of cannabis-based pharmaceuticals. For example, Canada and Uruguay have set themselves up as exporters of medical cannabis but face the obstacle of international trade in the form of different national regulations. The countries have to create systems that assure their products meet all of the safety and quality standards outlined internationally.
- Public Health & Education
30)Public Health and Pharmaceutical Medicine
Public health plays a highly relevant role in pharmaceutical medicine-areas of particular importance include:
Drug safety and drug efficacy: It is up to public health authorities like the FDA or WHO to assume responsibility for approval of drugs, ensuring that they are safe and effective and available for public access.
Disease Prevention and Treatment: Pharmaceutical industries manufacture vaccines, therapies, and prophylactic treatments in the management and elimination of diseases. Most public health programs find collaboration with these companies to ensure extensive production and utilization of the drugs.
Access and Equity: Ensuring that medicines reach underserved populations is a public health priority. This includes working on pricing strategies, global health initiatives, and ensuring essential drugs are available even in low-income areas.
Surveillance: Public health continues to monitor the side effects of drugs, long-term results, and appropriate actions in protecting populations. There is regulatory activities, market surveillance, data gathering on the efficacy of medicines among diverse populations.
44,45) Education pertaining to pharmaceutical medicine encompasses the following:
Medical Education: It calls for professional education and training for healthcare professionals, like doctors, nurses, and pharmacists, on a new medicine, its use, side effects, and the correct administration of the medicine. This is in relation to the proper usage of pharmaceuticals in care.
Public Awareness Campaigns: Public health campaigns educate communities to increase awareness about the importance of vaccines, the risks associated with non-prescription drug abuse, or the need for regular medication adherence, especially in chronic diseases like diabetes or hypertension.
Research and Development Education Academic institutes and research organizations have the role of training young minds in the field of pharmaceutical research, and clinical trials. These are the foundations for developing a better medical treatment and high standards of new medicines created for consumption.
• Ethical and social consideration
Ethical considerations in cannabis with THC-based research
41)Because legal landscapes vary so widely from state to state and because THC-containing cannabis is a federally controlled substance, research participants who report being habitual users may be at legal risk if their identity is disclosed. Researchers may apply for a certificate of confidentiality (CoC). NIH provides CoCs for unfunded or unregulated research under certain conditions, and FDA provides them for research done under an IND or IDE
11)Another ethical issue is impairment. A fundamental element of ethical research is obtaining informed consent from respondents. Some cannabis use research projects, for example case study 1 may require consenting participants who may be impaired at the time of consent. Care will be taken to ensure that the consent process is effective and that participants comprehend the research and are giving their free consent to participate. Depending on the level of impairment, their ability to understand and make sound decisions may be compromised
• Future direction:
47)The future direction of R&D of cannabis in medicine will be shaped by both scientific advancements in understanding the substance and by technological innovation. Here are some of the key areas where R&D is likely to evolve:
Understanding Cannabinoid Mechanisms
42)The endocannabinoid system (ECS): Understanding how cannabinoids such as THC, CBD, and others interact with the ECS better will make therapeutic applications in medicine much more refined. Scientists are researching how cannabinoids influence different biological systems and if certain receptors or pathways are possible targets for pain, inflammation, or neurological disorders.
Pharmacodynamics and Pharmacokinetics: Better pharmacokinetics (how the body absorbs, distributes, metabolizes, and excretes cannabinoids) and pharmacodynamics (how cannabinoids exert their effects) understandings will optimize dosing regimens and enhance therapeutic outcomes.
34,35,) Standardized Cannabis-Based Medications
Formulation of medicinal products: in the future, cannabis-based medicine will be made by producing consistent, standardized products. This will include the formulation of reliable pharmaceutical forms like capsules, oils, patches, and inhalers with specific cannabinoid profiles to treat different condoutcome
Cannabinoid combinations: further research will investigate the synergistic effects of combining various cannabinoids, such as THC, CBD, CBG, CBN and even other terpenes, in order to maximize therapeutic benefit while minimizing adverse effects.
Neurological Disorders
Epilepsy and Seizure Disorders: Therapeutic approaches combining CBD with other cannabinoids or only containing CBD, such as that in Epidiolex, are being prescribed for certain forms of epilepsy. Further research will likely see these treatments, based on their neuroprotective and anti-inflammatory effects, extended to conditions such as Parkinson’s disease, Alzheimer’s, multiple sclerosis, and migraines.
Mental health: Cannabis is used to treat anxiety, depression, PTSD, and other psychiatric conditions. Studying how cannabinoids can affect neurotransmitters and brain networks will bring targeted treatments for mental health into the spotlight.
38)Pain Management
Chronic Pain and Inflammation: Cannabis has been promising for chronic pain, especially in conditions like arthritis and fibromyalgia. Neuropathic pain also responds well to it. The R&D may be targeting an understanding of how cannabinoids interact with the pain pathways of the nervous system and their effectiveness in conjunction with other available pain management techniques.
Opioid Alternatives: The opioid crisis continues, so research involving cannabis as an alternative for pain relief, including the potential to mitigate opioid use or enhance opioid treatments with fewer side effects, continues to be explored.
Cancer and Oncology
In addition, cannabinoids may synergize with treatment protocols to enhance the effects of chemotherapy and radiation and reduce side effects of chemotherapy such as nausea and pain. Some research on cannabinoids also shows potential to have anti-tumor activity via prevention of proliferation of cancer cells, thus potentially altering cancer cell growth and metastasis. More research into these mechanisms may yield new approaches to cancer therapies and adjunct treatments.
48)Personalized Medicine and Precision Dosing
Genetic and Biomarker Research: Through R&D, it has been established how genetic variations may impact responses to cannabinoids. As genetic markers are identified, treatment with cannabis may be personalized to coordinate with a person’s unique biochemistry, yielding higher efficacy and fewer side effects.
AI and Data Analytics: Artificial intelligence and machine learning are expected to advance to the point where large datasets will enable the prediction of patients’ responses to cannabis-based treatments, thus optimizing therapy protocols for conditions.
Safety, Side Effects, and Long-Term Use
Chronic Use and Safety Profiles: Cannabis is viewed as a relatively safe drug although there is a great need for more information regarding chronic effects of its use in medicinally based uses, including risk potential for addiction, impairment in cognition, or psychoses in vulnerable individuals, such as adolescents or otherwise those with some predisposition towards psychiatry disorders.
Side Effects and Drug Interactions: Cannabis is commonly administered concomitantly with other drugs. A major focus of research will be to identify potential interactions and maximize safe use, especially for patients on complex regimens, including cancer and HIV.
42,46)International Policy and Regulatory Environment
Regulatory Approvals and Access: As more countries accept cannabis in practice, the development work will face changing regulatory frameworks. Approval processes for most medical cannabis-based drugs are slow and complicated in most regions. Simplifying these while maintaining safety and efficacy would be the way for future developments.
Clinical Trials and Evidence-Based Medicine: More well-designed, large-scale, randomized controlled trials (RCTs) are necessary to generate robust evidence for the clinical benefits of cannabis in medicine. This will support its broader acceptance and integration into mainstream healthcare systems.
56,57)Cannabis as a Preventative and Wellness Treatment
Preventative Medicine: Beyond treating diseases, cannabis-based products might emerge as preventative treatments, particularly for conditions like inflammation, autoimmune diseases, and neurodegenerative disorders.
Wellness: The interest in cannabis product use for general wellness purposes continues to grow, including stress relief, sleep improvement, and mood regulation. Research will examine how cannabis fits into holistic health strategies.
• CONCLUSION:
Evaluating the Regulation of Cannabis in Pharmaceutical Medicine is indicative of several key points that have to be considered for safe and effective use. As cannabis continues to gain recognition for its potential therapeutic benefits, regulation of the use of cannabis in medical contexts must take an appropriate balance between patient safety, science evidence, and public health concerns.
Scientific Evidence and Medical Efficacy: There is a increasing volume of scientific research that will form the basis of cannabis’s efficacy in addressing certain medical conditions, including chronic pain, epilepsy, and chemotherapy-induced nausea. Although considerable clinical studies and follow-up work are still pending in order to understand the scope of its therapeutic potential and risks, it would help shape evidence-based regulations.
Regulation Challenges: One of the main challenges in regulating cannabis for medicinal use is ensuring its quality, safety, and consistency. The lack of standardization in potency and the presence of contaminants in cannabis products pose significant risks. Regulatory bodies must implement strong quality control measures and enforce strict guidelines for manufacturing, testing, and distribution.
Legal and Ethical Issues: The legal framework for the use of cannabis is very complex with many differences in between the countries and regions or area within their countries. Patients will be appropriately accorded the needed access, appropriate consent, and the remit given to healthcare professionals who may prescribe cannabis-ethically.
Guidelines for Prescription: Prescription guidelines of cannabis should facilitate safe access for the patients but not misuse and abuse. The development of clear guidelines for healthcare providers on when and how to prescribe cannabis will be essential in ensuring that the appropriate care is provided to the patient without significant risks of dependence or diversion.
Public Health and Safety: While its use is rising in medical settings, there is a need to examine public health implications in relation to its impacts on mental health, the ability to function cognitively, and addiction rates. Such surveillance and data collection will be necessary for regulation adaptation as long-term effects of cannabis are learned better.
REFERENCES
- The Evolving Landscape of Cannabis Edibles April 2019Current Opinion in Food Science 28DOI:10.1016/j.cofs.2019.03.009
- The intersection between cannabis and cancer in the United States Author links open overlay panel Daniel W. Bowles a b c Cindy L. O’Bryant a d D. Ross Camidge a , Antonio Jimeno a b
- H Wall et al.Adverse health effects of non-medical cannabis use Lancet (2009)
- S. Aldington et al.Cannabis use and cancer of the head and neck: case–control study Otolaryngol Head Neck Surg(2008)
- May 2016Medical Marijuana Use in OncologyA Review Gianna Wilkie, BS1; Bachir Sakr, MD2; Tina Rizack, MD, MPH2 Author Affiliations Article Information JAMA Oncol. 2016;2(5):670-675. Doi:10.1001/jamaoncol.2016.0155
- The pharmacologic and clinical effects of medical cannabis 2013, Pharmacotherapy
- Medical cannabis regulation: an overview of models around the world with emphasis on the Brazilian scenario Maíra Ribeiro de Souza et al. J Cannabis Res. 2022.
- Front Pharmacol. 2023 Apr 7;14:1153584. Doi: 10.3389/fphar.2023.1153584 The evolving culture of medical cannabis in Canada for the management of chronic pain
- Regulating Cannabis Manufacturing: Applying Public Health Best Practices from Tobacco Control Daniel G Orenstein a, Stanton A Glantz b,iD
- Stability testing for medical Cannabis – What needs to be considered
- Let’s Talk About It: Cannabis RemediationFeb 23, 2024ByPatrick Bird
- Evolution and Classification of Cannabis sativa (Marijuana, Hemp) in Relation to Human UtilizationPublished 19 August 2015 Volume 81, pages 189–294, (2015) Cite this article
- Cannabis sativa: origin and history, glandular trichome development, and cannabinoid biosynthesis Ziyan Xie, Yaolei Mi, Lingzhe Kong, Maolun Gao, Shanshan Chen, Weiqiang Chen, Xiangxiao Meng, Wei Sun, Shilin Chen, Zhichao Xu Author Notes Horticulture Research, Volume 10, Issue 9, September 2023, uhad150, https://doi.org/10.1093/hr/uhad150
- Evolution and Classification of Cannabis sativa (Marijuana, Hemp) in Relation to Human Utilization
- Medical cannabis regulation: An overview of models around the world with emphasis on the Brazilian scenario Maíra Ribeiro de Souza, Amélia Teresinha Henriques, Renata Pereira Limberger Journal of Cannabis Research 4 (1), 33, 2022
- Legal and regulatory aspects of medical cannabis in the United States Genewoo Hong, Alexandra Sideris, Seth Waldman, Joe Stauffer, Christopher L Wu Anesthesia & Analgesia 138 (1), 31-41, 2024
- New developments in cannabis regulation Beau Kilmer Lisbon: EMCDDA, 2017
- 26 Stan. J.L. Bus. & Fin. 362 (2021) Legal Strategy during Legal Uncertainty: The Case of Cannabis Regulation.
- A Systematic Review and Narrative Synthesis of the Evolution of Adolescent and Young Adult Cannabis Consumption Before and After Legalization Author links open overlay panelA Lachance a d Richard E. Bélanger M.D. b c Mylène Riva Ph.D. a d Nancy A. Ross Ph.D. a
- Legal and Regulatory Issues Governing Cannabis and Cannabis-Derived Products in the United States.
- Regulatory Challenges and Opportunities in the Cannabis Industry.
- Randall, R., and O’Leary, A. (1998). Marijuana Rx: The Patients’ Fight for Medicinal Pot. New York, NY: Hachette Book Group.
- Russo, E. B. (2004). “History of cannabis as a medicine,” in The Medicinal Uses of Cannabis and Cannabinoids, eds G. Robson and Whittle (London: Pharmaceutical Press)..
- Single Convention on Narcotic Drugs (1961). Single Convention on Narcotic Drugs. Available at: https://www.incb.org/documents/NarcotiDrugs/1961Convention/convention_1961_en.pdf (accessed October 25, 2018).
- The Medicalization of Cannabis (2009). Wellcome Witnesses to Twentieth Century Medicine, Vol. 40. London: Wellcome Trust Centre for the History of Medicine.
- US Dep’t of Health and Human Services and US FDA (2016). Botanical Drug Development: Guidance for Industry. Silver Spring: US FDA. 26.Vantreese, V. L. (2002). Hemp support: evolution in EU regulation. J. Industr. Hemp 7, 17–31. Doi: 10.1300/j237v07n02_03
- Wang, M., Wang, Y. H., Avula, B., Radwan, M. M., Wanas, A. S., van Antwerp, J., et al. (2016). Decarboxylation study of acidic cannabinoids: a novel approach using ultra-highperformance supercritical fluid chromatography/photodiode array-mass spectrometry. Cannabis Cannabinoid Res. 1, 262–271. Doi: 10.1089/can.2016.0020
- Yeh, B. T. (2012). The Controlled Substances Act: Regulatory Requirements. Washington, DC: Congressional Research Service.
- Changes in Cannabis Use From 2014 to 2019 Among Young Adults in Washington State Author links open overlay panel Katarina Guttmannova PhD , Charles B. Fleming MA , Isaac C. Rhew PhD, MPH , Miranda L.M. Delawalla PhD, MPH , Anne M. Fairlie PhD , Mary E. Larimer PhD , Jason R. Kilmer PhD
- Cannabis use disorders among adults in the United States during a time of increasing use of cannabis Author links open overlay panel Wilson M. Compton a Beth Han b Christopher M. Jones c Carlos Blanco a
- Recreational cannabis legalization alters associations among cannabis use, perception of risk, and cannabis use disorder treatment for adolescents and young adults Author links open overlay panel Jeremy Mennis , Thomas P. McKeon , Gerald J. Stahler
- Planting the seed for marijuana use: Changes in exposure to medical marijuana advertising and subsequent adolescent marijuana use, cognitions, and consequences over seven years Author links open overlay panel Elizabeth J. D’Amico a , Anthony Rodriguez a , Joan S. Tucker a , Eric R. Pedersen a , Regina A. Shih b
- Trends in and correlates of medical marijuana use among adults in the United States Author links open overlay panel Beth Han a , Wilson M. Compton b , Carlos Blanco b , Christopher M. Jones a.
- Daily cannabis use, cannabis use disorder, and any medical cannabis use among US adults: Associations within racial, ethnic, and sexual minoritized identities in a changing policy context Author links open overlay panel Pia M. Mauro a , Morgan M. PhMeta-analysis Substance Use Systematic Review and Meta-Analysis: Medical and Recreational Cannabis Legalization and Cannabis Use Among Youth in the United States Author links open overlay panel Aditya K.S. Pawar MD a , Elizabeth S. Firmin BA b , Timothy E. Wilens MD b , Christopher J. Hammond MD,
- Systematic Review and Meta-Analysis: Medical and Recreational Cannabis Legalization and Cannabis Use Among Youth in the United States Author links open overlay panel Aditya K.S. Pawar MD a , Elizabeth S. Firmin BA b , Timothy E. Wilens MD b , Christopher J. Hammond MD, PhD c
- Effects of cannabis legalization on the use of cannabis and other substances.
- Cannabis use, health problems, and criminal offences in Germany: national and state-level trends between 2009 and 2021 Original Paper Open access Published: 19 March 2024 (2024) Cite this article
- Who seeks treatment for cannabis use? Registered characteristics and physical, psychological and psychosocial problem indicators among cannabis patients and matched controls Research article Open access Published: 22 June 2018 Volume 18, article number 780, (2018) Cite this article Download PDF.
- Assessing the Impact of Recreational Cannabis Legalization on Cannabis Use Disorder and Admissions to Treatment in the United States Published: 10 April 2023 Volume 10, pages 198– 209, (2023)
- Treatment demand for cannabis use problems: analyses of routine data from 30 European countries Original Paper Open access Published: 12 June 2024 (2024) Cite this article.
- History of Cannabis Regulation and Medicinal Therapeutics: It’s Complicated Author links open overlay panel Julie K. Johnson PhD Alexander Colby MA
- The history, evolution, and practice of cannabis and E-cigarette industries highlight necessary public health and public safety considerations Author links open overlay panel Alaina K. Holt a b , Justin L. Poklis c , Michelle R. Peace a
- Chemical constituents of marijuana: The complex mixture of natural cannabinoids Author links open overlay panel Mahmoud A. ElSohly a b , Desmond Slade a
- A history of United States cannabis law David V Patton Jl & HealTH 34, 1, 2020.
- Germany's evolving framework for cannabis legalization and regulation: Select comments based on science and policy experiences for public health Benedikt Fischera,b,c,d bfischer@sfu.ca ? Wayne Halle, f.
- Public health implications of legalising the production and sale of cannabis for medicinal and recreational use Lancet. 2019; 394:1580-1590
- Evolution of Marijuana Research at the Biopsychosocial Level: a General View September 2023International Journal of Mental Health and Addiction DOI:10.1007/s11469-023-01129-4
- Exploring perceptions toward biometric technology in service encounters: A comparison of current users and potential adopters January 2011Behaviour and Information Technology 32(3):1-14DOI:10.1080/0144929X.2011.553741
- Taking stock of progress: Cannabis legalization and regulation in Canada
- Cannabis retailer marketing strategies and regulatory compliance: A surveillance study of retailers in 5 US cities Author links open overlay panel Carla J. Berg a b , Katelyn F. Romm c d , Alexandria Pannell e , Priyanka Sridharan f , Tanvi Sapra f , Aishwarya Rajamahanty g , Yuxian Cui a Yan Wang a b , Y. Tony Yang b h , Patricia A. Cavazos-Rehg g.
- Federal Regulations of Cannabis for Public Health in the United States.
- A narrative review of molecular mechanism and therapeutic effect of Cannabidiol (CBD) January 2022Basic & Clinical Pharmacology & Toxicology 130(8) DOI:10.1111/bcpt.13710.


 Ankita Mahakal*
Ankita Mahakal*


 10.5281/zenodo.14886343
10.5281/zenodo.14886343